Abstract
Keratinocyte migration into skin wounds is the step of the healing process that correlates with the wound closure rate. Keratinocyte migration, and wound epithelialization are decreased when beta 2-adrenergic receptors (B2AR) are activated by 1 μM epinephrine/adrenaline, resulting in delayed wound healing in human and mouse skin. In the present study, we found paradoxically, that in a subset of keratinocyte strains exposure to low concentrations of epinephrine (0.1 nM) increased, rather than decreased, their migratory rate. We find that both the alpha- and the beta-adrenergic receptors are expressed in human keratinocytes, and expression of alpha-2 AR subtypes demonstrated for the first time. Therefore, we tested if the alpha-AR could be modulating the increased migratory response observed in these cell strains. By using specific inhibitors to alpha-AR, we demonstrated that blocking A2B-AR could reverse the rapid cell migration induced by the 0.1 nM epinephrine. Phosphorylation of ERK was elevated after 1–10 minutes of the low epinephrine treatment and the A2B-AR inhibitor blocked the ERK phosphorylation. The results suggest that both the A2B-AR and B2AR mediate keratinocyte migration, in which with a low level of epinephrine treatment, A2B-AR could alter the B2AR signals and regulate the migration rate.
Introduction
After wounding, processes of inflammation, tissue formation and remodeling, and wound re-epithelialization sequentially establish adequate healing [1, 2]. From our past studies, the G-protein coupled beta adrenergic adrenergic receptor (AR) mediates important wound healing processes including keratinocyte migration and wound epithelialization [3–6], alteration of fibroblast reparative phenotype [7, 8], sustaining an inflammatory environment within the wound by increasing neutrophil dwell times [9], and increasing inflammatory cytokines within the wound [9]. One of the endogenous ligands for ARs is the stress hormone, epinephrine/adrenaline. Because the beta 2AR (B2AR) is expressed in most skin resident cells, and the ligands epinephrine and norepinephrine are generated by skin upon wounding tissue [6], we and others have examined the effects of B2AR activation on keratinocyte migration, an important component of skin wound repair. When an individual is under stress, supra-physiological (50 nM—1 μM) concentrations of epinephrine are present in the circulation and these supraphysiological levels inhibit keratinocyte migration in vitro, and impairing healing in vitro, ex vivo and in vivo [3–6, 10]. Blocking the binding of the ligand to the beta AR by administration of beta AR antagonist, such as timolol, reverses the inhibition and improves healing in pre-clinical animal models [5, 11–13]. Indeed, beta AR blockade has been proposed as a therapeutic for non-healing wounds [14], and venous or diabetic ulcers [15–17].
However, the range of physiologic concentrations epinephrine in human serum is wide: as low as 0.06 nM during sleep, and 0.11 nM while awake [18], 0.2 nM in hospitalized, resting patients [19, 20], and rising to 8.2 nM during exercise [21], and as high as 56 nM during cardiac arrest [19]. Here we queried the response of keratinocytes to the lower end of the physiologic range of serum epinephrine concentration (0.1 nM) and found that keratinocyte strains from different individuals exhibited an increase, rather than the previously observed decrease, in migratory speed noted in cells exposed to higher concentrations of epinephrine (physiologic at 10 nM, stress at 50 nM or supra-physiologic at 1 μM) [5, 19, 22].
This type of biphasic response to AR stimulation has been reported in mouse (MC4-L5) and human (IBH-4, IBH-6 and MDA-MB-231) breast cancer cells, with a mitogenic response to 10−10 M epinephrine, but inhibition of growth at higher concentrations (>10−8 M) [20]. Co-treating the cells with low dose epinephrine and rauwolscine, an inhibitor of the alpha-2C adrenergic receptor, abolished the increase in proliferation induced by epinephrine alone [20], suggesting both the A2AR and B2AR regulate the cell proliferation response. Here we tested the hypothesis that low, physiologic concentrations (0.1 nM) of epinephrine can activate both the A2AR and the B2AR, resulting in increased, rather than decreased, migratory speed.
Materials and methods
The neonatal human keratinocytes (NHKs) were isolated [5] from the discarded foreskin from elective circumcision and collected from de-identified neonatal male donors at UC Davis hospital in Sacramento, CA under a protocol approved by the UC Davis Institutional Review Board (IRB) Administration. Seventeen strains of NHK were screened by the single cell migration assay [23] and 3 strains that demonstrated a divergent response of significantly accelerated migration at 0.1nM epinephrine concentration were identified for this study. Cell passages between 3–6 are used. NHKs were cultured in keratinocyte growth medium (KGM, EpiLife medium, human keratinocyte growth supplements and 1x antibiotic-antimycotic, Invitrogen, Carlsbad, CA). Cells were starved overnight by cultivation in half strength supplements before the migration assays, then treated with KGM alone (control) +/- (−)-Epinephrine (+)-bitartrate salt (epinephrine, Sigma-Aldrich, St. Louis, MO), yohimbine hydrochloride (yohimbine, a non-specific alpha 2-AR inhibitor, Sigma-Aldrich), BRL 44408 maleate salt (BRL44408, an A2A-AR inhibitor, Sigma-Aldrich), ARC239 dihydrochloride (ARC239, an A2B-AR inhibitor, Tocris Bioscience, Minneapolis, MN) and rauwolscine (an A2C-AR inhibitor, Sigma-Aldrich) for 30 minutes. Time-lapse images of 50–100 cells for the single cell migration assay were captured every 5 minutes for 1 hour and migratory speeds determined as described [23]. Trend lines were added in Excel. For scratch wound assays [24], confluent keratinocytes were treated with mitomycin C (10 μg/ml, 1 hr, Calbiochem, Burlington, MA) to inhibit cell proliferation. The wound area was measured by Image J. Total RNA was isolated from cultured NHK using the RNeasy® Mini Kit (Qiagen, Valencia, CA) and reverse transcription was performed on 2 ng RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative RT-PCR (TaqMan® Gene Expression Assays, Applied Biosystems, Hs01099503_s1 for ADRA2A, Hs00265090_s1 for ADRA2B, Hs03044628_s1 for ADRA2C, Hs02330048_s1 for ADRB1, and Hs00240532_s1 for ADRB2) was performed in duplicate on 0.056 ng cDNA in a reaction volume of 10 μl to determine AR expression in NHKs with normalization to GAPDH. The protein level of ARs and phosphorylated ERK was determined by Western blotting and 50 μg protein per lane was loaded on NuPAGE Novex 10% Bis-Tris gels (Invitrogen), transferred to Immun-Blot PVDF membranes (Invitrogen) and blocked for 1 hour at room temperature with 5% milk in tris-buffered saline (TBS). Blots were incubated with diluted primary antibodies overnight at 4°C followed by a 1-hour incubation with secondary antibodies at room temperature. Anti-A2A-AR (1: 50, Santa Cruz Biotech, Dallas, Texas), anti-A2B-AR (1: 5,000, Genex BioScience, Hayward CA), anti-A2C-AR (1: 100, Santa Cruz Biotech), anti-B2AR, (1: 2,500, Abcam, Cambridge, MA), anti-β-tubulin I, (1: 15,000, Sigma), anti P-ERK (1: 1,000), and anti ERK (1: 1,000), and HRP-conjugated secondary antibodies (1: 2,000–2,500, Cell Signaling, Danvers, MA) with enhanced chemiluminescence detection (GE Healthcare, Chicago, IL) were used to detect the target proteins. The Western blots for ARs in Fig 1C were imaged on X-ray films (Kodak, Rochester, NY), and the blots for phosphorylated ERK and total ERK in Fig 2C and 2D were imaged digitally (Odyssey, Li-Cor Bioscience, Lincoln, NE) for quantification. The time curves from the scratch assays were compared by two-way ANOVA in StatPlus and the Student T-test was used to compare the migration speeds and the intensity of Western blot bands in Fig 2.
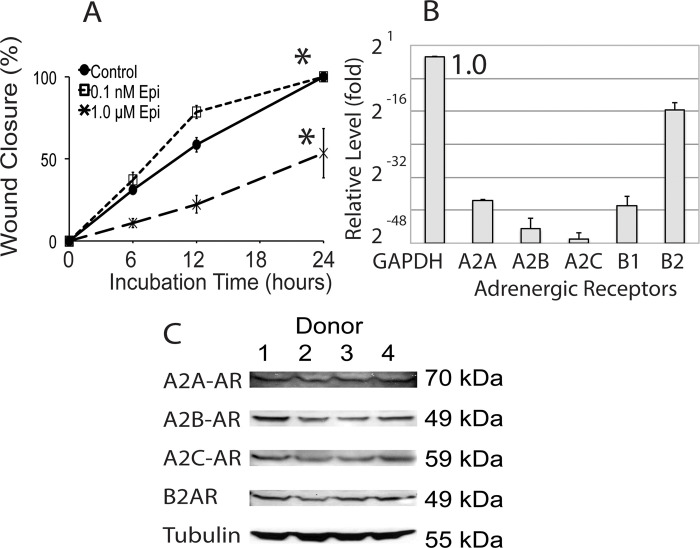
Fig 1. Migration patterns and expression of adrenergic receptors in human keratinocytes.
(A) An increase in migration rate at 12 hours was observed in scratch assays with the keratinocytes treated with 0.1 nM (low concentration) of epinephrine compared to higher, 1 μM concentration. (B) Quantitative RT-PCR (results normalized to GAPDH expression, set as 1.0; X-axis shown in log 2 scale) and (C) Western blotting was performed to detect the expression of adrenergic receptors in keratinocytes. In addition to the B2AR expression, alpha-AR subtypes are expressed in the keratinocytes at low levels. (N = 3 keratinocyte strains, mean +/- SE, * p<0.05).
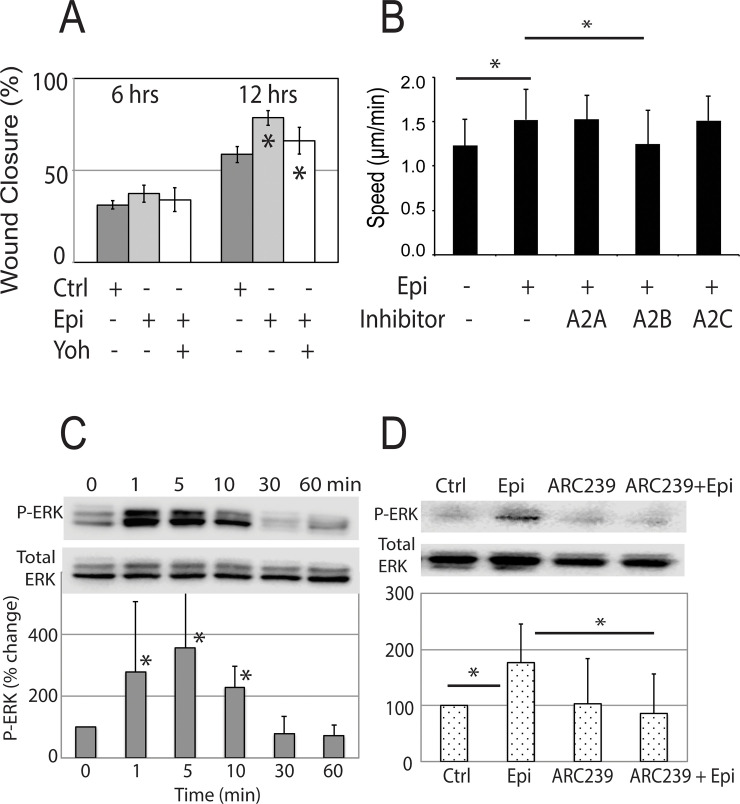
Fig 2. Inhibiting alpha-AR reversed the fast migration and ERK phosphorylation induced by low level of epinephrine.
Epinephrine-induced, fast keratinocyte migration could be reversed by co-treatment with an alpha-AR inhibitor (A) 10 μM yohimbine (Epi + Yoh) or (B) 10 μM specific inhibitors to the A2B-AR. In (B), the individual speeds of 100–150 cells were tracked in each group in the cell migration assay. (C) Phosphorylation of ERK was increased in keratinocyte lysates at different time points after the 0.1 nM epinephrine treatment. (D) When the A2B-AR specific inhibitor was added to the epinephrine treatment, the increased P-ERK signal was abolished. (N = 3 keratinocyte strains, mean +/- SD, * p<0.05).
Results
We previously demonstrated that supra-physiologic levels (1 μM) of epinephrine inhibit keratinocyte migration and wound healing [10]. However, a divergent response is seen in some strains when keratinocytes are exposed to lower, physiologic concentrations of epinephrine (0.1 nM). Scratch wound closure by keratinocyte cultures treated with low physiologic concentrations of epinephrine (0.1 nM) was increased by 34.1% relative to control untreated cultures, but was inhibited by 62.5% when treated with high concentrations of epinephrine (1 μM) (Fig 1A, 12 hrs). The results indicate that epinephrine may differentially modulate keratinocyte migrational speed, and thus potentially alter wound closure in different individuals.
Since epinephrine can also bind to the alpha AR, we hypothesized that their activation of these receptors could explain the divergent migratory response observed at low concentrations of epinephrine. Analysis of keratinocyte cultures from 4 random donors (Fig 1B) demonstrates that the mRNA for the beta 2-AR is the most abundantly expressed AR in human neonatal keratinocytes, while other subtypes, including A2AR are expressed at lower levels. Although the alpha-1 AR has been noted in the rat and human keratinocytes and is up-regulated after burn and nerve injury, and in the patients suffering from the complex regional pain syndrome [25–27], this is the first report of expression of A2AR subtypes in normal keratinocytes. Protein expression of the AR subtypes in keratinocytes was confirmed by Western blotting (Fig 1C).
To test the hypothesis that alpha-AR activation modulates the increase in keratinocyte migratory speed observed in cultures exposed to low physiologic concentrations of epinephrine, alpha-AR activation was blocked by pre-treating the cells in Fig 2 with the non-specific alpha 2-AR inhibitor yohimbine. Yohimbine reversed the accelerated keratinocyte migration induced by the low physiologic level of epinephrine, returning cell migratory speeds to that of untreated controls in the scratch migration assays at 12 hours (Fig 2A).
To determine which specific A2AR mediates the observed migratory response, we tested whether A2AR subtype specific antagonists, BRL44408 (A2A-AR inhibitor, 10 μM), ARC239 (A2B-AR inhibitor, 10 μM) or rauwolscine (A2C-AR inhibitor, 10 μM), could alter the epinephrine-induced increased migratory response (Fig 2B). The response could only be reversed by the A2B-AR antagonist ARC239, suggesting that the signaling pathway of A2B-AR modulates the B2AR-dependent keratinocyte migratory inhibition.
B2AR-induced inhibition of keratinocyte migration requires activation of the phosphatase PP2A that subsequently de-phosphorylates the p42/44 mitogen-activated protein kinase ERK, whose phosphorylation and activation mediates migration [5, 6]. Therefore, we examined the contribution of A2AR activation to ERK 1/2 phosphorylation by co-treatment of keratinocytes with epinephrine and ARC239. We observed a rapid (1–10 min) increase in ERK phosphorylation in response to low (0.1 nM) levels of epinephrine (Fig 2C) that returned to basal level after 30 minutes. The combined treatments of epinephrine and ARC239 reduced the ERK phosphorylation induced by low (0.1 nM) epinephrine concentrations (Fig 2D), suggesting that A2B-AR modulates B2AR signaling. Inhibiting A2B-AR activity antagonizes the B2AR-mediated ERK phosphorylation, resulting in the observed change cell migration speed.
Thus, in contrast to the decreased migration response of keratinocytes to stress-associated, supra-physiologic levels of epinephrine, here we demonstrate that a subset of strains exhibit a divergent response to low physiologic concentrations of epinephrine, with up-regulation of ERK 1/2 phosphorylation that is reversible by an A2B-AR inhibitor.
Discussion
The B2AR is highly expressed in undifferentiated, migrating keratinocytes [21, 28] in the basal layer of epidermis, and these cells can also generate catecholamine (epinephrine) ligands for the receptor [5, 19, 22]. Keratinocyte migration plays a critical role in wound re-surfacing [29, 30]. Therefore, the modulation of B2AR signaling in keratinocyte migration can play an important role in controlling the rate of wound healing.
Here we show that the B2AR signaling paradigm can be modulated by alpha adrenergic receptors, also expressed in the epidermis. We note that the adrenergic antagonists are not completely selective, and gene silencing could be a better approach to test the hypothesis. However, due to the inefficiency of depleting receptors in primary keratinocytes (for example only 50% knock down of expression of the epithelial sodium channel [31]) and a very limited generation time in cultured primary keratinocytes, even with knock down, it would be difficult to demonstrate convincingly that other receptors are not involved. Therefore, for this study we chose to use the more tractable pharmaceutical approach. Possible crosstalk between the alpha and beta ARs is observed in mouse cardiac and embryonic fibroblasts. The scaffold protein, arrestin, mediates the signaling between the 2 receptors and increases the phosphorylation of ERK possibly via a G-protein-independent signaling events [32, 33], suggesting that a combination of the drug treatments targeting the alpha and beta adrenergic receptors may compound or diminish the signaling and the efficacy of each drug on cardiac remodeling. Our results indicate that a subset of patients will have divergent responses to epinephrine in their skin wounds, and indeed, similar observations have been made in different clinical scenarios. For example, a small subset (16–19%) of patients receiving epinephrine for food-induced allergic anaphylaxis [34, 35] require multiple doses, suggesting that these individuals are not responsive to the initially administered low dose of epinephrine [35]. The expression levels of each of AR subtype, or their polymorphisms, as well as receptor internalization rates likely differ in each individual, which may account for the observed divergent responses.
BAR antagonists (beta blockers) are increasingly used topically to treat skin diseases. The non-specific antagonists for beta AR such as propranolol and timolol, are widely used for treating infantile hemangiomas [36–39]. Topical application of timolol is used to treat a host of dermatologic diseases [40, 41], including epidermolysis bullosa [42], Kaposi sarcoma [43, 44], acne and acne rosacea [45], and improving scar outcomes [46]. Timolol has also been found to be effective in treating chronic wounds [14–17]. However, this beta blockade improves healing in some, but not all, patients with chronic wounds [42, 47, 48]. Thus, the cell migration assay reported here could provide an in vitro tool to examine patients who may have the divergent reactions to epinephrine, and to determine the therapeutic strategy of epinephrine-derived treatments.
There are some limitations to this study. The keratinocytes used are derived from neonatal foreskin, due to the better replicative capacity of neonatal tissues. Thus, results are limited to cultured, rather than in vivo keratinocytes. Sexual dimorphism has been reported for B2AR responses [49–51], so confirming the observed results in keratinocytes derived from females will be important. The de-identified neonatal foreskins collected for the study do not provide any information regarding potential racial differences in responses, and this could be a factor to investigate especially in view of racial B2AR polymorphism differences [52–55].
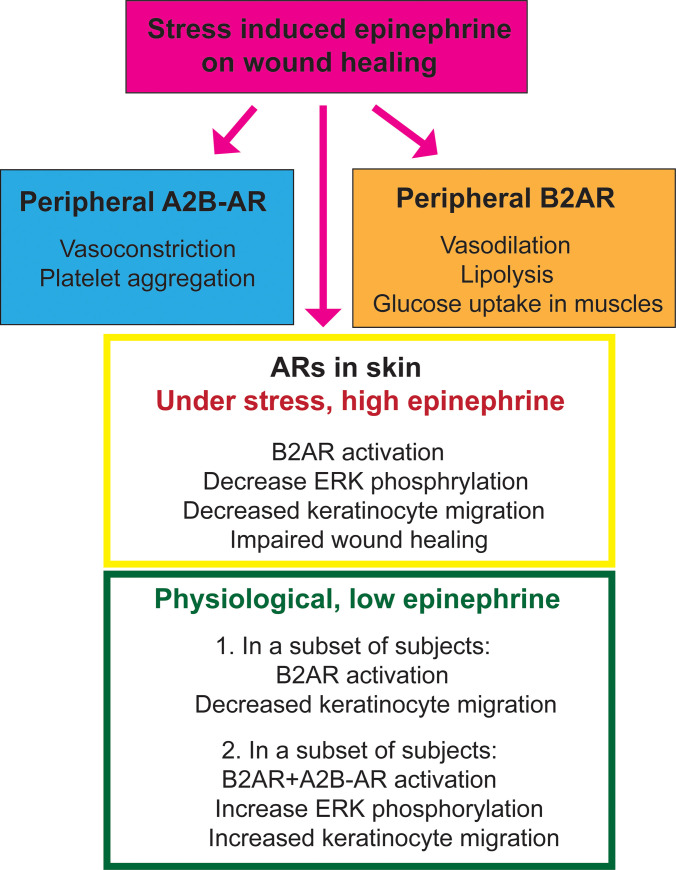
Taken together, the present study suggests an interaction between A2AR and B2AR in a subset of human keratinocytes, which influences the epinephrine-mediated keratinocyte migration. A summary of the peripheral functions of A2B-AR and B2AR in wound healing is shown in Fig 3. Future studies will investigate whether a combination of an A2B-AR agonist and B2AR antagonist can promote keratinocyte migration and improve wound healing.
Fig 3. A summary of functions of peripheral A2B-AR and B2AR in wound healing.
Supporting information
Acknowledgments
We would like to thank Dr. David Rocke (UC Davis) for assistance with statistical analyses.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21. doi: 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- 2.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75 [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Hoffman BB, Isseroff RR. Beta-adrenergic receptor activation inhibits keratinocyte migration via a cyclic adenosine monophosphate-independent mechanism. J Invest Dermatol. 2002;119(6):1261–8. doi: 10.1046/j.1523-1747.2002.19611.x [DOI] [PubMed] [Google Scholar]
- 4.Pullar CE, Grahn JC, Liu W, Isseroff RR. Beta2-adrenergic receptor activation delays wound healing. Faseb j. 2006;20(1):76–86. doi: 10.1096/fj.05-4188com [DOI] [PubMed] [Google Scholar]
- 5.Sivamani RK, Pullar CE, Manabat-Hidalgo CG, Rocke DM, Carlsen RC, Greenhalgh DG, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med. 2009;6(1):e12. doi: 10.1371/journal.pmed.1000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sivamani RK, Shi B, Griffiths E, Vu SM, Lev-Tov HA, Dahle S, et al. Acute wounding alters the beta2-adrenergic signaling and catecholamine synthetic pathways in keratinocytes. J Invest Dermatol. 2014;134(8):2258–66. doi: 10.1038/jid.2014.137 [DOI] [PubMed] [Google Scholar]
- 7.Pullar CE, Isseroff RR. Beta 2-adrenergic receptor activation delays dermal fibroblast-mediated contraction of collagen gels via a cAMP-dependent mechanism. Wound Repair Regen. 2005;13(4):405–11. doi: 10.1111/j.1067-1927.2005.130408.x [DOI] [PubMed] [Google Scholar]
- 8.Pullar CE, Le Provost GS, O’Leary AP, Evans SE, Baier BS, Isseroff RR. β2AR antagonists and β2AR gene deletion both promote skin wound repair processes. J Invest Dermatol. 2012;132(8):2076–84. doi: 10.1038/jid.2012.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MH, Gorouhi F, Ramirez S, Granick JL, Byrne BA, Soulika AM, et al. Catecholamine stress alters neutrophil trafficking and impairs wound healing by β2-adrenergic receptor-mediated upregulation of IL-6. J Invest Dermatol. 2014;134(3):809–17. doi: 10.1038/jid.2013.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steenhuis P, Huntley RE, Gurenko Z, Yin L, Dale BA, Fazel N, et al. Adrenergic signaling in human oral keratinocytes and wound repair. J Dent Res. 2011;90(2):186–92. doi: 10.1177/0022034510388034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang X, Li S, Xue XD, Chang F. Propranolol regulates ERK1/2 signaling pathway and promotes chronic wound healing in diabetic rats. Eur Rev Med Pharmacol Sci. 2019;23(10):4498–506. doi: 10.26355/eurrev_201905_17962 [DOI] [PubMed] [Google Scholar]
- 12.Zheng Z, Liu Y, Yang Y, Tang J, Cheng B. Topical 1% propranolol cream promotes cutaneous wound healing in spontaneously diabetic mice. Wound Repair Regen. 2017;25(3):389–97. doi: 10.1111/wrr.12546 [DOI] [PubMed] [Google Scholar]
- 13.Reidy JJ, Zarzour J, Thompson HW, Beuerman RW. Effect of topical beta blockers on corneal epithelial wound healing in the rabbit. Br J Ophthalmol. 1994;78(5):377–80. doi: 10.1136/bjo.78.5.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cahn BA, Kaur R, Hirt PA, Tchanque-Fossuo C, Dahle SE, Kirsner RS, et al. Use of Topical Timolol Maleate as Re-Epithelialization Agent for Treatment of Recalcitrant Wounds of Varying Etiologies. J Drugs Dermatol. 2020;19(12):1252–6. doi: 10.36849/JDD.2020.5306 [DOI] [PubMed] [Google Scholar]
- 15.Rai AK, Janani K, Rai R. Efficacy of Topical Timolol versus Saline in Chronic Venous Ulcers: A Randomized Controlled Trial. J Cutan Aesthet Surg. 2020;13(1):18–23. doi: 10.4103/JCAS.JCAS_13_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baltazard T, Senet P, Momar D, Picard C, Joachim C, Adas A, et al. Evaluation of timolol maleate gel for management of hard-to-heal chronic venous leg ulcers. Phase II randomised-controlled study. Ann Dermatol Venereol. 2021. doi: 10.1016/j.annder.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 17.Thomas B, Kurien JS, Jose T, Ulahannan SE, Varghese SA. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg Venous Lymphat Disord. 2017;5(6):844–50. doi: 10.1016/j.jvsv.2017.04.019 [DOI] [PubMed] [Google Scholar]
- 18.Dodt C, Breckling U, Derad I, Fehm HL, Born J. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension. 1997;30(1 Pt 1):71–6. doi: 10.1161/01.hyp.30.1.71 [DOI] [PubMed] [Google Scholar]
- 19.Wortsman J, Frank S, Cryer PE. Adrenomedullary response to maximal stress in humans. Am J Med. 1984;77(5):779–84. doi: 10.1016/0002-9343(84)90512-6 [DOI] [PubMed] [Google Scholar]
- 20.Pérez Piñero C, Bruzzone A, Sarappa MG, Castillo LF, Lüthy IA. Involvement of α2- and β2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br J Pharmacol. 2012;166(2):721–36. doi: 10.1111/j.1476-5381.2011.01791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schallreuter KU, Lemke KR, Pittelkow MR, Wood JM, Körner C, Malik R. Catecholamines in human keratinocyte differentiation. J Invest Dermatol. 1995;104(6):953–7. doi: 10.1111/1523-1747.ep12606218 [DOI] [PubMed] [Google Scholar]
- 22.Schallreuter KU, Wood JM, Lemke R, LePoole C, Das P, Westerhof W, et al. Production of catecholamines in the human epidermis. Biochem Biophys Res Commun. 1992;189(1):72–8. doi: 10.1016/0006-291x(92)91527-w [DOI] [PubMed] [Google Scholar]
- 23.Dasu MR, Ramirez SR, La TD, Gorouhi F, Nguyen C, Lin BR, et al. Crosstalk between adrenergic and toll-like receptors in human mesenchymal stem cells and keratinocytes: a recipe for impaired wound healing. Stem Cells Transl Med. 2014;3(6):745–59. doi: 10.5966/sctm.2013-0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pullar CE, Chen J, Isseroff RR. PP2A activation by beta2-adrenergic receptor agonists: novel regulatory mechanism of keratinocyte migration. J Biol Chem. 2003;278(25):22555–62. doi: 10.1074/jbc.M300205200 [DOI] [PubMed] [Google Scholar]
- 25.Drummond PD, Dawson LF, Finch PM, Drummond ES, Wood FM, Fear MW. Up-regulation of cutaneous α1-adrenoceptors after a burn. Burns. 2015;41(6):1227–34. doi: 10.1016/j.burns.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 26.Drummond ES, Dawson LF, Finch PM, Bennett GJ, Drummond PD. Increased expression of cutaneous α1-adrenoceptors after chronic constriction injury in rats. J Pain. 2014;15(2):188–96. doi: 10.1016/j.jpain.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 27.Finch PM, Drummond ES, Dawson LF, Phillips JK, Drummond PD. Up-regulation of cutaneous α1 -adrenoceptors in complex regional pain syndrome type I. Pain Med. 2014;15(11):1945–56. doi: 10.1111/pme.12548 [DOI] [PubMed] [Google Scholar]
- 28.Schallreuter KU. Epidermal adrenergic signal transduction as part of the neuronal network in the human epidermis. J Investig Dermatol Symp Proc. 1997;2(1):37–40. doi: 10.1038/jidsymp.1997.9 [DOI] [PubMed] [Google Scholar]
- 29.Usui ML, Underwood RA, Mansbridge JN, Muffley LA, Carter WG, Olerud JE. Morphological evidence for the role of suprabasal keratinocytes in wound reepithelialization. Wound Repair Regen. 2005;13(5):468–79. doi: 10.1111/j.1067-1927.2005.00067.x [DOI] [PubMed] [Google Scholar]
- 30.Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304(1):274–86. doi: 10.1016/j.yexcr.2004.10.033 [DOI] [PubMed] [Google Scholar]
- 31.Yang HY, Charles RP, Hummler E, Baines DL, Isseroff RR. The epithelial sodium channel mediates the directionality of galvanotaxis in human keratinocytes. J Cell Sci. 2013;126(Pt 9):1942–51. doi: 10.1242/jcs.113225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervantes D, Crosby C, Xiang Y. Arrestin orchestrates crosstalk between G protein-coupled receptors to modulate the spatiotemporal activation of ERK MAPK. Circ Res. 2010;106(1):79–88. doi: 10.1161/CIRCRESAHA.109.198580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9(5):373–86. doi: 10.1038/nrd3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oren E, Banerji A, Clark S, Camargo CA Jr., Food-induced anaphylaxis and repeated epinephrine treatments. Ann Allergy Asthma Immunol. 2007;99(5):429–32. doi: 10.1016/S1081-1206(10)60568-6 [DOI] [PubMed] [Google Scholar]
- 35.Järvinen KM, Sicherer SH, Sampson HA, Nowak-Wegrzyn A. Use of multiple doses of epinephrine in food-induced anaphylaxis in children. J Allergy Clin Immunol. 2008;122(1):133–8. doi: 10.1016/j.jaci.2008.04.031 [DOI] [PubMed] [Google Scholar]
- 36.Olsen GM, Hansen LM, Stefanko NS, Mathes E, Puttgen KB, Tollefson MM, et al. Evaluating the Safety of Oral Propranolol Therapy in Patients With PHACE Syndrome. JAMA Dermatol. 2020;156(2):186–90. doi: 10.1001/jamadermatol.2019.3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinha S, Lloyd MS. Propranolol for Surgeons in the Treatment of Infantile Hemangiomas. J Craniofac Surg. 2020;31(1):134–7. doi: 10.1097/SCS.0000000000005919 [DOI] [PubMed] [Google Scholar]
- 38.Chakkittakandiyil A, Phillips R, Frieden IJ, Siegfried E, Lara-Corrales I, Lam J, et al. Timolol maleate 0.5% or 0.1% gel-forming solution for infantile hemangiomas: a retrospective, multicenter, cohort study. Pediatr Dermatol. 2012;29(1):28–31. doi: 10.1111/j.1525-1470.2011.01664.x [DOI] [PubMed] [Google Scholar]
- 39.Chan H, McKay C, Adams S, Wargon O. RCT of timolol maleate gel for superficial infantile hemangiomas in 5- to 24-week-olds. Pediatrics. 2013;131(6):e1739–47. doi: 10.1542/peds.2012-3828 [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Tsai TF. The role of β-blockers in dermatological treatment: a review. J Eur Acad Dermatol Venereol. 2018;32(3):363–71. doi: 10.1111/jdv.14566 [DOI] [PubMed] [Google Scholar]
- 41.Yoon DJ, Kaur R, Gallegos A, West K, Yang H, Schaefer S, et al. Repurposing Ophthalmologic Timolol for Dermatologic Use: Caveats and Historical Review of Adverse Events. Am J Clin Dermatol. 2021;22(1):89–99. doi: 10.1007/s40257-020-00567-3 [DOI] [PubMed] [Google Scholar]
- 42.Chiaverini C, Passeron T, Lacour JP. Topical timolol for chronic wounds in patients with junctional epidermolysis bullosa. J Am Acad Dermatol. 2016;75(6):e223–e4. doi: 10.1016/j.jaad.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 43.Abdelmaksoud A, Filoni A, Giudice G, Vestita M. Classic and HIV-related Kaposi sarcoma treated with 0.1% topical timolol gel. J Am Acad Dermatol. 2017;76(1):153–5. doi: 10.1016/j.jaad.2016.08.041 [DOI] [PubMed] [Google Scholar]
- 44.Alcántara-Reifs CM, Salido-Vallejo R, Garnacho-Saucedo GM, Vélez García-Nieto A. Classic Kaposi’s sarcoma treated with topical 0.5% timolol gel. Dermatol Ther. 2016;29(5):309–11. doi: 10.1111/dth.12381 [DOI] [PubMed] [Google Scholar]
- 45.Al Mokadem SM, Ibrahim AM, El Sayed AM. Efficacy of Topical Timolol 0.5% in the Treatment of Acne and Rosacea: A Multicentric Study. J Clin Aesthet Dermatol. 2020;13(3):22–7. [PMC free article] [PubMed] [Google Scholar]
- 46.Dabiri G, Tiger J, Goreshi R, Fischer A, Iwamoto S. Topical timolol may improve overall scar cosmesis in acute surgical wounds. Cutis. 2017;100(1):E27–e8. [PubMed] [Google Scholar]
- 47.Larsen L, Tchanque-Fossuo CN, Gorouhi F, Boudreault D, Nguyen C, Fuentes JJ, et al. Combination therapy of autologous adipose mesenchymal stem cell-enriched, high-density lipoaspirate and topical timolol for healing chronic wounds. J Tissue Eng Regen Med. 2018;12(1):186–90. doi: 10.1002/term.2390 [DOI] [PubMed] [Google Scholar]
- 48.Tang JC, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38(1):135–8. doi: 10.1111/j.1524-4725.2011.02200.x [DOI] [PubMed] [Google Scholar]
- 49.de Coupade C, Gear RW, Dazin PF, Sroussi HY, Green PG, Levine JD. Beta 2-adrenergic receptor regulation of human neutrophil function is sexually dimorphic. Br J Pharmacol. 2004;143(8):1033–41. doi: 10.1038/sj.bjp.0705972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luzier AB, Nawarskas JJ, Añonuevo J, Wilson MF, Kazierad DJ. The effects of gender on adrenergic receptor responsiveness. J Clin Pharmacol. 1998;38(7):618–24. doi: 10.1002/j.1552-4604.1998.tb04468.x [DOI] [PubMed] [Google Scholar]
- 51.Al-Gburi S, Deussen A, Zatschler B, Weber S, Künzel S, El-Armouche A, et al. Sex-difference in expression and function of beta-adrenoceptors in macrovessels: role of the endothelium. Basic Res Cardiol. 2017;112(3):29. doi: 10.1007/s00395-017-0617-2 [DOI] [PubMed] [Google Scholar]
- 52.Salas-Martínez MG, Saldaña-Alvarez Y, Cordova EJ, Mendiola-Soto DK, Cid-Soto MA, Luckie-Duque A, et al. Genetic variability of five ADRB2 polymorphisms among Mexican Amerindian ethnicities and the Mestizo population. PLoS One. 2019;14(12):e0225030. doi: 10.1371/journal.pone.0225030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shahin MH, Rouby NE, Conrado DJ, Gonzalez D, Gong Y, Lobmeyer MT, et al. β(2) -Adrenergic Receptor Gene Affects the Heart Rate Response of β-Blockers: Evidence From 3 Clinical Studies. J Clin Pharmacol. 2019;59(11):1462–70. doi: 10.1002/jcph.1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai S, Hua L, Wang X, Liu Q, Bao Y. Association of a 4-Locus Gene Model Including IL13, IL4, FCER1B, and ADRB2 With the Asthma Predictive Index and Atopy in Chinese Han Children. J Investig Allergol Clin Immunol. 2018;28(6):407–134. doi: 10.18176/jiaci.0272 [DOI] [PubMed] [Google Scholar]
- 55.Ortega VE, Meyers DA. Pharmacogenetics: implications of race and ethnicity on defining genetic profiles for personalized medicine. J Allergy Clin Immunol. 2014;133(1):16–26. doi: 10.1016/j.jaci.2013.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.