A 70-year-old lady, from Vellore district of Tamil Nadu in south India, who was a known diabetic and hypertensive, presented to the Emergency Department (ED) with complaints of intermittent high-grade fever, generalized weakness, and myalgia for 3 days. One week earlier, she was admitted in a government hospital and evaluated for fever and myalgia of 2-days duration. Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was positive and she was confirmed to have COVID-19. Five days later, she came to our hospital for further management of the acute febrile illness. The initial working diagnosis at presentation to our ED was COVID-19 as she was recently tested positive and had persistent symptoms for 1 week. At admission, she was hemodynamically stable (pulse rate: 80 beats/minute; blood pressure: 120/80 mmHg; respiratory rate: 20 per minute; SpO2: 99% on room air). However, a detailed physical examination revealed an 8 x 5 mm sized, painless and nonpruritic eschar in the left infra-axillary egion [Figure 1]. Other systemic examination was within normal limits. Chest X-ray was normal. Thick and thin smears for malarial parasites and dengue serology (NS1, IgM, IgG) were negative. Blood culture showed no growth. Laboratory investigations showed thrombocytopenia and details are given in Table 1. As scrub typhus was strongly suspected, IgM ELISA (InBios International, Inc., Seattle, WA, USA) and 47 kDa q polymerase chain reaction (PCR) was performed. IgM ELISA was negative, whereas 47 kDa qPCR was positive (Ct value: 31). Further, the SYBR Green qPCR detecting the multicopy TraD gene specific for Orientia tsutsugamushi was performed as described by Chao et al.[1] As expected, the TraD real-time PCR (SYBR Green) also gave a positive signal (Ct value: 24). Nucleotide BLAST (Basic Local Alignment Search Tool; National Center for Biotechnology Information, U.S. National Library of Medicine, Bethesda, MD, USA) analysis of the 176 bp amplified TraD fragments showed 99.43% homology O. tsutsugamushi str Ikeda (CP044031). Homology of 98.29% was observed with other O. tsutsugamushi strains like Wuj/2014 (CP044031), UT176 (LS LS398547), TA686 (LS LS398549), Kato (LS LS398550), Gilliam (LS LS398551), UT76 (LS LS398552) and 97.71% with Karp (LS398548) and Boryong (AM494475). This confirmed the identity of the amplicon as Orientia tsutsugamushi. She was therefore diagnosed to have COVID-19 and co-infection with scrub typhus, which is endemic in our geographic locality. She was initiated on paracetamol and intravenous doxycycline for 1 week to which she responded well. Her clinical condition improved gradually and was discharged stable from hospital.
Figure 1.
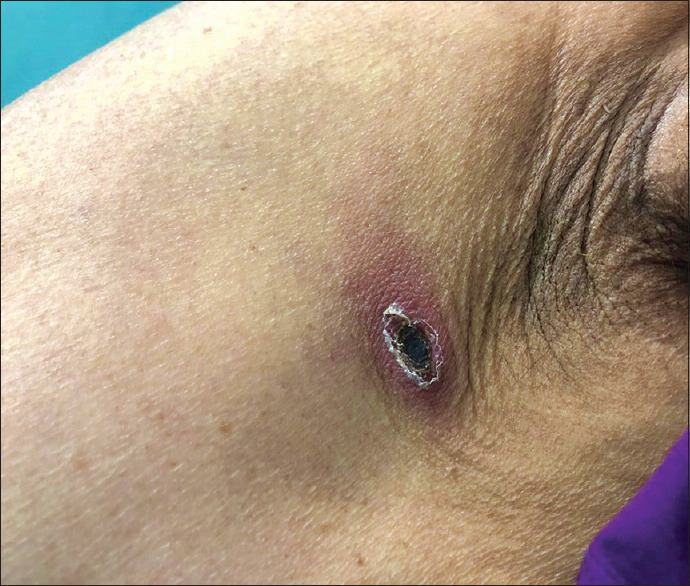
Eschar with an innermost black crust, erythematous patch in the periphery with a thin surface skin layer that was outlined by white scales
Table 1.
Laboratory blood investigations done in the present case
| Variables | Day 0 | Day 3 | Day 5 | Day 7 |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 11.6 | |||
| Total white blood cells count (/cumm) | 4300 | |||
| Neutrophils (/cumm) | 78 | |||
| Lymphocytes (/cumm) | 13 | |||
| Eosinophils (/cumm) | 7 | |||
| Basophils (/cumm) | 0 | |||
| Platelets count (/cumm) | 64000 | 48000 | 51000 | 124000 |
| Creatinine (mg%) | 0.59 | 0.43 | ||
| Sodium (Na+) (m mol/L) | 125 | 133 | 134 | |
| Potassium (K+), (m mol/L) | 3.8 | |||
| Serum Glutamic 0xaloacetic Transaminase (U/L) | 76 | |||
| Serum Glutamic Pyruvic Transaminase (U/L) | 47 | |||
| ALP (U/L) | 97 | |||
| Lactate dehydrogenase (LDH) (U/L) | 704 | |||
| D. Dimer (ng/ml) | 2964 | |||
| Ferritin (ng/ml) | 844.8 | |||
| C-Reactive Protein (CRP) | 113 |
SARS-CoV-2 emerged in December 2019 and the pandemic brought the World to a grinding halt. Clinical spectrum of COVID-19 ranges from asymptomatic carriers to critically ill patients. Fever is the most consistent clinical feature, followed by dry cough, dyspnea, myalgia, headache, and diarrhea.[2] It closely mimics acute febrile illnesses (AFI) like scrub typhus, malaria, dengue fever, and leptospirosis. India being a tropical country bears a high burden of febrile zoonotic/infectious endemics. Diagnosing the usual AFI in the backdrop of rapid spread of SARS CoV-2 infections has become a diagnostic challenge for most clinicians. There are specific government guidelines that state observation of the seasonal epidemic that can coexist with COVID-19. Literature search showed few reports of COVID-19 coinfection with dengue, malaria and also influenza A (H1NI1), but none with scrub typhus co-infection.[3,4,5]
Presence of a pathognomonic eschar, seen in 45-86% of patients, is the most vital indicator for the diagnosis of scrub typhus.[6] However, in the current setting of the pandemic, with doctors forced to wear uncomfortable personal protective equipment, a detailed physical examination to look for this vital clue may take a back seat, thus missing or delaying the diagnosis of scrub typhus.
In our case the classical eschar and laboratory investigations raised the suspicion towards a scrub typhus infection, hence IgM ELISA and 47 kDa qPCR and SYBR Green qPCR detecting the multicopy TraD gene specific for O. tsutsugamushi was done to confirm the diagnosis. This report emphasizes the need for a thorough physical and diagnostic evaluation of patients presenting with AFI during the ongoing pandemic. In the backdrop of COVID-19, diagnostic work-up and treatment plan of patients with AFI should depend upon the local prevalence of various other endemic diseases in the specific geographical area, keeping in mind the possibility of co-infections.
Declaration of patient consent
The authors certify that appropriate patient consent was obtained.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chao CC, Belinskaya T, Zhang Z, Jiang L, Ching WM. Assessment of a sensitive qPCR assay targeting a multiple-copy gene to detect Orientia tsutsugamushi DNA. Trop Med Infect Dis. 2019;4:113. doi: 10.3390/tropicalmed4030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant MC, Geoghegan L, Arbyn M, Mohammed Z, McGuinness L, Clarke EL, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15:e0234765. doi: 10.1371/journal.pone.0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verduyn M, Allou N, Gazaille V, Andre M, Desroche T, Jaffar M-C, et al. Co-infection of dengue and COVID-19: A case report. PLoS Negl Trop Dis. 2020;14:e0008476. doi: 10.1371/journal.pntd.0008476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sardar S, Sharma R, Alyamani TYM, Aboukamar M. COVID-19 and Plasmodium vivax malaria co-infection. IDCases. 2020;21:e00879. doi: 10.1016/j.idcr.2020.e00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuadrado-Payán E, Montagud-Marrahi E, Torres-Elorza M, Bodro M, Blasco M, Poch E, et al. SARS-CoV-2 and influenza virus co-infection. Lancet. 2020;395:e84. doi: 10.1016/S0140-6736(20)31052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kundavaram AP, Jonathan AJ, Nathaniel SD, Varghese GM. Eschar in scrub typhus: A valuable clue to the diagnosis. J Postgrad Med. 2013;59:177–8. doi: 10.4103/0022-3859.118033. [DOI] [PubMed] [Google Scholar]


