Abstract:
Epidemiological studies indicate that diabetes is the second most common comorbidity in COVID-19 (coronavirus disease 2019). Dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, exerts direct cardioprotective and nephroprotective effects. DARE-19 (Dapagliflozin in Respiratory Failure in Patients With COVID-19), an ongoing clinical trial, is designed to investigate the impact of dapagliflozin on COVID-19 progression. This article discusses the potential favorable impact of dapagliflozin on COVID-19 and its complications.
Key Words: COVID-19, SARS-CoV-2, diabetes, dapagliflozin, SGLT2i, DARE-19
INTRODUCTION
In December 2019, a sequence of pneumonia cases of unknown cause was documented in Wuhan, Hubei, China.1 Respiratory track samples revealed a novel coronavirus as the causative factor, named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).1 Diabetes is the second most common comorbidity in COVID-19 (coronavirus disease 2019).2 Severe COVID-19 frequently occurs in patients with comorbidities, such as cardiovascular disease, diabetes, hypertension, chronic lung disease, chronic kidney disease, obesity, and cancer.2,3
Individuals with diabetes and COVID-19 experience worse outcomes.4–8 Indeed, data from a Chinese retrospective study showed that patients with diabetes and COVID-19 had more severe pneumonia and greater mortality compared with those without (16.5% vs. 0%).9 Evidence from a survey in England showed that of patients dying from COVID-19 (n = 23,804), 32% had type 2 diabetes.10
Several scientific societies have issued recommendations on the administration of antidiabetic medications during COVID-19 pandemic.11–14 It has been demonstrated that COVID-19 increases the risk of diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state.15 Euglycemic DKA is a rare side effect associated with sodium-glucose co-transporter 2 inhibitors (SGLT2i).16,17 For this reason, specialists recommend that SGLT2i should be discontinued in patients with severe COVID-19 to reduce the risk of DKA.18 However, discontinuing SGLT2i is not recommended for outpatients in the absence of serious COVID-19.19,20
In this setting, a randomized, double-blind, placebo-controlled, phase 3 Dapagliflozin in Respiratory Failure in Patients with COVID-19 (DARE-19) study is evaluating the effect of dapagliflozin 10 mg versus placebo in reducing COVID-19 disease progression, complications, and all-cause mortality.21 Study population includes hospitalized patients with mild–moderate manifestations of COVID-19 and a history of at least one of the following: hypertension, type 2 diabetes, atherosclerotic cardiovascular disease, heart failure (HF), or chronic kidney disease (CKD) stage 3–4 (estimated glomerular filtration rate between 25 and 60 mL/min/1.73 m2). Therefore, both patients with and without diabetes are included. Cases of severe COVID-19 are excluded from the trial because of increased DKA risk.16 In addition, a randomized open-label trial, the TACTIC-E (mulTi-Arm Therapeutic Study in Pre-ICu Patients Admitted With COVID-19—Experimental Drugs and Mechanisms), is assessing the effect of a novel immunomodulatory agent, EDP1815, and a combination of dapagliflozin 10 mg and ambrisentan 5 mg as potential treatments for COVID-19.22 In this article, we aim to summarize the potential favorable impact of dapagliflozin on COVID-19 progression and associated systemic complications.
ROLE OF SGLT2i
SGLT2i block glucose and sodium reabsorption in the proximal renal tubule and consequently decrease glucose in patients with diabetes, reduce blood pressure and body weight, and have beneficial effects on HF and CKD.23–25 SGLT2i may exhibit multiple pleiotropic properties, including mitigation of inflammation and oxidative stress, improved myocardial and endothelial function, enhanced oxygen delivery, and increased diuresis.23,26,27
Potential Role of SGLT2i on COVID-19
(1) It has been reported that low cytosolic pH increases the probability of COVID-19 infection by affecting the angiotensin-converting enzyme 2 (ACE2)28 and that ACE2 activity is increased in an acidic environment29 (Fig. 1). Hydroxychloroquine may inhibit SARS-CoV-2 that attach to ACE2 by increasing the cytosolic pH.30,31 Angiotensin (Ang) II promotes Na+ reabsorption and H+ secretion through (Na+)/hydrogen (H+) membrane antiporter and therefore increases cytosolic pH.32 Aging, itself and by decreasing Ang II, and hypertension reduce cytosolic pH, and thus makes the virus binding to ACE2 easier.33–35 Furthermore, smoking and obesity may increase the probability of COVID-19 because of hypercapnia-mediated reduction of cytosolic pH.36 These data suggest that low cytosolic pH facilitates the binding of SARS-CoV-2 with ACE2 and subsequently may increase viral load.
FIGURE 1.
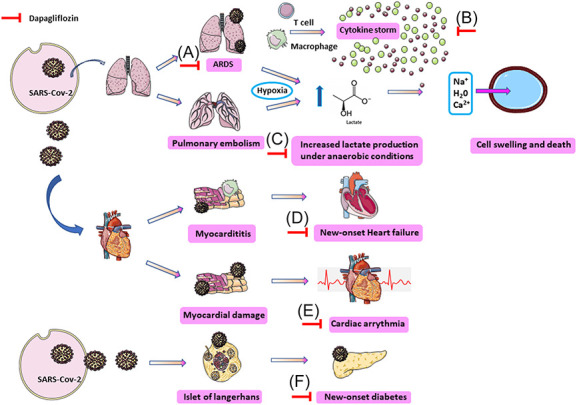
Potential beneficial role of SGLT2i in COVID-19 short-term and long-term complications. (A) SGLT2i seem to upregulate ACE2 receptors on the cells and thus increase the Ang 1–7 level that exert protective effect against ARDS caused by COVID-19; (B) SGLT2i reduce proinflammatory cytokines including those of COVID-19 cytokine storm, ie, IL-6, IL-10, and TNF-a; (C) ARDS and PE caused by COVID-19 lead to tissue hypoxia and subsequently increased lactate production. SGLT2i reduce lactate production and lactate/H+-symporter and NHE antiporter activity, and thus decrease Na+, water, and Ca2+ influx intracellularly through NHE and subsequent cell swelling and death; (D) anti-inflammatory properties of SGLT2i could likely alleviate myocarditis progression but no evidence exists to support this hypothesis. SGLT2i could potentially decrease the odds of new-onset HF caused by COVID-19 with the following mechanisms: (1) osmotic diuresis and natriuresis that decreases cardiac overload, (2) reduction of sarcoplasmic Ca2+ leak and thus increased myocardium contractility, (3) dampening of SNS, (4) reduction of oxidative stress and inflammation, (5) increased O2 delivery by triggering EPO secretion, (6) increased cardiac efficiency, and (7) blood pressure, HbA1c, body weight, and vascular stiffness decreasing; (E) SGLT2i decrease the arrhythmogenic risk caused by COVID-19 as they (1) mitigate inflammation and oxidative stress, (2) improve myocardial efficiency, (3) improve oxygen delivery, (4) increase reabsorption of magnesium and potassium from the tubular fluid, (5) improve cardiac metabolism, (6) exert sympathoinhibitory effect, and (7) reduce cardiac fibrosis and left ventricular hypertrophy, which provide a substrate for arrhythmia development; (F) SARS-CoV-2 can infect and damage the pancreatic alpha and beta cells, whereas the cytokine storm can aggravate this impairment and lead to new-onset diabetes. Dapagliflozin reduced the incidence of new-onset diabetes in participants with HFrEF without diabetes at baseline. ACE2, angiotensin-converting enzyme 2; Ang 1–7, angiotensin 1-7; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; EPO, erythropoietin; HbA1c, hemoglobin A1c; HFrEF, heart failure with reduced ejection fraction; IL-10, interleukin-10; IL-6, interleukin-6; Na+ HF, heart failure; NHE, Na+/H+ exchanger; PE, pulmonary embolism; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; SGLT2i, sodium-glucose co-transporter 2 inhibitors; SNS, sympathetic nervous system; TNF-a, tumor necrosis factor-a.
Severe virus infections, including COVID-19, may cause cytokine-mediated tissue damage and lactate dehydrogenase (LDH) release.37 Indeed, elevated LDH levels have been associated with worse prognosis in COVID-19.38 LDH is a cytosolic enzyme that increases in serum as infected cells break down. LDH induces lactate formation increases from pyruvate under anaerobic conditions.39 SARS-CoV-2 pulmonary infection results in high hypoxic environment due to tissue deoxygenation and increased lactate production.40 Na+/H+ exchanger (NHE) and lactate/H+ symporter play an important role in regulating intracellular pH.41 On elevated lactate levels in the extracellular area, the lactate/H+ symporter co-transports lactate and H+ intracellularly.41 This triggers NHE activation that extracts one H+ from the intracellular to the extracellular area and transports Na+, water, and Ca2+ inside the cell.41 As a consequence, the cell swells and dies.41
Dapagliflozin has been reported to reduce lactate levels by various mechanisms, ie, reduction of oxygen consumption in tissues.42,43 This lactate decrease in the extracellular area lowers the activation of lactate/H+ symporter and subsequently the cytosolic pH is maintained. In addition, dapagliflozin inhibits NHE and the subsequent flow of Na+ and Ca2+, preventing the cells from swelling and death.44
(2) SARS-CoV-2 enters into target cells after binding to ACE2.45 SGLT2i seem to upregulate ACE2 receptors on the cells.46,47 ACE2 increases the Ang 1–7 level. It has been proposed that increased Ang 1–7, a potent vasodilator, exerts protective effects against acute respiratory distress syndrome (ARDS) related to COVID-19.48 Indeed, animal experimental studies have shown that Ang 1–7 can significantly improve vascular endothelial function.49 Conversely, increased ACE2 can also trigger cardiac arrhythmias and myocarditis by increasing the cellular intrusion of SARS-CoV-2 into the heart tissue.50
In this context, a recent retrospective observational study (n = 717 hospitalized patients with COVID-19) showed that SGLT2i were significantly associated with a lower risk of mechanical ventilation [adjusted Relative risk (RR) = 0.03; 95% confidence interval (CI): 0.00–0.70] in patients with diabetes.51 However, another study in Spain (n = 2666 patients with type 2 diabetes admitted for COVID-19) showed that none of the at-home glucose-lowering drugs analyzed (metformin, DPP4i, insulin, metformin plus DPP4i, metformin plus SGLT2i, and metformin plus insulin) were significantly associated with in-hospital deaths; the composite outcome of the need for intensive care unit, mechanical ventilation, or in-hospital death; in-hospital complications; or a long-time hospital stay.52
Another question is whether increased ACE2 levels from SGLT2i might contribute in increased susceptibility to COVID-19.53 In a propensity score–matched cohort study with active comparators and a negative control outcome in a large UK-based primary care data set, participants prescribed SGLT2i (n = 9948) were compared with those prescribed dipeptidyl peptidase-4 inhibitors (DPP4i) (n = 14,917). The incidence rate of confirmed or clinically suspected COVID-19 was not significantly different between users of SGLT2i and users of DPP4i (adjusted HR 0.92, 95% CI 0.66–1.29).54 It was concluded that clinicians may safely use SGLT2i in the everyday care of people with diabetes during the COVID-19 pandemic.
COVID-19 CLINICAL SPECTRUM AND THE ROLE OF DAPAGLIFLOZIN
COVID-19, New-Onset Diabetes and Dapagliflozin
New-onset diabetes has been observed in patients with COVID-1955 (Fig. 1). In a population of 453 patients with COVID-19, 94 developed new-onset diabetes [defined as initial recognized fasting plasma glucose ≥7 mmol/L and HbA1c ≥48 mmol/mol (6.5%) at hospital admission].56 One study showed that SARS-CoV-2 can infect and damage the pancreatic alpha and beta cells, whereas the cytokine storm can aggravate this impairment.57 These data support that COVID-19 can lead to new-onset diabetes. Conversely, in a prespecified exploratory analysis from DAPA-HF trial (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure), dapagliflozin reduced by 32% [HR = 0.68 (95% CI, 0.50–0.94); P = 0.019] the incidence of new-onset diabetes in participants without diabetes at baseline (n = 2605).58 Decreasing the risk of new-onset diabetes may be of benefit in patients with COVID-19 without diabetes at the time of infection.
COVID-19, Immune Response Alterations and Dapagliflozin
Hyperglycemia is the principal reason of attenuated innate immunity; it decreases neutrophil chemotaxis, phagocytosis, and adherence to endothelium.59 This makes the first-line defense against SARS-CoV-2 inadequate and cells become more vulnerable to SARS-CoV-2–related inflammatory response.60 In general, better diabetes control is associated with more efficient immune response.61
Diabetes is associated with low-grade chronic inflammation due to increased secretion of cytokines and adipose tissue hormones, such as leptin, tumor necrosis factor-a (TNF-a), and interleukin-6 (IL-6).62–64 As a result, if individuals with diabetes get infected by SARS-CoV-2, the underlying chronic inflammatory state may enhance the cytokine storm associated with COVID-19, ie, an increased secretion of IL-6, IL-10, and TNF-a, with potential organ damage.9 SGLT2i decrease proinflammatory cytokines, including those involved in cytokine storm of COVID-19.65
In addition, diabetes is considered as a hypercoagulable state because of increased platelet aggregation and stimulation and increased levels of clotting factors and fibrinolysis inhibitors as well as endothelium dysfunction.66,67 COVID-19 has been linked with thrombotic and coagulation abnormalities, which can clinically be manifested as pulmonary embolism and deep vein thrombosis.68–70 Furthermore, in severe SARS-CoV-2 infection, histopathological studies demonstrated direct viral infection of endothelial cells, endotheliitis with inflammation response and secondary endothelial dysfunction, and microthrombi formation.71–73 However, SLGT2i have been demonstrated to improve endothelial function.74,75 Interestingly, empagliflozin and dapagliflozin reduced in vitro platelet activation, potentially through NHE inhibition and thus may prevent thrombosis.76 However, in a meta-analysis of 29 randomized controlled trials (RCTs) (n = 56,035 patients with type 2 diabetes), no significant association between SGLT2i and risk of deep vein thrombosis, pulmonary embolism, and venous thromboembolism was found.77 It remains uncertain how SGLT2i affect thromboembolic events, and multiple mechanistic studies are ongoing to elucidate their impact on vascular system.
COVID-19, Cardiovascular System and Dapagliflozin
A recent study demonstrated that the coexistence of cardiovascular disease and diabetes further increased COVID-19 mortality risk.10 One multihospital cohort study in New York City demonstrated that of hospitalized patients with COVID-19 (n = 2736), 36% had myocardial injury.78 Cardiovascular presentation of COVID-19 includes myocarditis, cardiac arrhythmias, acute coronary syndrome, and death.79,80 Whether the cardiac damage is provoked directly by the virus or is associated with the immunologic response remains a testable hypothesis.81
COVID-19, Myocarditis, Heart Failure, and Dapagliflozin
COVID-19 myocarditis is associated with cytokine storm and probably SARS-CoV-2 entry into cardiomyocytes by binding to ACE2.82 Anti-inflammatory properties of SGLT2i could likely alleviate myocarditis progression, but until now no evidence exists to support this hypothesis.83
Interestingly, Puntmann et al84 demonstrated that of patients recently recovered from COVID-19 (n = 100), 78% had demonstrable cardiac injury, 60% had active myocardial inflammation in cardiovascular magnetic resonance imaging, and 76% had detectable high-sensitivity troponin after 71 days from COVID-19 diagnosis. Indeed, recently recovered patients had lower left ventricular ejection fraction, higher left ventricle volumes, higher left ventricle mass, and late gadolinium enhancement compared with controls.84 In the same line, postmortem pathological findings of deceased individuals demonstrated (1) increased activity of 6 proinflammatory genes in heart tissue with SARS-CoV-2 infection compared with hearts with no SARS-CoV-2, (2) detection of virus in the heart of 24 patients (61.5%), and (3) active viral replication in interstitial cells or macrophages.85 Furthermore, COVID-19–related myocarditis and cytokine storm may increase the odds for HF.86 These findings indicate that COVID-19, even if resolved, might lead to long-term residual left ventricular dysfunction and inflammation and thus evolve to new-onset HF.84–87
To this end, SGLT2i could play a crucial role in preventing HF progression in these patients. DAPA-HF trial (n = 4744) demonstrated that among patients with HF and a reduced ejection fraction, dapagliflozin reduced the risk of worsening HF or death from cardiovascular causes versus placebo, regardless of the presence or absence of diabetes.24 Especially, dapagliflozin compared with placebo reduced the risk of worsening HF or cardiovascular death by 27% [hazard ratio (HR), 0.73 (95% CI, 0.60–0.88)] in patients without diabetes and by 25% in patients with diabetes [HR, 0.75 (95% CI, 0.63–0.90)].88 These data support a potential beneficial role of SGLT2i in HF of patients with COVID-19 without diabetes as well. Possible underlying mechanisms include (1) osmotic diuresis and natriuresis that lower cardiac overload,89 (2) reduction of sarcoplasmic Ca2+ leak that increases cardiac contractility,90 (3) dampening the sympathetic nervous system,91 (4) reduction of inflammation and oxidative stress,74,92–94 (5) increased oxygen delivery to the heart through triggering renal erythropoietin secretion,94,95 (6) increased cardiac efficiency,96–99 and (7) blood pressure, body weight, A1c, and vascular stiffness reduction.100,101
COVID-19, Arrythmias and Dapagliflozin
COVID-19 has been implicated in cardiac arrhythmias, especially in critically ill patients.102,103 These include supraventricular tachycardia,104 atrial fibrillation (AF),103 atrial flutter (AFL),105 complete heart block,105,106 cardiac arrest,103 polymorphic ventricular tachycardia,103 multifocal ventricular tachycardia,106 and sinus tachycardia.107 Interestingly, in a study of 137 patients with COVID-19 in tertiary hospitals in China, almost 7.3% reported palpitations as one of the presenting symptoms108. In another study, 16.7% of patients with COVID-19 were documented to have arrhythmias, commonly in the intensive care unit setting (44.4%)109. Sustained episodes of ventricular tachycardia/ventricular fibrillation occurred in 5.9% of 187 hospitalized patients with COVID-19.79 Possible mechanisms for arrhythmogenesis in COVID-19 include (1) myocarditis,102,110 (2) hypoxia induced from direct viral injury on pulmonary system,102,111 (3) myocardial ischemia,86 (4) myocardial strain due to pulmonary hypertension, (5) increased IL-6 and IL-1β that are potentially proarrhythmic factors,102 (6) electrolyte decompensation and intravascular volume disturbance from diarrhea, vomiting, and/or renal injury, and (8) drug side effects.112
In the DECLARE TIMI 58 trial, dapagliflozin reduced the risk of new AF/AFL events by 19% (HR: 0.81, 95% CI 0.68–0.95, P = 0.009), the risk of atrial tachycardia by 20% (HR: 0.80, 95% CI 0.68–0.94), the risk of supraventricular tachyarrhythmia/tachycardia by 17% (HR: 0.83, 95% CI 0.71–0.97), and the total number of AF/AFL events by 17% (HR: 0.77, 95% CI 0.64–0.92) in high-risk patients with type 2 diabetes.113 Similarly, one real-world study demonstrated that SGLT2i administration was associated with a lower risk of new-onset arrhythmias in patients with type 2 diabetes compared with those not receiving SGLT2i.114 SGLT2i may (1) mitigate inflammation and oxidative stress, (2) improve myocardial efficiency, and (3) improve oxygen delivery, all of which may be important in preventing AF/AFL.23,26,27 The EMPA-HEART CardioLink-6 trial, a 6-month double-blind, randomized, placebo-controlled trial in patients with type 2 diabetes and coronary artery disease demonstrated that left ventricular mass index was reduced by 2.6 g/m2 with empagliflozin and by 0.01 g/m2 with placebo from baseline to the 6-month visit (P = 0.01).115 In this context, the Losartan Intervention for Endpoint Reduction in Hypertension trial has shown that left ventricular mass regression is independently associated with a reduction in new heart failure and new AF.116 Moreover, dapagliflozin may exert antiarrhythmic effects through (1) increasing reabsorption of magnesium and potassium from the tubular fluid,100 (2) improving cardiac metabolism,100 (3) exerting sympathoinhibitory effect,117,118 and (4) reducing cardiac fibrosis and left ventricular hypertrophy, which provide a substrate for arrhythmia development.119,120
COVID-19, Renal System and Dapagliflozin
Acute kidney injury (AKI) has been observed in hospitalized patients with COVID-19 with a prevalence as high as 46%.121 In a retrospective observational study in New York (n = 5449), 1993 (36.6%) patients with COVID-19 developed AKI.122 Another study reported that of 62 hospitalized patients COVID-19 not on dialysis, 10% required kidney replacement therapies.123 The exact underlying mechanism is not well understood. COVID-19 is proposed to cause AKI with the following mechanisms: (1) direct viral infection of the endothelial cells of the glomerular capillary loop,124 (2) direct tubular or glomerular injury,57,125,126 (3) acute ischemic tubular necrosis induced by systemic collapse and/or COVID-19–related prothrombotic state, and (4) inflammatory syndrome-mediated renal injury.127 In one of the largest autopsy studies on 26 patients with COVID-19 in China, renal tissue biopsies of 3 patients had glomerular thrombi, 3 pigmented tubular casts, and 7 viral-like particles under electron microscopy.125
SGLT2i have well-recognized nephroprotective properties that might play a key role in COVID-19–related AKI.128–130 RCTs have demonstrated that SGLT2i administration do not increase AKI risk.131–134 Actually, dapagliflozin was associated with significantly lower AKI risk (HR 0.69, 95% CI 0.55–0.87).132 A recently published study (n = 39,094) demonstrated that SGLT2i initiation was associated with a 21% reduction in the 90-day risk of AKI compared with DPP4i (weighted risk ratio: 0.79, 95% CI 0.64–0.98).135 A meta-analysis of 3 large RCTs of cardiovascular outcomes with SGLT2i demonstrated that AKI was reduced by 34% with SGLT2i administration (HR: 0.66, 95% confidence interval 0.54–0.80) compared with placebo.131 A systematic review and meta-analysis of RCTs (n = 38,723) reported that SGLT2i use was associated with a 25% reduction of AKI versus placebo.136 These data suggest a beneficial role of SGLT2i on AKI and hopefully in the setting of COVID-19–related AKI.
In the cardiovascular and renal outcomes trials of SGLT2i, renoprotective properties of SGLT2i seem to extend beyond glycemic control.76,134 Notably, SGLT2i have been proposed to favor renal function by activating tubuloglomerular feedback and reducing intrarenal hypoxia.137,138 Two large cardiovascular and renal outcomes trials, EMPA-KIDNEY and DAPA-CKD, were designed to demonstrate the potential benefit of SGLT2i in CKD.139,140 EMPA-KIDNEY, a randomized double-blind trial, investigates the effect of empagliflozin on kidney disease progression or cardiovascular death versus placebo on top of standard of care in patients (n = 6000) with preexisting CKD with and without type 2 diabetes.139 DAPA-CKD, a phase III randomized double-blind trial, demonstrated that dapagliflozin compared with placebo significantly reduced time to first occurrence of a composite renal end point (estimated glomerular filtration rate decline >50%, end-stage renal disease or renal death) or cardiovascular death in patients (n = 4304) with CKD stages 2–4 regardless of diabetes status.25 These glucose-independent, salutary effects of SGLT2i on CKD might favor renal function in patients with COVID-19 with or without diabetes.
SGLT2i AND ADVERSE EVENTS DURING COVID-19
SGLT2i are associated with adverse events, including DKA, hypovolemia, and low blood pressure, as well as genital mycotic infections that can be exacerbated by COVID-19.141
COVID-19, DKA and Dapagliflozin
DKA has been reported more frequently among patients with COVID-19 with diabetes.142 Infection-related conditions potentially contribute to the development of DKA, including starvation, dehydration due to high fever, vomiting and/or diarrhea, and release of insulin-antagonistic hormones, such as catecholamines and cortisol. In addition, volume depletion and low blood pressure can be amplified by SGLT2i.132–134 As a result, DKA risk among SGLT2i-treated patients might be high in severe COVID-19.143 In this regard, a number of SGLT2i-related cases of euglycemic DKA in patients with COVID-19 have been reported.144–146
COVID-19, Dapagliflozin and Low Pressure
COVID-19 is associated with respiratory failure, shock, multiorgan failure, and tend to decrease blood pressure due to natriuresis.147,148 Thus, SGLT2i administration could aggravate shock caused by severe COVID-19.
COVID-19, Dapagliflozin and Urogenital System Mycotic Infections
SGLT2i have been linked with a higher incidence of urogenital fungal infections.23,149,150 Antibiotics, and dexamethasone, commonly used in patients with COVID-19 are known to also predispose to fungal infections.151
COVID-19, Dapagliflozin and Drug Interactions
As of October 3, 2020, no meaningful interactions for empagliflozin or dapagliflozin were documented, whereas canagliflozin may potentially interact with lopinavir-ritonavir (Kaletra) requiring efficient drug-dosing modifications (https://www.covid19-druginteractions.org/).
CONCLUSIONS
SGLT2i could play a key role in reducing COVID-19 progression and prevent its short-term and long-term complications, mainly by offering cardioprotection and nephroprotection. SGLT2i seem to upregulate ACE2 receptors and thus increase the Ang 1–7, which exert protective effects against ARDS related to COVID-19. Furthermore, SGLT2i may reduce proinflammatory cytokines, including those of COVID-19 cytokine storm, ie, IL-6, IL-10, and TNF-a. Interestingly, SGLT2i could reduce the risk of new-onset diabetes observed in COVID-19. In addition, they could alleviate myocarditis, arrhythmogenesis, HF progression, and kidney injury in these patients. DARE-19 will show if this hypothesis is true.
Footnotes
E. Hatziagelaki has participated in educational, research, and advisory activities sponsored by MSD, Eli Lilly, Bristol/AstraZeneca, Novo Nordisk, Menarini, Win Medica, Boehringer-Ingelheim, Sanofi, Novartis, and Elpen; E. Liberopoulos has participated in educational, research, and advisory activities sponsored by Astra-Zeneca, MSD, Lilly, Bayer, Amgen, Sanofi, Boehringer-Ingelheim, Novartis, Novo Nordisk, and Servier. The remaining authors report no conflicts of interest.
G. Anastasiou: Conceptualization, Methodology, Investigation, and Writing—original draft. E. Hatziagelaki: Writing—review and editing. E. Liberopoulos: Visualization, Conceptualization, Methodology, Investigation, Supervision, Project administration, and Writing—Review and editing.
Contributor Information
Georgia Anastasiou, Email: anastgeorgia@hotmail.com.
Erifili Hatziagelaki, Email: erihat@med.uoa.gr.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of covid-19 in New York city. N Engl J Med. 2020;382:2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentile S, Strollo F, Ceriello A. COVID-19 infection in Italian people with diabetes: lessons learned for our future (an experience to be used). Diabetes Res Clin Pract. 2020;162:108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoian AP, Banerjee Y, Rizvi AA, et al. Diabetes and the COVID-19 pandemic: how insights from recent experience might guide future management. Metab Syndr Relat Disord. 2020;18:173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoian AP, Papanas N, Prazny M, et al. Incretin-based therapies role in COVID-19 era: evolving insights. J Cardiovasc Pharmacol Ther. 2020;25:494–496. [DOI] [PubMed] [Google Scholar]
- 9.Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katulanda P, Dissanayake HA, Ranathunga I, et al. Prevention and management of COVID-19 among patients with diabetes: an appraisal of the literature. Diabetologia. 2020;63:1440–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceriello A, Stoian AP, Rizzo M. COVID-19 and diabetes management: what should be considered? Diabetes Res Clin Pract. 2020;163:108151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patoulias D, Papadopoulos C, Katsimardou A, et al. Sodium-glucose cotransporter 2 inhibitors and major COVID-19 outcomes: promising mechanisms, conflicting data, and intriguing clinical decisions. Diabetes Ther. 2020;11:3003–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rayman G, Lumb A, Kennon B, et al. Guidelines for the management of diabetes services and patients during the COVID-19 pandemic. Diabet Med. 2020;37:1087–1089. [DOI] [PubMed] [Google Scholar]
- 16.FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. 2015. Available at: https://www.fda.gov/media/92185/download. Accessed October 3, 2020. [Google Scholar]
- 17.Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koufakis T, Metallidis S, Zebekakis P, et al. Sodium-glucose cotransporter 2 inhibitors in the era of COVID-19 pandemic: is the benefit to risk ratio still favorable? J Diabetes Sci Technol. 2020;14:745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartmann-Boyce J, Morris E, Goyder C, et al. Managing diabetes during the COVID-19 pandemic. CEBM. 2020. Available at: https://www.cebm.net/covid-19/managing-diabetes-during-the-covid-19-pandemic/. [Google Scholar]
- 20.Papadokostaki E, Tentolouris N, Liberopoulos E. COVID-19 and diabetes: what does the clinician need to know? Prim Care Diabetes. 2020;14:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S.. National Library of Medicine. Dapagliflozin in Respiratory Failure in Patients with COVID-19 (DARE-19) [Internet]. Available at: https://www.clinicaltrials.gov/ct2/show/NCT04350593. Accessed May 10, 2020 [Google Scholar]
- 22.Lu IN, Kulkarni S, Fisk M, et al. muLTi-Arm Therapeutic study in pre-ICu patients admitted with Covid-19-Experimental drugs and mechanisms (TACTIC-E): a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zelniker TA, Braunwald E. Cardiac and renal effects of sodium-glucose Co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:1845–1855. [DOI] [PubMed] [Google Scholar]
- 24.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 25.Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 26.Kidokoro K, Cherney DZI, Bozovic A, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019;140:303–315. [DOI] [PubMed] [Google Scholar]
- 27.Sano M, Goto S. Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation. 2019;139:1985–1987. [DOI] [PubMed] [Google Scholar]
- 28.Cure E, Cumhur Cure M. Comment on “Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19.” J Med Virol. 2020;92:1423–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoian AP, Catrinoiu D, Rizzo M, et al. Hydroxychloroquine, COVID-19 and diabetes. Why it is a different story. Diabetes Metab Res Rev. 2021;37:e3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye M, Flores G, Batlle D. Angiotensin II and angiotensin-(1-7) effects on free cytosolic sodium, intracellular pH, and the Na+-H+ antiporter in vascular smooth muscle. Hypertension. 1996;27:72–78. [DOI] [PubMed] [Google Scholar]
- 33.Kniess RA, Mayer MP. The oxidation state of the cytoplasmic glutathione redox system does not correlate with replicative lifespan in yeast. NPJ Aging Mech Dis. 2016;2:16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon HE, Choi BS. The renin-angiotensin system and aging in the kidney. Korean J Intern Med. 2014;29:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Resnick LM, Gupta RK, Sosa RE, et al. Intracellular pH in human and experimental hypertension. Proc Natl Acad Sci U S A. 1987;84:7663–7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rios EJ, Fallon M, Wang J, et al. Chronic hypoxia elevates intracellular pH and activates Na+/H+ exchange in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L867–L874. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Outschoorn UE, Prisco M, Ertel A, et al. Ketones and lactate increase cancer cell “stemness,” driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via Metabolo-Genomics. Cell Cycle. 2011;10:1271–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farhana A, Lappin SL. Biochemistry, lactate dehydrogenase (LDH), 2020. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 40.Rello J, Storti E, Belliato M, et al. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur Respir J. 2020;55:2001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu D, Kraut JA. Potential role of NHE1 (sodium-hydrogen exchanger 1) in the cellular dysfunction of lactic acidosis: implications for treatment. Am J Kidney Dis. 2011;57:781–787. [DOI] [PubMed] [Google Scholar]
- 42.Cure E, Cumhur Cure M. Comment on sodium-glucose Co-transporter 2 inhibitors and heart failure. Am J Cardiol. 2020;125:1602. [DOI] [PubMed] [Google Scholar]
- 43.Tang Y, Sun Q, Bai XY, et al. Effect of dapagliflozin on obstructive sleep apnea in patients with type 2 diabetes: a preliminary study. Nutr Diabetes. 2019;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye Y, Jia X, Bajaj M, et al. Dapagliflozin attenuates Na(+)/H(+) exchanger-1 in cardiofibroblasts via AMPK activation. Cardiovasc Drugs Ther. 2018;32:553–558. [DOI] [PubMed] [Google Scholar]
- 45.Vaduganathan OV M, Michel T, McMurray JJV, et al. Renin–angiotensin–aldosterone system inhibitors in patients with covid-19. N Engl J Med. 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cure E, Cumhur Cure M. Comment on “Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic?” J Hypertens. 2020;38:1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawanami D, Matoba K, Takeda Y, et al. SGLT2 inhibitors as a therapeutic option for diabetic nephropathy. Int J Mol Sci. 2017;18:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92:726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas MC, Pickering RJ, Tsorotes D, et al. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res. 2010;107:888–897. [DOI] [PubMed] [Google Scholar]
- 50.Cure E, Cumhur Cure M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabetes Metab Syndr. 2020;14:349–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dalan R, Ang LW, Tan WYT, et al. The association of hypertension and diabetes pharmacotherapy with COVID-19 severity and immune signatures: an observational study. Eur Heart J Cardiovasc Pharmacother. 2020:pvaa098. doi: 10.1093/ehjcvp/pvaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez-Belmonte LM, Torres-Pena JD, Lopez-Carmona MD, et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study. BMC Med. 2020;18:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cure E, Cumhur Cure M. Can dapagliflozin have a protective effect against COVID-19 infection? A hypothesis. Diabetes Metab Syndr. 2020;14:405–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sainsbury C, Wang J, Gokhale K, et al. Sodium-glucose co-transporter-2 inhibitors and susceptibility to COVID-19: a population-based retrospective cohort study. Diabetes Obes Metab. 2021;23:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubino F, Amiel SA, Zimmet P, et al. New-onset diabetes in covid-19. N Engl J Med. 2020;383:789–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251:228–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inzucchi SE, Docherty KF, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, et al. ; DAPA-HF Investigators and Committees. Dapagliflozin and the incidence of type 2 diabetes in patients with heart failure and reduced ejection fraction: an exploratory analysis from DAPA-HF. Diabetes Care. 2021;44:586–594. [DOI] [PubMed] [Google Scholar]
- 59.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26:259–265. [DOI] [PubMed] [Google Scholar]
- 60.Yan Y, Yang Y, Wang F, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8:e001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hine JL, de Lusignan S, Burleigh D, et al. Association between glycaemic control and common infections in people with Type 2 diabetes: a cohort study. Diabet Med. 2017;34:551–557. [DOI] [PubMed] [Google Scholar]
- 62.Arnalich F, Hernanz A, López-Maderuelo D, et al. Enhanced acute-phase response and oxidative stress in older adults with type II diabetes. Horm Metab Res. 2000;32:407–412. [DOI] [PubMed] [Google Scholar]
- 63.Kado S, Nagase T, Nagata N. Circulating levels of interleukin-6, its soluble receptor and interleukin-6/interleukin-6 receptor complexes in patients with type 2 diabetes mellitus. Acta Diabetol. 1999;36:67–72. [DOI] [PubMed] [Google Scholar]
- 64.Pickup JC, Chusney GD, Thomas SM, et al. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291–300. [DOI] [PubMed] [Google Scholar]
- 65.Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: the potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018;44:457–464. [DOI] [PubMed] [Google Scholar]
- 66.Carr ME. Diabetes mellitus: a hypercoagulable state. J Diabetes Complications. 2001;15:44–54. [DOI] [PubMed] [Google Scholar]
- 67.Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with COVID-19 in wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142:114–128. [DOI] [PubMed] [Google Scholar]
- 70.Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020;56:2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jung F, Kruger-Genge A, Franke RP, et al. COVID-19 and the endothelium. Clin Hemorheol Microcirc. 2020;75:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pons S, Fodil S, Azoulay E, et al. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El-Daly M, Pulakazhi Venu VK, Saifeddine M, et al. Hyperglycaemic impairment of PAR2-mediated vasodilation: prevention by inhibition of aortic endothelial sodium-glucose-co-Transporter-2 and minimizing oxidative stress. Vascul Pharmacol. 2018;109:56–71. [DOI] [PubMed] [Google Scholar]
- 75.Mancini SJ, Boyd D, Katwan OJ, et al. Canagliflozin inhibits interleukin-1beta-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci Rep. 2018;8:5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spigoni V, Fantuzzi F, Carubbi C, et al. Sodium-glucose cotransporter 2 inhibitors antagonize lipotoxicity in human myeloid angiogenic cells and ADP-dependent activation in human platelets: potential relevance to prevention of cardiovascular events. Cardiovasc Diabetol. 2020;19:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang A, Yang K, Wang T, et al. Effects of sodium-glucose cotransporter 2 inhibitors on risk of venous thromboembolism in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2020;36:e3174. [DOI] [PubMed] [Google Scholar]
- 78.Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu PP, Blet A, Smyth D, et al. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142:68–78. [DOI] [PubMed] [Google Scholar]
- 82.Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Das L, Dutta P. SGLT2 inhibition and COVID-19: the road not taken. Eur J Clin Invest. 2020;50:e13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;15:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lindner D, Fitzek A, Brauninger H, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uriel N, Sayer G, Clerkin KJ. Myocardial injury in COVID-19 patients: the beginning or the end? J Am Coll Cardiol. 2020;76:547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yancy CW, Fonarow GC. Coronavirus disease 2019 (COVID-19) and the heart-is heart failure the next chapter? JAMA Cardiol. 2020;5:1216–1217. [DOI] [PubMed] [Google Scholar]
- 88.Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hallow KM, Helmlinger G, Greasley PJ, et al. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479–487. [DOI] [PubMed] [Google Scholar]
- 90.Mustroph J, Wagemann O, Lucht CM, et al. Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Fail. 2018;5:642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scheen AJ. Effect of SGLT2 inhibitors on the sympathetic nervous system and blood pressure. Curr Cardiol Rep. 2019;21:70. [DOI] [PubMed] [Google Scholar]
- 92.Mulder S, Heerspink HJL, Darshi M, et al. Effects of dapagliflozin on urinary metabolites in people with type 2 diabetes. Diabetes Obes Metab. 2019;21:2422–2428. [DOI] [PubMed] [Google Scholar]
- 93.Yaribeygi H, Atkin SL, Butler AE, et al. Sodium-glucose cotransporter inhibitors and oxidative stress: an update. J Cell Physiol. 2019;234:3231–3237. [DOI] [PubMed] [Google Scholar]
- 94.Ye Y, Bajaj M, Yang HC, et al. SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther. 2017;31:119–132. [DOI] [PubMed] [Google Scholar]
- 95.Maruyama T, Takashima H, Oguma H, et al. Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technol Ther. 2019;21:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73:1931–1944. [DOI] [PubMed] [Google Scholar]
- 97.Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. [DOI] [PubMed] [Google Scholar]
- 98.Verma S, Rawat S, Ho KL, et al. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci. 2018;3:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horton JL, Davidson MT, Kurishima C, et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4:e124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:422–434. [DOI] [PubMed] [Google Scholar]
- 101.Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lazzerini PE, Boutjdir M, Capecchi PL. COVID-19, arrhythmic risk, and inflammation: mind the gap!. Circulation. 2020;142:7–9. [DOI] [PubMed] [Google Scholar]
- 103.Dherange P, Lang J, Qian P, et al. Arrhythmias and COVID-19: a review. JACC Clin Electrophysiol. 2020;6:1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vidovich MI. Transient brugada-like ECG pattern in a patient with coronavirus disease 2019 (COVID-19). JACC Case Rep. 2020;2:1245–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seecheran R, Narayansingh R, Giddings S, et al. Atrial arrhythmias in a patient presenting with coronavirus disease-2019 (COVID-19) infection. J Investig Med High Impact Case Rep. 2020;8:2324709620925571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He J, Wu B, Chen Y, et al. Characteristic electrocardiographic manifestations in patients with COVID-19. Can J Cardiol. 2020;36:966 e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133:1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peretto G, Sala S, Rizzo S, et al. Arrhythmias in myocarditis: state of the art. Heart Rhythm. 2019;16:793–801. [DOI] [PubMed] [Google Scholar]
- 111.Kolettis TM. Coronary artery disease and ventricular tachyarrhythmia: pathophysiology and treatment. Curr Opin Pharmacol. 2013;13:210–217. [DOI] [PubMed] [Google Scholar]
- 112.Giudicessi JR, Noseworthy PA, Friedman PA, et al. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19). Mayo Clin Proc. 2020;95:1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zelniker TA, Bonaca MP, Furtado RHM, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141:1227–1234. [DOI] [PubMed] [Google Scholar]
- 114.Chen HY, Huang JY, Siao WZ, et al. The association between SGLT2 inhibitors and new-onset arrhythmias: a nationwide population-based longitudinal cohort study. Cardiovasc Diabetol. 2020;19:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Verma S, Mazer CD, Yan AT, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140:1693–1702. [DOI] [PubMed] [Google Scholar]
- 116.Devereux RB, Dahlof B, Gerdts E, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110:1456–1462. [DOI] [PubMed] [Google Scholar]
- 117.Matthews VB, Elliot RH, Rudnicka C, et al. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens. 2017;35:2059–2068. [DOI] [PubMed] [Google Scholar]
- 118.Wan N, Rahman A, Hitomi H, et al. The effects of sodium-glucose cotransporter 2 inhibitors on sympathetic nervous activity. Front Endocrinol (Lausanne). 2018;9:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Durak A, Olgar Y, Degirmenci S, et al. A SGLT2 inhibitor dapagliflozin suppresses prolonged ventricular-repolarization through augmentation of mitochondrial function in insulin-resistant metabolic syndrome rats. Cardiovasc Diabetol. 2018;17:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. [DOI] [PubMed] [Google Scholar]
- 121.Chan L, Chaudhary K, Saha A, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Malberti F, Pecchini P, Marchi G, et al. When a nephrology ward becomes a COVID-19 ward: the Cremona experience. J Nephrol. 2020;33:625–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kissling S, Rotman S, Gerber C, et al. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98:228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Farouk SS, Fiaccadori E, Cravedi P, et al. COVID-19 and the kidney: what we think we know so far and what we don't. J Nephrol. 2020;33:1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heerspink HJL, Kosiborod M, Inzucchi SE, et al. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94:26–39. [DOI] [PubMed] [Google Scholar]
- 129.Lim HJ, Lee HH, Kim AJ, et al. Renin-angiotensin-aldosterone system blockade in critically ill patients is associated with increased risk for acute kidney injury. Tohoku J Exp Med. 2016;238:17–23. [DOI] [PubMed] [Google Scholar]
- 130.Lapi F, Azoulay L, Yin H, et al. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ. 2013;346:e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Menne J, Dumann E, Haller H, et al. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: a systematic review and meta-analysis. PLoS Med. 2019;16:e1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 133.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 134.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 135.Iskander C, Cherney DZ, Clemens KK, et al. Use of sodium-glucose cotransporter-2 inhibitors and risk of acute kidney injury in older adults with diabetes: a population-based cohort study. CMAJ. 2020;192:E351–E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7:845–854. [DOI] [PubMed] [Google Scholar]
- 137.Dekkers CCJ, Gansevoort RT, Heerspink HJL. New diabetes therapies and diabetic kidney disease progression: the role of SGLT-2 inhibitors. Curr Diab Rep. 2018;18:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rajasekeran H, Cherney DZ, Lovshin JA. Do effects of sodium-glucose cotransporter-2 inhibitors in patients with diabetes give insight into potential use in non-diabetic kidney disease? Curr Opin Nephrol Hypertens. 2017;26:358–367. [DOI] [PubMed] [Google Scholar]
- 139.National Library of Medicine (U.S.). (2019, January 31- 2022, October 31). EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin). ClinicalTrials.gov Identifier: NCT03594110. Available at: https://www.clinicaltrials.gov/ct2/show/NCT03594110. Accessed October 3, 2020. [Google Scholar]
- 140.Heerspink HJL, Stefansson BV, Chertow GM, et al. Rationale and protocol of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transpl. 2020;35:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Singh M, Kumar A. Risks associated with SGLT2 inhibitors: an overview. Curr Drug Saf. 2018;13:84–91. [DOI] [PubMed] [Google Scholar]
- 142.Li J, Wang X, Chen J, et al. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Armeni E, Aziz U, Qamar S, et al. Protracted ketonaemia in hyperglycaemic emergencies in COVID-19: a retrospective case series. Lancet Diabetes Endocrinol. 2020;8:660–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ozer O, Yorulmaz G. Euglycemic diabetic ketoacidosis associated with empagliflozin use in the course of the SARS-cov-2 pandemic. J Coll Physicians Surg Pak. 2020;30:110–111. [DOI] [PubMed] [Google Scholar]
- 145.Fang J, Genco M, Caskey RN. COVID-19 precipitating euglycaemic diabetic ketoacidosis with SGLT2 inhibitor use. Eur J Case Rep Intern Med. 2020;7:001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Batista DV, Vieira CAFA, Costa TA, Lima EG. COVID-19-associated euglycemic diabetic ketoacidosis in a patient with type 2 diabetes on SGLT2 inhibitor: a case report. Diabetol Int. 2020;1–4. doi: 10.1007/s13340-020-00473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 148.Goldenberg RM, Berard LD, Cheng AYY, et al. SGLT2 inhibitor-associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis. Clin Ther. 2016;38:2654–2664 e1. [DOI] [PubMed] [Google Scholar]
- 149.Hirji I, Andersson SW, Guo Z, et al. Incidence of genital infection among patients with type 2 diabetes in the UK General Practice Research Database. J Diabetes Complications. 2012;26:501–505. [DOI] [PubMed] [Google Scholar]
- 150.Figueiredo IR, Rose SCP, Freire NB, et al. Use of sodium-glucose cotransporter-2 inhibitors and urinary tract infections in type 2 diabetes patients: a systematic review. Rev Assoc Med Bras. 2019;65:246–252. [DOI] [PubMed] [Google Scholar]
- 151.Brossart P, Kotthoff P. Dexamethasone promotes fungal infection by inhibition of APC activation with beta-glucans via STAT-3 and NF-κb. Blood. 2016;128:3710. [Google Scholar]


