Abstract:
Statin therapy has been recently suggested as possible adjuvant treatment to improve the clinical outcome in patients with coronavirus disease 2019 (COVID-19). The aim of this study was to describe the prevalence of preadmission statin therapy in hospitalized patients with COVID-19 and to investigate its potential association with acute distress respiratory syndrome (ARDS) at admission and in-hospital mortality. We retrospectively recruited 467 patients with laboratory-confirmed COVID-19 admitted to the emergency department of 10 Italian hospitals. The study population was divided in 2 groups according to the ARDS diagnosis at admission and in-hospital mortality. A multivariable regression analysis was performed to assess the risk of ARDS at admission and death during hospitalization among patients with COVID-19. A competing risk analysis in patients taking or not statins before admission was also performed. ARDS at admission was reported in 122 cases (26.1%). There was no statistically significant difference for clinical characteristics between patients presenting with and without ARDS. One hundred seven patients (18.5%) died during the hospitalization; they showed increased age (69.6 ± 13.1 vs. 66.1 ± 14.9; P = 0.001), coronary artery disease (23.4% vs. 12.8%; P = 0.012), and chronic kidney disease (20.6% vs. 11.1%; P = 0.018) prevalence; moreover, they presented more frequently ARDS at admission (48.6% vs. 19.4%; P < 0.001). At multivariable regression model, statin therapy was not associated neither with ARDS at admission nor with in-hospital mortality. Preadmission statin therapy does not seem to show a protective effect in severe forms of COVID-19 complicated by ARDS at presentation and rapidly evolving toward death.
Key Words: COVID-19, SARS-CoV-2, statin therapy, mortality, acute distress respiratory syndrome
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly pathogenic human coronavirus recently recognized as the cause of the coronavirus disease 2019 (COVID-19), which spread rapidly form China to other countries, reaching devastating pandemic proportion.1 Italy is the one of the hardest hit countries by COVID-19, with more than 540,000 laboratory-confirmed cases by October 27, 2020.2,3
Acute distress respiratory syndrome (ARDS) is one of the most frequent encountered complications of COVID-19 and has been associated with significantly lower survival during patients' hospitalization.4,5 Although its pathophysiology is not completely understood, the interplay between inflammation, immune response, and coagulation seems to have a central role.6
Statins exert many pleiotropic effects, in addition to the lowering of serum cholesterol levels, as anti-inflammatory and immunomodulatory properties in several clinical conditions, including viral infections.7 Previous studies showed a reduced hospitalization rate and mortality in subjects on treatment with statins affected by flu diseases.8 Therefore, some authors hypothesized a protective role of these agents on the clinical outcome of patients affected by COVID-199–12
Whether statin therapy might influence the risk of life-threatening complications, including ARDS, and mortality in patients infected by SARS-CoV-2 has not yet clearly established. The aim of this multicenter observational study was to evaluate the prevalence of preadmission statin therapy among COVID-19 patients and to investigate the association between statin use and ARDS at admission and in-hospital mortality.
MATERIALS AND METHODS
We retrospectively enrolled all consecutive patients with laboratory-confirmed COVID-19 admitted to 10 Italian Hospitals from February 2020 to April 2020. COVID-19 diagnosis was initially based on the World Health Organization criteria, and all cases were confirmed by real-time reverse transcriptase-polymerase chain reaction analysis of throat swab specimens.13
This study was conducted according to the Declaration of Helsinki and approved by the institutional ethics committees. The requirement for informed consent from individual patients was waived because of the observational retrospective design of the study.
At admission, all patients underwent medical history, physical examination, laboratory evaluation, chest x-ray, and/or computed tomography. Information on patient baseline characteristics, clinical course (admission in intensive care unit and respiratory support measures), and in-hospital complications (ARDS, acute cardiac injury, myocardial infarction, acute heart failure, and pulmonary embolism) were systematically recorded. ARDS was defined according to the Berlin definition.14 The number of patients who had died or recovered was recorded. No patient was still hospitalized at the time of the analysis (June 13, 2020).
The COVID-19 population was divided into 2 groups according to the evidence of ARDS at admission and to the occurrence of death during hospitalization. The prevalence of statin therapy has been compared between these groups.
The primary study outcome was ARDS at admission; the secondary outcome was the occurrence of death for any cause during the hospitalization. Daily assumption of statins was verified at the entrance through medical history. Discontinuation of statin therapy before hospitalization was considered as an exclusion criterion.
Statistical Analysis
Distribution of continuous data was tested with the Kolmogorov–Smirnov and the Shapiro-Wilk test. Normally distributed variables were expressed as mean ± SD, whereas non-normal distributed ones as median and interquartile range. Categorical variables were reported as numbers and percentages. Continuous normally distributed variables were compared by using the Student t-test; differences between non-normally distributed variables were tested with the Mann–Whitney U test. Categorical variables were compared with the χ2 test, or the Fisher exact test, when appropriate. Based both on the results of the comparative analysis and on previous findings from the literature, we adjusted for all variables potentially affecting the outcome and/or asymmetrically distributed between statin and nonstatin groups. A propensity score weighted multivariable logistic regression model was performed; the propensity score values were calculated by using a logistic regression with the dependent variable being the likelihood of receiving statin treatment. The independent variables included in the model were as follows: sex, smoke, chronic obstructive pulmonary disease, hypertension, diabetes, heart failure, atrial fibrillation, stroke, and chronic kidney disease (CKD). The balance of the propensity score between the 2 groups before and after weighting was assessed for each variable. Age and coronary artery disease (CAD) were excluded from the propensity score model because of substantial imbalance after weighting. Propensity-weighted multivariable regression models for both the study outcome measures were then fitted using independent variables statin use, age, and CAD. The risk of collinearity was assessed and excluded by using the variance inflation factors. The unadjusted and adjusted risk ratios (RRs) for the outcomes of interest were presented with their 95% confidence intervals (CIs). Furthermore, a competing risk analysis for death or discharge in patients taking or not statins before admission was also performed and showed using Kaplan–Meier survival curves. For all test, a P value < 0.05 was considered statistically significant. Analysis was performed by using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
During the period of interest, 467 patients were admitted with diagnosis of COVID-19. The baseline characteristics of the study population are summarized in Table 1. The mean age was 66.9 ± 14.6 years; 294 (63%) were men. Home statin therapy was reported in 167 patients (35.8%). Statin-treated patients were older than those not taking statins (70.6 ± 12 vs. 64.8 ± 15.4; P = 0.001) and showed higher prevalence of hypertension (79.6% vs. 52%; P = 0.001), diabetes (49.7% vs. 14.3%, P = 0.001), CAD (32.3% vs. 5.7%; P = 0.001), CKD (22.2% vs. 8.3%; P = 0.001), heart failure (11.4% vs. 5.3%; P = 0.028), dyslipidemia (67.2% vs. 2.3%; P = 0.001), previous stroke (13.2% vs. 2.7%; P = 0.001), and concomitant use of antiplatelet drugs (36.5% vs. 26.7%; P = 0.034). ARDS was reported at admission in 122 cases (26.1%). There was no statistically significant difference for demographic and clinical characteristics between patients with and without ARDS at admission (Table 2).
TABLE 1.
Clinical Characteristic of the Study Population According to Treatment With Statins
| Overall Population (N = 467) | No Statin Therapy (N = 300) | Statin Therapy (N = 167) | P | |
| Males, n (%) | 294 (63.0) | 179 (59.7) | 115 (68.9) | 0.061 |
| Age, yr | 66.88 (14.55) | 64.82 (15.41) | 70.58 (12.03) | <0.001 |
| Smoker, n (%) | 79 (16.9) | 33 (11.0) | 46 (27.5) | <0.001 |
| Hypertension, n (%) | 289 (61.9) | 156 (52.0) | 133 (79.6) | <0.001 |
| Diabetes mellitus, n (%) | 123 (26.3) | 43 (14.3) | 80 (47.9) | <0.001 |
| Dyslipidemia, n (%) | 119 (25.5) | 7 (2.3) | 112 (67.1) | <0.001 |
| Atrial fibrillation, n (%) | 65 (13.9) | 35 (11.7) | 30 (18.0) | 0.081 |
| Heart failure, n (%) | 35 (7.5) | 16 (5.3) | 19 (11.4) | 0.028 |
| Previous stroke, n (%) | 42 (9.0) | 20 (6.7) | 22 (13.2) | 0.029 |
| CKD, n (%) | 62 (13.3) | 25 (8.3) | 37 (22.2) | <0.001 |
| CAD, n (%) | 71 (15.2) | 17 (5.7) | 54 (32.3) | <0.001 |
| COPD, n (%) | 90 (19.3) | 44 (14.7) | 46 (27.5) | 0.001 |
| Antiplatelet therapy, n (%) | 141 (30.2) | 80 (26.7) | 61 (36.5) | 0.034 |
| Anticoagulant therapy, n (%) | 60 (12.8) | 35 (11.7) | 25 (15.0) | 0.380 |
| Ezetimibe therapy, n (%) | 22 (4.7) | 2 (0.7) | 20 (12.0) | <0.001 |
| PCSK-9 inhibitors therapy, n (%) | 12 (2.6) | 2 (0.7) | 10 (6.0) | 0.001 |
| ACEIs/ARBs therapy, n (%) | 204 (43.7) | 112 (37.3) | 96 (57.5) | <0.001 |
COPD, chronic obstructive pulmonary disease.
TABLE 2.
Clinical Characteristic of the Study Population According to the Presence or Not of ARDS at Admission
| Patients Without ARDS (N = 345) | Patients With ARDS (N = 122) | P | |
| Males, n (%) | 209 (60.6) | 85 (69.7) | 0.093 |
| Age, mean (SD) | 66.43 (14.93) | 68.16 (13.37) | 0.258 |
| Smoker, n (%) | 58 (16.8) | 21 (17.2) | 1.000 |
| Hypertension, n (%) | 210 (60.9) | 79 (64.8) | 0.515 |
| Diabetes mellitus, n (%) | 90 (26.1) | 33 (27.0) | 0.930 |
| Dyslipidemia, n (%) | 87 (25.2) | 32 (26.2) | 0.921 |
| Atrial fibrillation, n (%) | 46 (13.3) | 19 (15.6) | 0.644 |
| Heart failure, n (%) | 26 (7.5) | 9 (7.4) | 1.000 |
| Previous stroke, n (%) | 32 (9.3) | 10 (8.2) | 0.862 |
| CKD, n (%) | 43 (12.5) | 19 (15.6) | 0.475 |
| CAD, n (%) | 53 (15.4) | 18 (14.8) | 0.989 |
| COPD, n (%) | 69 (20.0) | 21 (17.2) | 0.591 |
| Antiplatelet therapy, n (%) | 100 (29.0) | 41 (33.6) | 0.400 |
| Anticoagulant therapy, n (%) | 43 (12.5) | 17 (13.9) | 0.795 |
| Statin therapy, n (%) | 126 (36.5) | 41 (33.6) | 0.640 |
| Ezetimibe therapy, n (%) | 18 (5.2) | 4 (3.3) | 0.535 |
| PCSK-9 inhibitors therapy, n (%) | 9 (2.6) | 3 (2.5) | 1.000 |
| ACEIs/ARBs therapy, n (%) | 153 (44.3) | 51 (41.8) | 0.63 |
COPD, chronic obstructive pulmonary disease.
During hospitalization, 107 deaths were reported (18.5%). Deceased patients showed a significantly increased age (69.6 ± 13.1 vs. 66.1 ± 14.9; P = 0.001), CAD (23.4% vs. 12.8%; P = 0.012), and CKD (20.6% vs. 11.1%; P = 0.018) prevalence compared with those who survived; no difference in the use of statins was observed (43% vs. 33.6%; P = 0.096; Table 3). Moreover, they presented more frequently ARDS at admission (48.6% vs. 19.4%; P < 0.001).
TABLE 3.
Clinical Characteristics of Patients Survived and Deceased During Hospitalization
| Survived (N = 360) | Not Survived (N = 107) | P | |
| Males, n (%) | 214 (59.4) | 80 (74.8) | 0.006 |
| Age, mean (SD) | 66.08 (14.88) | 69.57 (13.06) | 0.029 |
| Hypertension, n (%) | 217 (60.3) | 72 (67.3) | 0.231 |
| Smoke, n (%) | 59 (16.4) | 20 (18.7) | 0.681 |
| Diabetes mellitus, n (%) | 89 (24.7) | 34 (31.8) | 0.184 |
| Dyslipidemia | 86 (23.9) | 33 (30.8) | 0.186 |
| Atrial fibrillation, n (%) | 46 (12.8) | 19 (17.8) | 0.251 |
| Heart failure, n (%) | 24 (6.7) | 11 (10.3) | 0.300 |
| Previous stroke, n (%) | 32 (8.9) | 10 (9.3) | 1.000 |
| CKD, n (%) | 40 (11.1) | 22 (20.6) | 0.018 |
| CAD, n (%) | 46 (12.8) | 25 (23.4) | 0.012 |
| COPD, n (%) | 68 (18.9) | 22 (20.6) | 0.806 |
| Antiplatelet therapy, n (%) | 107 (29.7) | 34 (31.8) | 0.775 |
| Anticoagulant therapy, n (%) | 45 (12.5) | 15 (14.0) | 0.804 |
| Statin therapy, n (%) | 121 (33.6) | 46 (43.0) | 0.096 |
| Ezetimibe therapy, n (%) | 12 (3.3) | 10 (9.3) | 0.020 |
| PCSK-9 inhibitors therapy, n (%) | 8 (2.2) | 4 (3.7) | 0.601 |
| ACEIs/ARBs therapy, n (%) | 149 (41.4) | 55 (51.4) | 0.067 |
COPD, chronic obstructive pulmonary disease.
No significant differences for ARDS at admission [24.6% vs. 27.0%; risk ratio (RR): 0.91, CI: 0.64–1.24, P = 0.564] and all-cause death (27.5% vs. 20.3%; RR: 1.34, CI: 0.97–1.78, P = 0.077) were observed (Table 4).
TABLE 4.
RR of Adverse Events Between Patients Treated or Not With Statin Before Hospitalization
| No Statin Therapy, N (%) | Statin Therapy, N (%) | RR | CI | P | |
| ARDS at admission | 81 (27.0) | 41 (24.6) | 0.91 | 0.64–1.24 | 0.564 |
| In-hospital mortality | 61 (20.3) | 46 (27.5) | 1.34 | 0.97–1.78 | 0.077 |
Figure 1 shows the proportion of ARDS and in-hospital mortality according to home therapy with statins.
FIGURE 1.
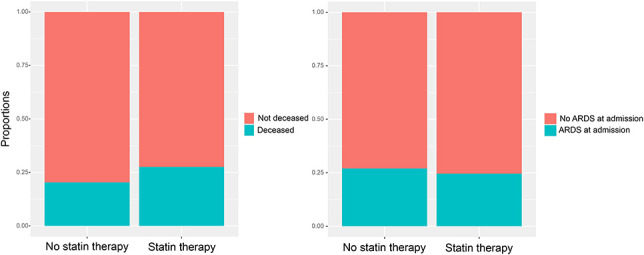
Proportion of ARDS at admission and in-hospital mortality according to the statin therapy among patients with COVID-19.
The distribution of the propensity score values before and after weighting in statin and nonstatin groups is plotted in Figure 2.
FIGURE 2.
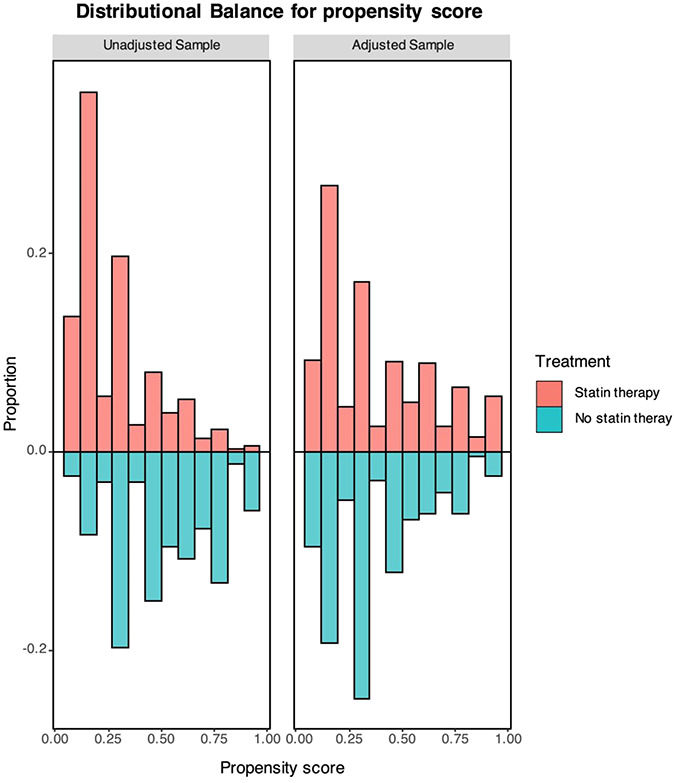
Distributional balance of the propensity score values before and after weighting between study groups.
The propensity score weighted multivariable logistic regression analysis showed that statin therapy and age were associated neither with ARDS at admission nor with the occurrence of death during hospitalization. Conversely, CAD was not associated with ARDS at admission but was independently associated with in-hospital mortality (P = 0.027, Table 5).
TABLE 5.
Multivariable Regression Models for the Risk of ARDS at Admission and In-Hospital Mortality
| Parameter | RR | CI | P | |
| ARDS at admission | Statin | 1.06 | 0.68–1.55 | 0.793 |
| Age | 1.00 | 0.68–1.55 | 0.789 | |
| CAD | 0.69 | 0.35–1.24 | 0.231 | |
| In-hospital mortality | Statin | 0.96 | 0.56–1.53 | 0.860 |
| Age | 1.00 | 0.99–1.02 | 0.770 | |
| CAD | 1.83 | 1.08–2.67 | 0.027 |
Figure 3 shows the Kaplan–Meier survival analysis estimating the competing risk of death or discharge in patients taking or not statins before the admission. A nonsignificant trend toward a higher risk of death (P = 0.066) and a lower rate of discharge bordering on significance (P = 0.050) were observed.
FIGURE 3.
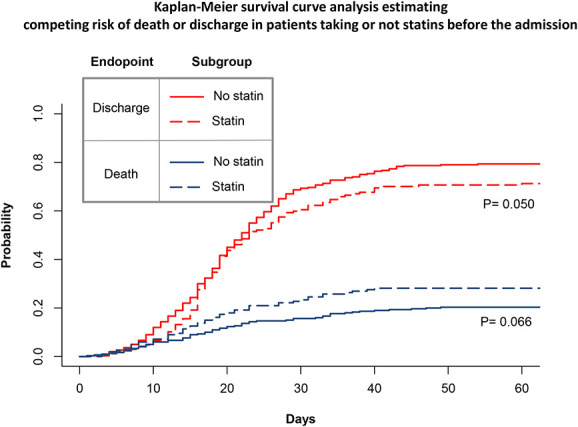
Kaplan–Meier survival curve analysis estimating competing risk of death or discharge in patients taking or not statins.
DISCUSSION
The main findings of this study can be summarized as follows: more than one-third of patients admitted for COVID-19 was on treatment with statins; the statin therapy was associated with higher prevalence of cardiovascular comorbidities or previous cardiovascular events; however, it did not correlate with the risk of severe adverse events including ARDS at admission and in-hospital mortality.
Statins have been proposed as adjuvant agents for infection, given the increasing real-world evidences about the statins use and the lowering of infectious disease-related mortality.15 In particular, in a retrospective case‐control study including 1520 patients with laboratory‐confirmed H1N1 influenza, the outpatient statins were associated with a 28% reduction in the severity of illness.16 Moreover, the statin use has been shown associated with a 41% reduction in 30-day mortality among 3043 patients hospitalized with laboratory-confirmed influenza.17 The beneficial effect of statins in reducing influenza virus mortality and morbidity may be related to their capacity of altering the host cell receptors for virus internalization and lipid rafts for virus replication18. Moreover, statins determine the downregulation of the Rho/Rho kinase pathway and the reduction of proinflammatory cytokines and chemokines.
Previous laboratory findings showed that statins might increase the expression of angiotensin-converting enzyme 2, the receptor for the coronavirus, in laboratory animals19; however, more recent report based on molecular docking analysis showed that statins might inhibit SARS-CoV-2 entry into host cells by directly binding the main protease of the coronavirus.20
These laboratory contrasting effects are similar to those shown by ACE inhibitors and ARBs on the physiopathology of SARS-CoV-2 infection21 and led to the same dilemma about the clinical application of these therapies in the contest of COVID-19.
Three previous meta-analyses of observational studies compared clinical outcomes in statin users versus nonusers gave conflicting results: 2 demonstrated a positive impact,22,23 whereas the other failed to show any significant differences in prognosis.24 The divergent reported data might be related to the high degree of heterogeneity between the included studies and prevent any definite conclusions on the effects of statin therapy on COVID-19 clinical outcomes.
The pathogenesis of ARDS in the clinical context of COVID-19 may be related to the direct effect of SARS-CoV-2 on alveolar epithelial cells and to indirect effects of infection-related hypoxia, both conditions predisposing to thrombotic events; moreover, a severe inflammatory response leading to leading to microvascular endothelium dysfunction and disseminated intravascular coagulation may occur in patients with COVID-19.6,18,25
Based on the hypothesis that COVID-19-induced ARDS may be driven by inflammatory and procoagulant state, we evaluated if the preadmission statin therapy might impact on the clinical course and prognosis of hospitalized patients with COVID-19. To this aim, we focused on the assumption of statins before admission. Patients previously treated with statins, but who had discontinued before hospitalization, were excluded.
We did not consider the assumption of statins after the admission because many patients may have discontinued statin treatment during the hospitalization (eg, patients admitted in the intensive care unit (ICU) who underwent invasive mechanical ventilation). Although, CT scan was generally conducted many times during hospitalization, the timing of computed tomography could vary between patients and centers and might constitute a potential bias. Therefore, we used ARDS at admission as a primary outcome measure because each patient was screened for the presence of ARDS at admission.
In the present analysis, preadmission statin therapy was not associated with the clinical outcome of patients with COVID-19 for ARDS developing and survival. Our results do not support the hypothesis that statins may mitigate SARS-CoV2 immuno-mediated inflammatory response, which is involved in ARDS pathophysiology. Likewise, there was no association with the occurrence of fatal outcome during hospital course.
The lack of the beneficial effect of statins among our study population might be related to their inhibiting activity on toll-like receptors and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, which carries the potential risk of exacerbating compensatory immune signals and poor disease outcome.26 Elevated baseline plasma IL-18 was associated with higher mortality in sepsis-induced ARDS among patients on rosuvastatin treatments.27 Moreover, the concomitant use of corticosteroids in outpatients setting28 and during the hospitalization might make statins unable to determine a significant immunomodulatory action or vascular protection.
Our results should be interpreted in light of the limitations related to the retrospective observational nature of the study and the potential beneficial role of adjuvant treatment, and preadmission statin therapy should be investigated in phase III clinical trial. Owing to the aim of this study, the major limitation is related to the lack of information on statin type and dosage before hospitalization. The retrospective nature of this registry and the clinical context of the COVID-19 pandemic in overwhelmed areas (eg, Bergamo) may have affected the collection and granularity of data, such as information on statin intensity, and the verification of daily assumption of statins through prescriptions. Another limitation concerns the relatively small number of patients included in the study. Although the multicenter study design might have improved the generalizability of data, we cannot exclude type II error related to the small sample size. In fact, unless the RRs for ARDS at admission (1.06) and for mortality (0.96) related to statins were close to the identity, the relatively wide CIs (0.68–1.55 for ARDS at admission; 0.56–1.53 for mortality) included possible differences >20%, that could be considered clinically significant. Thus, we cannot exclude that the study is underpowered. The selective inclusion of patients who needed hospitalization might also represent a potential bias of this study because only symptomatic patients were ascertained. The potential beneficial effect of preadmission statin therapy on clinical course and outcome of hospitalized patients with infected by SARS-CoV-2 needs further investigation by larger prospective studies.
CONCLUSION
Although our results need confirmation by prospective studies including a larger population, statin therapy does not seem to show a protective effect on the occurrence of severe forms of COVID-19 characterized by ARDS at admission and rapidly evolving toward death during the hospitalization.
Footnotes
The authors report no conflicts of interest.
Contributor Information
Angelo Silverio, Email: angelosilverio1988@gmail.com.
Fernando Scudiero, Email: fernandoscudiero@gmail.com.
Emilio Attena, Email: emilioattena@hotmail.it.
Antonello D'Andrea, Email: antonellodandrea@libero.i.
Luigi Nunziata, Email: nunziata.luigi@alice.it.
Guido Parodi, Email: parodiguido@gmail.com.
Dario Celentani, Email: dario.celentani@gmail.com.
Ferdinando Varbella, Email: fvarbella@aslto3.piemonte.it.
Stefano Albani, Email: albani.aosta@gmail.com.
Giuseppe Musumeci, Email: giuseppe.musumeci@gmail.com.
Pierpaolo Di Micco, Email: pdimicco@libero.it.
Marco Di Maio, Email: marcodimaio88@gmail.com.
REFERENCES
- 1.Hui DS, EIA, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Coronavirus Disease 2019 (COVID-19). Situation Report. 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200425-sitrep-96-covid-19.pdf?sfvrsn=a33836bb_4. Accessed November 3, 2020. [Google Scholar]
- 3.Silverio A, Di Maio M, Ciccarelli M, et al. Timing of national lockdown and mortality in COVID-19: the Italian experience. Int J Infect Dis. 2020;100:193–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo V, Bottino R, Carbone A, et al. COVID-19 and heart: from clinical features to pharmacological implications. J Clin Med. 2020;9:1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost FJ, Petersen H, Tollestrup K, et al. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest. 2007;131:1006–1011. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Wen X, Peng J, et al. Systematic review and meta-analysis on the association between outpatient statins use and infectious disease-related mortality. PLoS One. 2012;7:e51548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bifulco M, Gazzerro P. Statins in coronavirus outbreak: it's time for experimental and clinical studies. Pharmacol Res. 2020;156:104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang XJ, Qin JJ, Cheng X, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32:176–187.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Nava G, Trelles-Garcia DP, Yanez-Bello MA, et al. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit Care. 2020;24:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. 2020. Available at: https://www.who.int/docs/defaultsource/coronaviruse/clinical-management-of-novel cov.pdf. Accessed February 15, 2020.
- 14.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. [DOI] [PubMed] [Google Scholar]
- 15.Brett SJ, Myles P, Lim WS, et al. Pre-admission statin use and in-hospital severity of 2009 pandemic influenza A(H1N1) disease. PLoS One. 2011;6:e18120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandermeer ML, Thomas AR, Kamimoto L, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205:13–19. [DOI] [PubMed] [Google Scholar]
- 17.Mehrbod P, Omar AR, Hair-Bejo M, et al. Mechanisms of action and efficacy of statins against influenza. Biomed Res Int. 2014;2014:872370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Micco P, Russo V, Carannante N, et al. Clotting factors in COVID-19: epidemiological association and prognostic values in different clinical presentations in an Italian cohort. J Clin Med. 2020;9:1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin YH, Min JJ, Lee JH, et al. The effect of fluvastatin on cardiac fibrosis and angiotensin-converting enzyme-2 expression in glucose-controlled diabetic rat hearts. Heart Vessels. 2017;32:618–627. [DOI] [PubMed] [Google Scholar]
- 20.Reiner Ž, Hatamipour M, Banach M, et al. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch Med Sci. 2020;16:490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084–H1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheen AJ. Statins and clinical outcomes with COVID-19: meta-analyses of observational studies. Diabetes Metab. 2020:101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hariyanto TI, Kurniawan A. Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14:1613–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo V, Di Maio M, Attena E, et al. Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: a multicenter observational study. Pharmacol Res. 2020;159:104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dashti-Khavidaki S, Khalili H. Considerations for statin therapy in patients with COVID-19. Pharmacotherapy. 2020;40:484–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers AJ, Guan J, Trtchounian A, et al. Association of elevated plasma interleukin-18 level with increased mortality in a clinical trial of statin treatment for acute respiratory distress syndrome. Crit Care Med. 2019;47:1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo V, Piccinocchi G, Mandaliti V, et al. Cardiovascular comorbidities and pharmacological treatments of COVID-19 patients not requiring hospitalization. Int J Environ Res Public Health. 2020;18:102. [DOI] [PMC free article] [PubMed] [Google Scholar]


