Abstract
BACKGROUND
Hypertonic saline (HTS) and mannitol are effective in reducing intracranial pressure (ICP) after severe traumatic brain injury (TBI). However, their simultaneous effect on the cerebral perfusion pressure (CPP) and ICP has not been studied rigorously.
OBJECTIVE
To determine the difference in effects of HTS and mannitol on the combined burden of high ICP and low CPP in patients with severe TBI.
METHODS
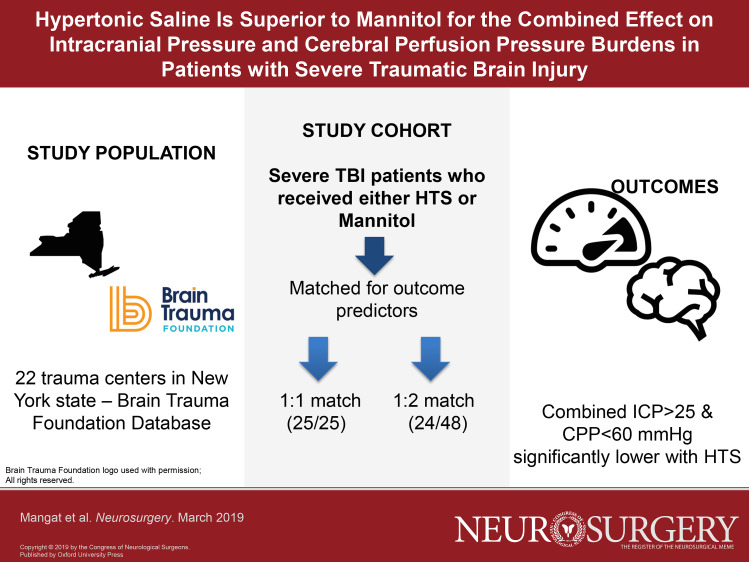
We performed a case–control study using prospectively collected data from the New York State TBI-trac® database (Brain Trauma Foundation, New York, New York). Patients who received only 1 hyperosmotic agent, either mannitol or HTS for raised ICP, were included. Patients in the 2 groups were matched (1:1 and 1:2) for factors associated with 2-wk mortality: age, Glasgow Coma Scale score, pupillary reactivity, hypotension, abnormal computed tomography scans, and craniotomy. Primary endpoint was the combined burden of ICPhigh (> 25 mm Hg) and CPPlow (< 60 mm Hg).
RESULTS
There were 25 matched pairs for 1:1 comparison and 24 HTS patients matched to 48 mannitol patients in 1:2 comparisons. Cumulative median osmolar doses in the 2 groups were similar. In patients treated with HTS compared to mannitol, total number of days (0.6 ± 0.8 vs 2.4 ± 2.3 d, P < .01), percentage of days with (8.8 ± 10.6 vs 28.1 ± 26.9%, P < .01), and the total duration of ICPhigh + CPPlow (11.12 ± 14.11 vs 30.56 ± 31.89 h, P = .01) were significantly lower. These results were replicated in the 1:2 match comparisons.
CONCLUSION
HTS bolus therapy appears to be superior to mannitol in reduction of the combined burden of intracranial hypertension and associated hypoperfusion in severe TBI patients.
Keywords: Cerebral perfusion pressure, Hypertonic saline, Intracranial pressure, Mannitol, Traumatic brain injury
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- AIS
American Spinal Injury Association Impairment Scale
- CBF
cerebral blood flow
- CPP
cerebral perfusion pressure
- CT
computed tomography
- GCS
Glasgow Coma Scale
- HTS
hypertonic saline
- ICP
intracranial pressure
- IQR
interquartile range
- ISS
injury severity score
- TBI
traumatic brain injury
Traumatic brain injury (TBI) is a significant cause of morbidity and mortality in developed countries and is emerging as a major cause of death in developing countries. More than 500 000 individuals with TBI suffer permanent neurological disability and 50 000 die each year in the United States.1
Surgical and medical management of severe TBI has undergone significant advancement in the last decades as evidenced by decreasing mortality. Development and compliance with the Brain Trauma Foundation guidelines (hereafter referred to as Guidelines) for management of severe TBI has been key in significantly reducing mortality.2 While primary surgical intervention is aimed at evacuating extra-axial hematomas, the overarching principle of medical management is to treat elevated intracranial pressure (ICP) and decreased cerebral perfusion pressure (CPP).3-6 There are several therapies employed for achieving these, and use of hyperosmotic therapy is often first line in emergent treatment of discrete episodes of elevated ICP as well as episodes of cerebral herniation, due to the favorable therapeutic risk–benefit profile.7-12
Data on the use of hyperosmotic agents have focused on lowering elevated ICP, while the effect on CPP has been considered a secondary endpoint and not rigorously studied. An ideal hyperosmotic agent should simultaneously lower ICP and maintain or improve CPP. The previous Guidelines included level II recommendations for the use of mannitol for treating intracranial hypertension, while no recommendation existed supporting the use of HTS due to lack of evidence.13 The current iteration of the Guidelines published in 2016 state “while there is increasing use of hypertonic saline as an alternative hyperosmotic agent there is insufficient evidence available from comparative studies to support a formal recommendation.”6
Several studies have demonstrated the efficacy of HTS and mannitol in ICP reduction.8,10,12,14-20 However, debate continues on the superiority of either agent and there exists no data on the effect of these agents on the combination of high ICP and low CPP. In the current study, we examined the effect of HTS and mannitol on the combination of cumulative daily periods of ICP > 25 mm Hg (ICPhigh) and CPP < 60 mm Hg (CPPlow), during the entire treatment of intracranial hypertension in patients with severe TBI. We have used a pragmatic approach and examined cumulative burdens of simultaneously high ICP and low CPP over the entire period of acute TBI rather than a single dose–response basis or sole effect on ICP alone, in an effort to clarify overall superiority of either agent. We also compared the effects of the 2 agents on CPP to discern if either agent was associated with lowered cerebral perfusion related to systemic effects on diuresis, intravascular volume, and cardiac output, since mannitol is a diuretic and HTS is not.
METHODS
Study Design and Setting
This is a case–control study using prospectively collected data from the TBI-trac® database (Brain Trauma Foundation, New York, New York) established to monitor compliance with existing Guidelines in the management of severe TBI in New York State. The database was supported by the New York State Department of Health and implemented across 20 level I and 2 level II trauma centers. In addition to monitoring compliance to Guidelines, the database also allowed for the testing of clinical hypotheses that could improve upon the current guidelines as part of a quality improvement program. Clinical information about patients with severe TBI was entered into the database prospectively by trained nurse coordinators at participating institutions. The information includes data from the prehospital period, the emergency department, and the first 10 d in the intensive care unit along with 2-wk mortality. No patient identifiers were entered into the database to maintain patient confidentiality. The protocol was either approved by or exempt from review by each participating institutional review board. No patient consent was required for inclusion in the TBI registry.
Participants
Data from patient admissions between June 6, 2000 and August 21, 2008 when the database was discontinued were reviewed. The study population included adult patients (>16 yr) who had suffered a severe TBI and were hospitalized for at least 5 d. Patients were included if they received only 1 hyperosmolar agent, either HTS or mannitol, for the treatment of intracranial hypertension. When both agents were used, data were not available regarding the reasons for utilizing the second agent, ie, it was used based on physician preference, drug availability, or treatment failure. Therefore, patients who received both agents were excluded from data analysis to prevent erroneous conclusions. Patients were also excluded if they met any of the following criteria on day 1: Glasgow Coma Scale (GCS) score of ≥ 9, motor score of 6, GCS score of 3 with bilateral fixed and dilated pupils, arrival at the trauma center 24 h or more following injury, and death on day 1. Patients with a do-not-resuscitate/do-not-intubate order or an advanced directive requesting no heroic measures were also excluded. Mannitol doses were recorded by range per day (0-50, 51-100, 101-200, 201-600 g/d). HTS doses were converted to similar osmolar ranges, and medians were compared between HTS and mannitol. Injury severity scores (ISS) were compared.
Patient Matching
Patients were matched for day of ICP monitor insertion as well as known predictors of 2-wk mortality after severe TBI namely age, initial GCS score, hypotension on day 1, pupillary reactivity, abnormalities on computed tomography (CT) scan and extra-axial surgical lesions.21 Since the HTS group was not very large, we performed exact matching for differences in baseline characteristics between the 2 groups.22 In order to include maximal numbers of matched pairs, age and baseline CT abnormality were not included in the matching since their standardized difference was <20% and the P-value was > .05 (ie they were balanced between the 2 groups), and consequently we evaluated the these variables between the 2 groups after matching. Additional to the 1:1 matching, for sensitivity analyses we also performed a 1:2 matching. Patients without matches or with missing or erroneous data were excluded.
Outcome Variables
The primary outcome measure was the burden of combined high ICP and low CPP (ICPhigh + CPPlow) as measured by total burden and cumulative burden. Total burden was represented by the total number of days of ICPhigh + CPPlow. The cumulative ICPhigh + CPPlow burden was calculated as number of days with ICPhigh + CPPlow as a percentage of total days of ICP monitoring. In addition, the cumulative number of hours with ICPhigh + CPPlow was calculated. We also examined the total, cumulative, and averaged daily CPPlow burden and associated systemic hypotension and vasopressor use during the period of ICP monitoring.
Statistical Analysis
The comparison is made between 2 groups of patients receiving either HTS or mannitol. Propensity score matching was not possible due to the small sample sizes; we therefore used an exact matching approach. Baseline differences between the groups were assessed using 2 methods. First, continuous variables were compared using the 2 sample t-test, and categorical variables were compared using chi-square or Fisher exact test. Second, the standardized difference, which is the difference in mean in units of the pooled standard deviation between patients in the 2 groups, was computed. A standardized difference of >20% has been suggested to represent meaningful imbalance between groups, as described previously.23 Either a standardized difference of >20% or a P-value of < .05 was used to determine baseline differences between the 2 groups.
The differences in outcome measures between matched pairs were evaluated using the Wilcoxon signed rank test for continuous variables and the Cochran–Mantel–Haenszel test for categorical variables. All statistical tests were 2-sided, and P < .05 was considered statistically significant. Analyses were performed using SAS version 9.3 software (SAS Institute Inc, Cary, North Carolina).
RESULTS
Patient Selection and Matching
Data from 22 trauma centers were available. We identified 2641 patients with severe TBI, of whom 512 met inclusion criteria (Figure 1). A total of 35 patients received only HTS as an osmotic agent and 477 received mannitol only. Eight patients were excluded in the HTS group: 7 had missing or wrong data entered. Of the 27 remaining patients who received HTS, exact matches in the mannitol group were found for 25 patients and these were included for the 1:1 matching and data analysis. In the mannitol group, all patients received 20% mannitol; in the HTS group 24 patients received 3% HTS as a bolus while 1 patient received 23.4% HTS as a bolus (osmolar dose was similar between 3% and 23.4% HTS). In the 1:2 matching group, 1 further HTS patient did not have a corresponding match in the mannitol group; therefore, in this group 24 HTS patients were matched to 48 mannitol patients.
FIGURE 1.
Flowchart of patient selection and inclusion.
Baseline demographics and key characteristics were similar in the 2 groups (1:1; 1:2 matching; Table 1). In each group, age and incidence of abnormal CT were not statistically different, whereas GCS score, pupillary abnormality, hypotension on day 1, craniotomy, and day of ICP monitor insertion were matched for and therefore identical. Duration of ICP monitoring (Tables 2 and 3) in the matched groups was not statistically different. In the 1:1 match, 3 patients (2 in HTS and 1 in mannitol group) underwent ICP monitoring with normal CT scans based on level III guideline recommendation, though only 1 did not develop intracranial hypertension at any time. In the 1:2 group, there were 6 patients (2 in HTS and 4 in mannitol group) with normal CT who underwent ICP monitoring, and 3 did not develop intracranial hypertension. Differences in the cumulative median dose ranges for HTS (1101-3300 mOsm) and mannitol (551-1100 mOsm) were not statistically different (P = .19).
TABLE 1.
Baseline Characteristics of the 2 Study Groups After 1:1 and 1:2 Matching for Predictive Variables
| 1:1 Match | 1:2 Match | ||||
|---|---|---|---|---|---|
| HTS | Mannitol | HTS | Mannitol | ||
| (n = 25) | (n = 25) | (n = 24) | (n = 48) | P-value* | |
| Age (years) (mean ± SD) | 34.96 ± 5.41a | 36.68 ± 16.90a | 35.29 ± 15.65b | 32.75 ± 13.61b | .96a .51b |
| GCS score (mean ± SD) | 5.40 ± 1.55† | 5.33 ± 1.54† | |||
| Abnormal pupils % | 16.0† | 16.7† | |||
| Hypotension on day 1% | 16.0† | 16.7† | |||
| Craniotomy % | 24.0† | 25.0† | |||
| Day of ICP insertion (mean ± SD) | 1.16 ± 0.47† | 1.08 ± 0.28† | |||
| Abnormal CT% | 92.0c | 96.0c | 91.7† | .56c | |
HTS, hypertonic saline; SD, standard deviation; GCS, Glasgow Coma Scale; ICP, intracranial pressure; CT, computed tomography.
*The P values were calculated using the Wilcoxon signed-rank or Cochran–Mantel–Haenszel test for paired data.
†matched variables. a,b,c p-values for corresponding variable comparisons.
TABLE 2.
ICP and CPP Burden Comparisons Between HTS and Mannitol Groups in 1:1 Matched Group
| HTS (n = 25) | Mannitol (n = 25) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Med (IQR) | Mean ± SD | Med (IQR) | P-value | |
| ICP monitoring duration (days) | 6.4 ± 2.7 | 5 (4-9) | 7.7 ± 2.7 | 9 (7-10) | .09 |
| No. of days with ICPhigh + CPPlow | 0.6 ± 0.8 | 0 (0-1) | 2.4 ± 2.3 | 2 (0-4) | <.01* |
| % days with ICPhigh + CPPlow | 8.8 ± 10.6 | 0 (0-20) | 28.1 ± 26.9 | 22.2 (0-40) | <.01* |
| Total hours with ICPhigh + CPPlow | 11.12 ± 14.11 | 7 (1-18) | 30.56 ± 31.89 | 18 (10-44) | .01* |
| No. of days with CPPlow | 2.0 ± 1.7 | 1 (1-3) | 3.6 ± 2.8 | 3 (2-5) | .03* |
| % of days with CPPlow | 32.8 ± 23.9 | 30 (20-42.9) | 48.6 ± 44.7 | 33.3 (20-80) | .11 |
| Total hours with CPPlow | 8.88 ± 11.89 | 6 (1-13) | 18.96 ± 21.89 | 11 (3-23) | .06 |
| Averaged daily duration of CPPlow (hours) | 1.5 ± 2.2 | 0.7 (0.3-1.9) | 2.8 ± 3.9 | 1.1 (0.4-3.5) | .20 |
HTS, hypertonic saline; SD, standard deviation; IQR, interquartile range; ICP, intracranial pressure; CPP, cerebral perfusion pressure. *p < .05.
TABLE 3.
ICP and CPP Burden Comparisons Between HTS and Mannitol Groups in 1:2 Matched Group
| HTS (n = 24) | Mannitol (n = 48) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Med (IQR) | Mean ± SD | Med (IQR) | P-value | |
| ICP monitoring duration (days) | 6.6 ± 2.6 | 5.5 (4.5-9.5) | 7.1 ± 2.70 | 8 (5-10) | .46 |
| No. of days with ICPhigh + CPPlow | 0.7 ± 0.8 | 0 (0-1) | 2.2 ± 2.1 | 1.5 (0.5-4) | <.01* |
| % days with ICPhigh + CPPlow | 9.2 ± 10.7 | 0 (0-20) | 30.2 ± 26.7 | 23.6 (5-50) | <.01* |
| Total hours with ICPhigh + CPPlow | 11.54 ± 14.26 | 7 (1-18) | 32.98 ± 35.56 | 17 (8.5-49) | <.01* |
| No. of days with CPPlow | 2.0 ± 1.8 | 1 (1-3) | 3.6 ± 2.6 | 3 (2-5) | .01* |
| % of days with CPPlow | 32.1 ± 24.1 | 27.5 (20-41.4) | 57.1 ± 52.6 | 50 (21.1-80) | .01* |
| Total hours with CPPlow | 9.21 ± 12.03 | 6 (1-15) | 18.85 ± 19.19 | 12 (4-28.5) | .01* |
| Averaged daily duration of CPPlow (hours) | 1.5 ± 2.2 | 0.7 (0.3-1.9) | 3.4 ± 5.1 | 1.9 (0.6-4) | .01* |
HTS, hypertonic saline; SD, standard deviation; IQR, interquartile range; ICP, intracranial pressure; CPP, cerebral perfusion pressure. *p < .05.
ICPhigh + CPPlow Burden
In the 1:1 match, total number of days with ICPhigh + CPPlow burden (0.6 ± 0.8 vs 2.4 ± 2.3 d, P < .01) and cumulative ICPhigh + CPPlow burden (% days) were significantly lower in the HTS group (8.8 ± 10.6 vs 28.1 ± 26.9%, P < .01; Figure 2). The total duration of ICPhigh + CPPlow burden was also lower in the HTS group (11.12 ± 14.11 vs 30.56 ± 31.89 h, P = .01; Table 2). Similarly, in the 1:2 match the total number of days with ICPhigh + CPPlow (0.7 ± 0.8 vs 2.2 ± 2.1 d, P < .01), cumulative ICPhigh + CPPlow burden (% days; 9.2 ± 10.7 vs 30.2 ± 26.7%, P < .01), and total hours of ICPhigh + CPPlow burden (11.54 ± 14.26 vs 32.98 ± 25.56 h, P < .01) were all significantly lower in the HTS group (Table 3).
FIGURE 2.
Effects of HTS and mannitol on ICPhigh + CPPlow.
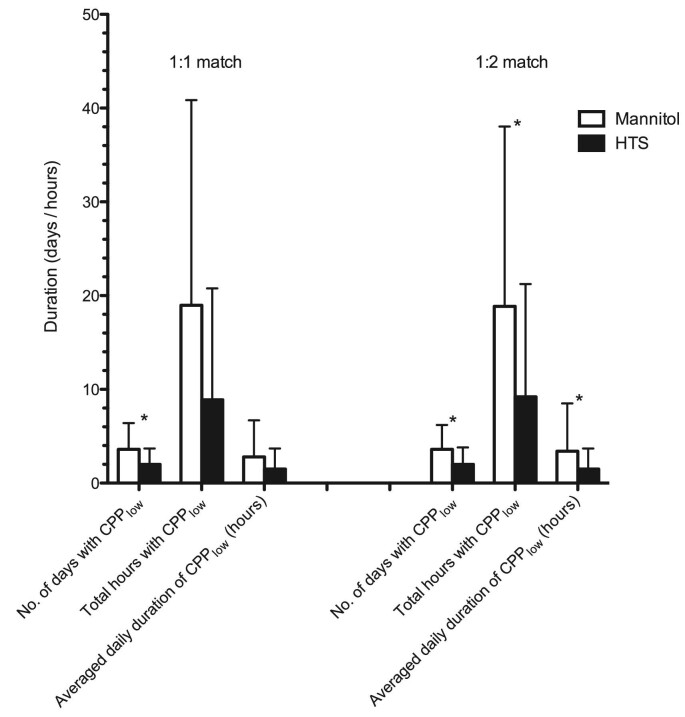
CPPlow Burden
In the HTS group of the 1:1 match, the number of days with CPPlow was significantly lower than in the mannitol group (2.0 ± 1.7 vs 3.6 ± 2.8 d, P = .03; Figure 3). Cumulative CPPlow burden (% days; 32.8 ± 23.9 vs 48.6 ± 44.7%, P = .11), total hours with CPPlow (8.88 ± 11.89 vs 18.96 ± 21.89 h, P = .055), and averaged daily CPPlow burden (1.5 ± 2.2 vs 2.8 ± 3.9 h/d, P = .20) were lower in the HTS group, but this difference was not statistically significant (Table 2).
FIGURE 3.
Effects of HTS and mannitol on CPPlow.
However, in the larger group comparison (1:2 match), total number of days with CPPlow (2.0 ± 1.8 vs 3.6 ± 2.6 d, P = .01), cumulative CPPlow burden (% days; 32.1 ± 24.1 vs 57.1 ± 52.6%, P = .01), total hours with CPPlow (9.21 ± 12.03 vs 18.85 ± 19.19 h, P = .01), as well as the daily averaged CPPlow (1.5 ± 2.2 vs 3.4 ± 5.1 h/d, P = .01) were significantly lower in the HTS group compared with the mannitol group (Table 3).
Hypotension and Vasopressor Use
In the 1:1 match, cumulative number of hours with systolic blood pressure < 90 mm Hg was 0 h (interquartile range [IQR] 0-1) in HTS group and 0 h (IQR 0-1) in mannitol group (P = .69). Similarly cumulative duration of mean arterial pressure < 80 mm Hg was 28 h (IQR 13-46) in the HTS group and 32 (IQR 10-52) in the mannitol group (P > .99). The difference was not statistically significant for either parameter. This was similar in the 1:2 matched group (Table 4). Use of vasopressors was documented in ranges per day (0, 1-8, 9-17, 17-24 h); median category values for duration of vasopressor use were not statistically significantly different between HTS (1 h, IQR 1-1.5) and mannitol group (1 h, IQR 1-1.5; P = .97) in 1:1 as well as the 1:2 matched groups.
TABLE 4.
Comparisons of Incidence of Hypotension and Vasopressor Use Between HTS and Mannitol Groups in 1:1 and 1:2 Matched Groups
| HTS | Mannitol | ||
|---|---|---|---|
| Mean (IQR) | Mean (IQR) | P-value | |
| 1:1 match | |||
| Cumulative duration of SBP < 90 mm Hg (hours) | 0 (0-1) | 0 (0-1) | .69 |
| Cumulative duration of MAP < 80 mm Hg (hours) | 28 (13-46) | 32 (10-52) | .99 |
| Median duration category value for vasopressor usea | 1 (1-1.5) | 1 (1-1.5) | .97 |
| 1:2 match | |||
| Cumulative duration of SBP < 90 mm Hg (hours) | 0 (0-1) | 0 (0-1) | .98 |
| Cumulative duration of MAP < 80 mm Hg (hours) | 27 (12.5-45.5) | 20.5 (10-44) | .55 |
| Median duration category value for vasopressor usea | 1 (1-2.25) | 1 (1-1) | .23 |
HTS, hypertonic saline; SBP, systolic blood pressure; MAP, mean arterial pressure; IQR, interquartile range.
aCategories for vasopressor use: 1 = 0 h, 2 = 1-8 h, 3 = 9-17 h, 4 = 18-24 h administered.
Overall Injury Severity
ISS were not available for all patients. In the 1:1 group, 23 HTS-treated patients and 11 mannitol-treated patients had ISS recorded, while in the 1:2 group, 22 of 24 HTS and 21 of 48 mannitol-treated patients had ISS recorded. In the 1:1 match, median ISS was 35 (IQR 22-38) in HTS and 33 (IQR 26-45) in mannitol groups (P = .36); and American Spinal Injury Association Impairment Scale (AIS) for head was 5 (IQR 3-5) in HTS and 5 (IQR 4-5) in mannitol groups, respectively (P = .27). In the 1:2 cohort, ISS was 36.5 (IQR 22-38) in HTS and 29 (25-45) in mannitol-treated patients (P = .68); and AIS head was 5 (IQR 3-5) and 5 (IQR 4-5) in HTS and mannitol-treated patients, respectively (P = .41). In addition, in all but one patient, AIS head was the highest score compared to other subscores.
DISCUSSION
In this study, we utilized a pragmatic approach in comparing the cumulative effect of HTS and mannitol on the combined durations of high ICP (> 25 mm Hg) and low CPP (< 60 mm Hg): ICPhigh + CPPlow over the entire clinical course of ICP monitoring after severe TBI. Our data demonstrate that in severe TBI patients, HTS use was associated with a greater reduction in the total number of days, the percentage of days, and total hours of ICPhigh + CPPlow burden when compared to mannitol. In addition, the CPPlow burden was significantly lower in HTS-treated patients compared with those who received mannitol. We matched patients in the 2 comparison groups for clinical predictors that impact 2-wk mortality after severe TBI. The results are consistent when compared in a 1:1 and 1:2 match between HTS and mannitol patients respectively for ICPhigh + CPPlow burden, and strengthened for CPPlow burden by comparison of 1:2 matched groups.
We have previously reported on HTS superiority in reducing ICP burden while observing no difference in mortality between HTS and mannitol-treated groups.20 Data from several other studies also demonstrate the superiority of HTS in reducing ICP in periods of intracranial hypertension.10,14-19,24-26 While reducing ICP is a key treatment endpoint, ensuring adequate cerebral perfusion is also essential in the management of intracranial hypertension in patients with TBI. In the uninjured brain, cerebral autoregulation ensures an adequate cerebral blood flow (CBF) over a range of CPP. TBI may impair autoregulation, thereby making CBF more dependent upon CPP, and the extent of this disruption correlates with outcome.27,28 Current guidelines recommend a CPP of 60 to 70 mm Hg, while avoiding an elevated CPP with vasopressors as it contributes to increased incidence of respiratory failure.29 An ideal hyperosmotic agent must decrease ICP as well as ensure maintenance of adequate CPP. Data on the combined effects on ICP and CPP by mannitol and HTS are sparse.
HTS and mannitol share some common mechanisms of action in reducing increased ICP. They enable osmotic fluid shifts, increase cardiac output, improve capillary blood laminar flow, and increase perfusion in ischemic areas by dehydrating endothelial cells.30-33 While these are cited as predominant mechanisms for ICP reduction, several of these also improve CBF and CPP.34,35 And improvement in cerebral perfusion further improves cerebral oxygenation.10,26
In a cohort of 11 patients, in instances of ICP < 20 mm Hg, mannitol administration did not affect ICP or cerebral hemodynamics; with ICP > 20 mm Hg, increased CPP was not reflected in improvements in brain parenchymal oxygenation or jugular oxygenation.35 However, in these patients the initial CPP was not below the threshold of 60 mm Hg. In 6 patients with ICP > 25 mm Hg and CPP < 60 mm Hg who were given a total of 32 infusions of hypertonic/hyperoncotic saline (7.5% HTS combined with 6% hydroxyethyl starch), ICP was lowered 44% and CPP increased 38% at 30 min after administration.12 Vialet et al studied the response to isovolumic HTS and mannitol in 10 patients each; the number and duration of episodes of ICP > 25 mm Hg were significantly lower in the HTS group, whereas the number and duration of episodes of CPP < 60 mm Hg were not significantly different even though the osmolar load and measured osmolality were higher in the HTS group.36 In a similar study with 10 patients in each arm, Francony et al17 administered single equiosmolar dose (255 mOsm) of either 20% mannitol or 7.45% HTS. Mannitol was noted to have a similar reduction in ICP as with HTS up to 120 min, whereas both CPP and mean flow velocities by transcranial Doppler were significantly higher in the patients who received mannitol. However, the baseline CPP in these patients was 75 ± 15 mm Hg and 81 ± 12 mm Hg in the mannitol and HTS groups, respectively, and was not subthreshold. Oddo et al26 reported on the treatment of 42 episodes (28 with mannitol, 14 with HTS) of intracranial hypertension in 12 patients. While both agents were associated with decreased ICP, patients who received HTS had a significant increase in CPP from baseline, which was also not below threshold (63 ± 15 mm Hg to 76 ± 17 mm Hg at 120 min), and this increase was not seen in patients who received mannitol. In addition, HTS patients had a significant increase in brain oxygenation. Similarly, 2 further studies showed increased CPP after HTS therapy; however, one study had only an HTS arm while in the other study, patients were treated at a threshold ICP of 15 mm Hg and averaged baseline values were > 70 mm Hg.10,25 The latter study by Cottenceau et al25 was prospective, which showed an significant increase in CPP in both HTS and mannitol groups that was not different when examined for the combined effect of infusion group and post-treatment measurement time, though CBF was higher in the HTS group. In meta-analyses, Kamel et al37 reported on 5 trials that included patients with non-TBI, showing HTS as a superior agent in reducing ICP, but no data on CPP were reported. Meanwhile, the Cochrane review concluded that mannitol may increase mortality after severe TBI compared to HTS.38 More recently, a pooled data analysis from 3 trials showed continuous hyperosmolar HTS therapy was associated with improved survival over bolus HTS therapy,24 and a prospective clinical trial is underway.39
The above studies are the most meticulous publications examining the effects of HTS and mannitol on ICP and CPP after TBI. As can be seen, these studies are small, have overlap in HTS and mannitol utilization in the same patients, which may potentially include a residual effect from the agent previously dosed, or examine CPP effects in patients who did not have a critically low CPP (< 60 mm Hg) and would not derive any clear benefit from further CPP augmentation. Therefore, we strictly used the burden of CPP < 60 mm Hg as an endpoint by itself as well as in combination with ICP > 25 mm Hg.
We have also addressed a time period bias seen in previous studies, which examined only immediate effects on ICP and CPP changes following single-dose treatment but ignore the delayed effects. Mannitol is a diuretic and there are concerns that it causes subsequent delayed diuresis.17,40,41 However, in our data, we did not observe any difference in occurrence of hypotension between groups, suggesting the effects of HTS may be predominantly in lowering ICP and thereby also reducing incidence of low CPP, without a significant effect on blood pressure. Even brief episodes of intracranial hypertension and cerebral hypoperfusion measured as area under the curve for ICPhigh and CPPlow correlate with poor outcome.42,43 To address the cumulative effects of such brief episodes as well as a possible decreased dose–response to hyperosmotics after repeated doses, we chose to compare the total burden of ICP and CPP during the entire treatment period. It appears from our data that the effects of HTS and mannitol are clearly different when total burden is examined.
Limitations of the Study
First, this is a retrospective analysis of data, and does not allow for the sample to be powered to the highest level of analysis. However, the data were collected prospectively in a systematic and consistent manner. Second, our patient sample size is small due to the low utilization of HTS; however, this is the largest cohort of severe TBI patients studied for ICP and CPP effects of mannitol and HTS. Due to the small number of HTS patients available, we selected patients with no treatment crossover and performed rigorous patient matching to strengthen our results. Third, information about other treatments, practice patterns of individual centers, and reasons for selection of a particular hyperosmotic agent are not known. Therefore, physician bias or other confounders cannot be entirely excluded. While ISS were not available for the entire group, from the available data severity of injury appears similar in the 2 groups, and more importantly, in all but one patient cranial injury was the most severe injury suffered. During the 8-yr period of data collection and in the period since, the ICP threshold changed from 25 to 22 mm Hg, while CPP thresholds have not changed; we assume that the small difference in threshold would not impact the results. Finally, information on adverse events is not available. However, there are some reported in the literature that would warrant close attention, such as renal failure, cardiac failure from volume overload, and severe hypernatremia. By nature of design and data availability, confounding cannot be completely excluded, though as detailed we have made a systematic effort to minimize this effect.
The results of this study suggest that HTS use is associated with lower combined burden of “high ICP and low CPP” in severe TBI patients suffering intracranial hypertension. Whether this has a significant effect on mortality can only be determined in a large randomized controlled trial.
CONCLUSION
HTS bolus therapy for raised ICP was associated with lower incidence and duration of the combination of raised ICP and reduced CPP burden in patients with severe TBI and intracranial hypertension. In addition, patients treated with HTS had lower incidence and duration of low CPP. These data suggest that HTS is superior to mannitol in the treatment of intracranial hypertension after severe TBI. Larger prospective validation is required to determine whether these benefits also result in improvement in patient outcomes.
Disclosures
Dr Gerber and Ms Wu were supported in part by funds from the Clinical and Translational Science Center (CTSC), National Center for Advancing Translational Sciences (NCAS) grant no. UL1-TR000457-06. Financial support was received from the New York State Department of Health, the Brain Trauma Foundation, and the New York-Presbyterian Hospital TBI fund. Dr Ghajar is president of the Brain Trauma Foundation. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
The authors thank the New York State Department of Health, the Brain Trauma Foundation and the New York-Presbyterian Hospital TBI fund for their financial support.
Notes
Parts of these results were presented in poster form (top 10 posters) at the annual meeting of the Neurocritical Care Society, National Harbor, Maryland, September 17, 2016.
Neurosurgery Speaks! Audio abstracts available for this article at www.neurosurgery-online.com.
COMMENT
Despite a lot of recent study comparing hypertonic saline to mannitol there remains uncertainty as to which agent is superior. Unfortunately many of these studies have compared apples to oranges with the osmolalities of the administered agents differing. In addition, specific clinical scenarios such as shock and hyponatremia require additional study to determine the superior agent in these conditions. The work presented here advances our understanding of the clinical effects of mannitol vs. hypertonic saline.
The New York tbi-trac® database (Brain Trauma Foundation) has made important contributions to traumatic brain injury care. It has provided key evidence supporting both intracranial pressure (ICP) and cerebral perfusion pressure (CPP) directed care, and has also suggested dramatic improvements in outcome in relation to adoption of the Brain Trauma Foundation guidelines.1 Among the many patients in this database, some were treated only with mannitol or hypertonic saline, facilitating valuable comparisons. In a previous work examining these patients the authors demonstrated that hypertonic saline was more effective than mannitol at controlling ICP.2 Given the concern with mannitol's diuretic effects the authors have now additionally and appropriately analyzed the effect of mannitol and hypertonic saline on CPP and the composite outcome measure ICPhigh + CPPlow. The BEST: TRIP study has suggested that clinicians are perhaps too focused on ICP alone,3 so this analysis is a welcome one. The results of the performed case-control study led the authors to conclude that hypertonic saline performed better than mannitol with respect to CPP and ICPhigh + CPPlow.
Although this manuscript provides valuable information, it has shortcomings. As CPP is derived by subtracting ICP from the mean arterial pressure (MAP) it is initially unclear whether noted effects relate to ICP or MAP. This is especially true of the composite outcome ICPhigh + CPPlow. The authors found that hypotension was not significantly different between the groups so it is presumed that the effect ultimately seen in this study related to a difference in ICP control (as presented in their previous manuscript2). The suggestion that the two hyperosmolar agents do not differentially affect MAP is quite valuable. There is an important caveat here, though, which the reader should be aware of. Note that patients were matched for burden of hypotension in the first 24 h. Although the total burden of hypotension (including that subsequent to the first 24 h) was the metric incorporated into the outcome measures, matching for this outcome variable should be considered in interpreting the study. The study likely also suffers from some residual confounding - although the osmolality of administered solutions, vasopressor administration and the injury severity score did not differ significantly between the groups they remain potential confounds. Indeed, there was a statistical trend (P = .19) to hypertonic saline treated patients receiving more osmoles. The fact that the data is 10 y old is also a consideration.
It is an increasingly rare day that I use mannitol in my practice but I do find some patients respond better to it than hypertonic saline. Indeed, mannitol should have a superior concentration gradient as it is not normally found in the body. Some unpublished, objective data at my disposal suggests that most neurotraumatologists have also switched to predominant use of hypertonic saline. Better quality data will ultimately be needed to change the guideline recommendations, however. Many clinicians, in my experience, neglect to replace fluid losses associated with mannitol administration and doing so would likely eliminate a lot of the concern with hypotension with this agent. Do not forget that in the second edition of the Brain Trauma Foundation Guidelines for the Management of Severe Traumatic Brain Injury4 mannitol was recommended as a resuscitation fluid given its initial volume expanding effects, so it should not be withheld in a hypotensive, herniating patient if hypertonic saline is not available.
Gregory W. J. Hawryluk
Salt Lake City, Utah
References
- 1. Gerber LM, Chiu YL, Carney N, Hartl R, Ghajar J. Marked reduction in mortality in patients with severe traumatic brain injury. J Neurosurg. 2013;119(6):1583-1590. [DOI] [PubMed] [Google Scholar]
- 2. Mangat HS, Chiu YL, Gerber LM, Alimi M, Ghajar J, Hartl R. Hypertonic saline reduces cumulative and daily intracranial pressure burdens after severe traumatic brain injury. J Neurosurg. 2015;122(1):202-210. [DOI] [PubMed] [Google Scholar]
- 3. Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367(26):2471-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Brain Trauma Foundation. The American Association of Neurological Surgeons. The joint section on neurotrauma and critical care. initial management. J Neurotrauma. 2000;17(6-7):463-469. [DOI] [PubMed] [Google Scholar]
Neurosurgery Speaks (Audio Abstracts)
Listen to audio translations of this paper's abstract into select languages by choosing from one of the selections below.
REFERENCES
- 1. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury. J Head Trauma Rehabil. 2006;21(5):375-378. [DOI] [PubMed] [Google Scholar]
- 2. Gerber LM, Chiu YL, Carney N, Hartl R, Ghajar J. Marked reduction in mortality in patients with severe traumatic brain injury. J Neurosurg. 2013;119(6):1583-1590. [DOI] [PubMed] [Google Scholar]
- 3. Bullock MR, Chesnut R, Ghajar J et al. Surgical management of acute epidural hematomas. Neurosurgery. 2006;58(3 suppl):S7-15; discussion Si-iv. [PubMed] [Google Scholar]
- 4. Bullock MR, Chesnut R, Ghajar J et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58(3 Suppl):S16-24; discussion Si-iv. [PubMed] [Google Scholar]
- 5. Bullock MR, Chesnut R, Ghajar J et al. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006;58(3 suppl):S25-46; discussion Si-iv. [DOI] [PubMed] [Google Scholar]
- 6. Carney N, Totten AM, O’Reilly C et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6-15. [DOI] [PubMed] [Google Scholar]
- 7. Koenig MA, Bryan M, Lewin JL 3rd, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70(13):1023-1029. [DOI] [PubMed] [Google Scholar]
- 8. Eskandari R, Filtz MR, Davis GE, Hoesch RE. Effective treatment of refractory intracranial hypertension after traumatic brain injury with repeated boluses of 14.6% hypertonic saline. J Neurosurg. 2013;119(2):338-346. [DOI] [PubMed] [Google Scholar]
- 9. Worthley LI, Cooper DJ, Jones N. Treatment of resistant intracranial hypertension with hypertonic saline. J Neurosurg. 1988;68(3):478-481. [DOI] [PubMed] [Google Scholar]
- 10. Rockswold GL, Solid CA, Paredes-Andrade E, Rockswold SB, Jancik JT, Quickel RR. Hypertonic saline and its effect on intracranial pressure, cerebral perfusion pressure, and brain tissue oxygen. Neurosurgery. 2009;65(6):1035-1042; discussion 1041-1032. [DOI] [PubMed] [Google Scholar]
- 11. Mortazavi MM, Romeo AK, Deep A et al. Hypertonic saline for treating raised intracranial pressure: literature review with meta-analysis. J Neurosurg. 2012;116(1):210-221. [DOI] [PubMed] [Google Scholar]
- 12. Hartl R, Ghajar J, Hochleuthner H, Mauritz W. Hypertonic/hyperoncotic saline reliably reduces ICP in severely head-injured patients with intracranial hypertension. Acta Neurochir Suppl. 1997;70:126-129. [DOI] [PubMed] [Google Scholar]
- 13. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons et al. Guidelines for the management of severe traumatic brain injury. II. Hyperosmolar therapy. J Neurotrauma. 2007;24(suppl 1):S14-S20. [DOI] [PubMed] [Google Scholar]
- 14. Ware ML, Nemani VM, Meeker M, Lee C, Morabito DJ, Manley GT. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study. Neurosurgery. 2005;57(4):727-736; discussion 727-736. [PubMed] [Google Scholar]
- 15. Shackford SR, Bourguignon PR, Wald SL, Rogers FB, Osler TM, Clark DE. Hypertonic saline resuscitation of patients with head injury. J Trauma. 1998;44(1):50-58. [DOI] [PubMed] [Google Scholar]
- 16. Schatzmann C, Heissler HE, Konig K et al. Treatment of elevated intracranial pressure by infusions of 10% saline in severely head injured patients. Acta Neurochir Suppl. 1998;71:31-33. [DOI] [PubMed] [Google Scholar]
- 17. Francony G, Fauvage B, Falcon D et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. 2008;36(3):795-800. [DOI] [PubMed] [Google Scholar]
- 18. James HE. Methodology for the control of intracranial pressure with hypertonic mannitol. Acta neurochir. 1980;51(3-4):161-172. [DOI] [PubMed] [Google Scholar]
- 19. Muizelaar JP, Lutz HA 3rd, Becker DP. Effect of mannitol on ICP and CBF and correlation with pressure autoregulation in severely head-injured patients. J Neurosurg. 1984;61(4):700-706. [DOI] [PubMed] [Google Scholar]
- 20. Mangat HS, Chiu YL, Gerber LM, Alimi M, Ghajar J, Hartl R. Hypertonic saline reduces cumulative and daily intracranial pressure burdens after severe traumatic brain injury. J Neurosurg. 2015;122(1):202-210. [DOI] [PubMed] [Google Scholar]
- 21. Farahvar A, Gerber LM, Chiu YL, Carney N, Hartl R, Ghajar J. Increased mortality in patients with severe traumatic brain injury treated without intracranial pressure monitoring. J Neurosurg. 2012;117(4):729-734. [DOI] [PubMed] [Google Scholar]
- 22. Rosenbaum PR. Optimal matching for observational studies. J Am Statist Assoc. 1989;84(408):1024-1032. [Google Scholar]
- 23. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- 24. Asehnoune K, Lasocki S, Seguin P et al. Association between continuous hyperosmolar therapy and survival in patients with traumatic brain injury - a multicentre prospective cohort study and systematic review. Crit Care. 2017;21(1):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cottenceau V, Masson F, Mahamid E et al. Comparison of effects of equiosmolar doses of mannitol and hypertonic saline on cerebral blood flow and metabolism in traumatic brain injury. J Neurotrauma. 2011;28(10):2003-2012. [DOI] [PubMed] [Google Scholar]
- 26. Oddo M, Levine JM, Frangos S et al. Effect of mannitol and hypertonic saline on cerebral oxygenation in patients with severe traumatic brain injury and refractory intracranial hypertension. J Neurol Neurosurg Psychiatry. 2009;80(8):916-920. [DOI] [PubMed] [Google Scholar]
- 27. Bouma GJ, Muizelaar JP, Bandoh K, Marmarou A. Blood pressure and intracranial pressure-volume dynamics in severe head injury: relationship with cerebral blood flow. J Neurosurg. 1992;77(1):15-19. [DOI] [PubMed] [Google Scholar]
- 28. Hiler M, Czosnyka M, Hutchinson P et al. Predictive value of initial computerized tomography scan, intracranial pressure, and state of autoregulation in patients with traumatic brain injury. J Neurosurg. 2006;104(5):731-737. [DOI] [PubMed] [Google Scholar]
- 29. Robertson CS, Valadka AB, Hannay HJ et al. Prevention of secondary ischemic insults after severe head injury. Crit Care Med. 1999;27(10):2086-2095. [DOI] [PubMed] [Google Scholar]
- 30. Nath F, Galbraith S. The effect of mannitol on cerebral white matter water content. J Neurosurg. 1986;65(1):41-43. [DOI] [PubMed] [Google Scholar]
- 31. Wisner DH, Schuster L, Quinn C. Hypertonic saline resuscitation of head injury. J Trauma. 1990;30(1):75-78. [DOI] [PubMed] [Google Scholar]
- 32. Burke AM, Quest DO, Chien S, Cerri C. The effects of mannitol on blood viscosity. J Neurosurg. 1981;55(4):550-553. [DOI] [PubMed] [Google Scholar]
- 33. Willerson JT, Curry GC, Atkins JM, Parkey R, Horwitz LD. Influence of hypertonic mannitol on ventricular performance and coronary blood flow in patients. Circulation. 1975;51(6):1095-1100. [DOI] [PubMed] [Google Scholar]
- 34. Muizelaar JP, Wei EP, Kontos HA, Becker DP. Mannitol causes compensatory cerebral vasoconstriction and vasodilation in response to blood viscosity changes. J Neurosurg. 1983;59(5):822-828. [DOI] [PubMed] [Google Scholar]
- 35. Hartl R, Bardt TF, Kiening KL, Sarrafzadeh AS, Schneider GH, Unterberg AW. Mannitol decreases ICP but does not improve brain-tissue pO2 in severely head-injured patients with intracranial hypertension. Acta Neurochir Suppl. 1997;70:40-42. [DOI] [PubMed] [Google Scholar]
- 36. Vialet R, Albanese J, Thomachot L et al. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 mL/kg 7.5% saline is more effective than 2 mL/kg 20% mannitol. Crit Care Med. 2003;31(6):1683-1687. [DOI] [PubMed] [Google Scholar]
- 37. Kamel H, Navi BB, Nakagawa K, Hemphill JC 3rd, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: A meta-analysis of randomized clinical trials. Crit Care Med. 2011;39(3):554-559. [DOI] [PubMed] [Google Scholar]
- 38. Wakai A, McCabe A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2013;8(8):CD001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roquilly A, Lasocki S, Moyer JD et al. COBI (COntinuous hyperosmolar therapy for traumatic Brain-Injured patients) trial protocol: a multicentre randomised open-label trial with blinded adjudication of primary outcome. BMJ Open. 2017;7(9):e018035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bentsen G, Breivik H, Lundar T, Stubhaug A. Hypertonic saline (7.2%) in 6% hydroxyethyl starch reduces intracranial pressure and improves hemodynamics in a placebo-controlled study involving stable patients with subarachnoid hemorrhage. Crit Care Med. 2006;34(12):2912-2917. [DOI] [PubMed] [Google Scholar]
- 41. de Felippe J Jr., Timoner J, Velasco IT, Lopes OU, Rocha-e-Silva M Jr. Treatment of refractory hypovolaemic shock by 7.5% sodium chloride injections. Lancet North Am Ed. 1980;2(8202):1002-1004. [DOI] [PubMed] [Google Scholar]
- 42. Sheth KN, Stein DM, Aarabi B et al. Intracranial pressure dose and outcome in traumatic brain injury. Neurocrit Care. 2013;18(1):26-32. [DOI] [PubMed] [Google Scholar]
- 43. Kahraman S, Dutton RP, Hu P et al. Automated measurement of “pressure times time dose” of intracranial hypertension best predicts outcome after severe traumatic brain injury. J Trauma. 2010;69(1):110-118. [DOI] [PubMed] [Google Scholar]