Abstract
Neurite outgrowth involves reciprocal signaling interactions between tumor cells and nerves where invading tumor cells have acquired the ability to respond to pro-invasive signals within the nerve environment. Neurite outgrowth could serve as a mechanism leading to invasion of cancer cells into the nerve sheath and subsequent metastasis. Snail transcription factor can promote migration and invasion of prostate cancer cells. We hypothesized that prostate cancer cell interaction with nerve cells will be mediated by Snail expression within prostate cancer cells. For this study we utilized various prostate cancer cell lines: C4-2 non-silencing (NS, control); C4-2 Snail shRNA, (stable Snail knockdown); LNCaP Neo (empty vector control) and LNCaP Snail (stably over-expressing Snail). Cancer cell adhesion and migration towards nerve cells (snF96.2 or NS20Y) was examined by co-culture assays. Conditioned media (CM) collected from C4-2 cells was cultured with nerve cells (PC-12 or NS20Y) for 48 hours followed by qualitative or quantitative neurite outgrowth assay. Our results showed that cancer cells expressing high levels of Snail (LNCaP Snail/C4-2 NS) displayed significantly higher migration adherence to nerve cells, compared to cells with lower levels of Snail (LNCaP Neo/C4-2 Snail shRNA). Additionally, LNCaP Snail or C4-2 NS (Snail-high) CM led to a higher neurite outgrowth compared to the LNCaP Neo or C4-2 Snail shRNA (Snail-low). In conclusion, Snail promotes migration and adhesion to nerve cells, as well as neurite outgrowth via secretion of soluble factors. Therefore, targeting cancer cell interaction with nerves may contribute to halting prostate cancer progression/metastasis.
Keywords: Snail, Prostate Cancer, Neurite Outgrowth, Nerve, Neurite, Cancer Cell
1 ∣. INTRODUCTION
The critical issue arising from prostate cancer is its tendency to metastasize and acquire the ability to exceed confinement from one particular area, further developing in other organs of the body. Men who die of prostate cancer often exhibit aggressive metastatic disease [1]. Here, we investigate a potential mechanism that may function to propagate metastatic progression. Nerves provide cancer cells a path of low resistance to exit the prostate that is independent of metastasis via the circulatory system [2]. Neural functions and tissues exert important functions on cancer initiation and development [3]. A scientific study showed that 6-Hydroxydopamine, a neurotoxic synthetic organic compound used by researchers to selectively destroy dopaminergic and noradrenergic neurons in the brain, significantly antagonized the growth rate of certain tumors and induced neuronal changes on the sympathetic nervous system to augment the growth of neuroblastoma [4]. This suggested that a stable and unimpaired functional sympathetic nervous system is necessary for the productivity and development of certain tumors [4]. Peripheral nerve fibers have also been found to innervate some tumors [3]. In primary and pre-metastatic organs, cancer cells actively establish connections with nerve fibers and receive signals from the nervous system [3].
Immuno-histochemical studies have shown peripheral nerve fibers within cancer specimens such as colorectal as well as prostate [5]. Neurons of dorsal root ganglia also have the ability to interact and extend toward cancer cells [6]. Peripheral nerve invasion (PNI) induced by cancer is an independent factor for poor prognosis in some cancer patients [2]. Based on experimental studies, it is believed that neurite outgrowth serves as an alternative mechanism that promotes cancer aggressiveness and metastatic spread [2].
Neurons are excessively polarized cells that have two structurally and functionally defined components which are identified as axons or dendrites [7, 8]. Axonal extension or elongation, as well as dendritic generation are essential for nerve development [7]. There are four distinct stages in neurite outgrowth: (1) During stage one, filopodia initially extend around the circumference of the cell body, (2) During the second stage, the nerve cell then forms a number of morphologically premature neurites that continuously extend and retract, (3) During stage three, neurites generate and grow at a faster pace from the nerve cell body to develop into axons, and (4) In the final stage, which is considered neurite formation, the remaining neurites proceed to endure the growth process and continue to further develop into dendrites [7]. Neurons contain subcellular gradients in forces and material, in which actin flows backward rapidly in the growth cone periphery, while microtubules flow forward in bulk along the axon [9]. Overall, neurite outgrowth is a process where axons extend from the cell body of a nerve and form elongated branch-like extensions where they secrete various growth promoting molecules that enable them to send messages, signals, and interact with other cell types. Axonal guidance molecules such as Netrins belong to a conserved family of secreted proteins having regional homology to laminins, and are able to regulate axonal outgrowth [10]. Neurons also secrete various survival and chemo attractants such as neuropeptides and neurotransmitters. However, cancer cells possess the capability to carry out the same functions by secreting these same factors that may create a micro-environment where both the nerve and cancer cell can thrive. Cancer cells secrete neurotrophic factors such as Nerve Growth Factor (NGF), Brain Derived Neurotrophic Factor, Ciliary Growth Factor and Glial Cell Derived Growth Factor [10]. The tumor may utilize the nerve as another means of survival and mobility via the secretion of various proteins and molecules. More recent experimental studies determined that structural synapses form between glioma cells and neurons in the tumor microenvironment, resulting in a positive feedback loop in which the glioma cells promote neuronal excitability that increases glioma growth [11]. Therefore, neuronal activity is evolving as a critical regulator of glioma progression. Alternatively, the tumor cells themselves can behave as neural cells as has been observed in patient-derived glioblastoma multiforme (GBM) stem cells where experimental studies have demonstrated cell projections from GBM stem cells identical to the morphology of neurites; these outgrowths facilitate resistance to radiation therapy [12]. We have also previously shown that Snail transcription factor overexpression in prostate cancer cells promotes neuroendocrine differentiation which includes neurite-like extensions from the prostate cancer cells and expression of neuronal markers such as neuron specific enolase and chromogranin A [13].
As prostate cancer advances, the disease acquires more mechanisms of independence, self-sustenance and motility that contribute to the perturbation of canonical cellular behavior. SNAIL1 or Snail is a zinc-finger protein that can down-regulate cell adhesion proteins such as E-cadherin by binding several E-boxes located in the promotor region, thereby inducing the Epithelial mesenchymal transition (EMT) process [14]. In addition, Snail functions in the repression of tight junction proteins such as claudin, and occludin [15]. Snail can mediate increase in expression of mesenchymal markers such as vimentin, and fibronectin leading to cancer cell migration and invasion [14]. Immuno-histochemical studies on a prostate cancer tissue microarray showed that Snail staining is associated with Gleason grade, with increasing expression from benign prostatic hyperplasia (BPH) to prostate cancer bone metastasis [16]. Our goal is to delineate the role of Snail transcription factor in human prostate cancer interaction with the microenvironment, specifically nerve cells at the initial site. Our data indicates that Snail-expressing prostate cancer cells display higher migration towards and adhesion to nerve cells. Additionally, Snail promotes neurite outgrowth in multiple nerve cells, which can be abrogated by endogenous Snail knockdown. This reveals a novel role for Snail in prostate cancer cell interactions with nerve cells.
2 ∣. MATERIALS AND METHODS
2.1. Reagents
RPMI, DMEM media and penicillin/streptomycin were purchased from Corning, New York, NY. Fetal Bovine Serum (FBS) was purchased from Fisher Scientific, Waltham, MA. Omnipur 10X PBS was purchased from Millipore Sigma, Darmstadt, Germany. Neurite outgrowth staining kit and dextrose charcoal-stripped serum (DCC) were purchased from Thermo Fisher, Waltham, MA. Anti- rat monoclonal Snail antibody was from Cell Signaling, Danvers, MA. Actin and secondary mouse antibody were purchased from Santa Cruz Biotechnology, Dallas, TX. G418 (aminoglycoside antibiotic) was purchased from Calbiochem, Burlington, MA. Luminata Forte ECL reagent and the protease inhibitor cocktail were from Roche Molecular Biochemicals, Indianapolis, IN. Rat tail collagen I was from BD Biosciences (Franklin Lakes, NJ).
2.2. Cell lines
NS20Y nerve cells were purchased from Millipore Sigma., Darmstadt, Germany. LNCaP prostate cancer cell line, PC-12 nerve cells, and sNF96.2 nerve cells were obtained from American Tissue Cell Culture (ATCC), Manassas, Virginia. NS20Y nerve cells are derived from mouse neuroblastoma, while sNF96.2 nerve cells are derived from a human malignant peripheral nerve. The cell lines were tested for mycoplasma before use and those purchased have been previously validated by the respective companies using short tandem repeat (STR) profiling. C4-2 (aggressive sub-line derived from LNCaP) was kindly provided and authenticated by Dr. Leland Chung, Cedars Sinai Medical Center, Los Angeles, CA. C4-2 and LNCaP cell lines were cultured and maintained in RPMI complete media supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S). NS20Y and sNF96.2 nerve cells were cultured in DMEM complete media containing 10% FBS and 1% P/S PC-12, a rat pheochromocytoma cell line (American Tissue Culture Collection) was cultured in F12 K media containing 5% fetal bovine serum and 10% horse serum and incubated in a humidified 5% CO2 incubator. LNCaP cells stably overexpressing Snail were generated as previously described [13] where several clones were generated, and representative clones LNCaP Neo5 (empty vector control) and LNCaP Snail 18 (Snail overexpression) utilized in this study were cultured in RPMI complete media containing 400 μg/ml G418. C4-2 cells with non-silencing vector (C4-2 NS) or stable Snail knockdown using 3 different shRNA clones was previously generated and published showing that C4-2 E8 clone shows the most efficient knockdown [17], so this is the clone we are using in this study and also naming it as C4-2 Snail shRNA; these cells were also maintained in RPMI complete media containing 400 μg/ml G418. All cells were maintained at 37°C with 5% CO2 in a humidified incubator.
2.3. Measurement cell adherence and proliferation in real-time
We used xCELLigence system (E-plates) to measure cell adherence and proliferation in real-time following the manufacturer instructions (Agilent, USA). The xCELLigence system measured cell adherence and proliferation of C4-2 NS and C4-2 Snail shRNA cells by seeding 2X104 cells per well using E-Plate. We used 10% FBS-fresh DMEM for this set of experiments. Impedance value of each well was automatically recorded every 15 minutes while incubated in 37°C incubator for 120 hours. As previously recommended, data for cell adherence were normalized at 40 min. We used GraphPad Prism to measure the rate of cell growth by calculating the slope of the line between two critical time points (60 hours to 80 hours).
2.4. Measurement of cell migration and invasion in real time
We used xCELLigence system (CIM-plates) to measure cell migration and invasion in real-time following the manufacturer instructions. Briefly, cells (C4-2 NS and C4-2 Snail shRNA) were starved using 1% FBS-media for 15 hours prior to conducting the migration experiment. The upper chamber (UC) of the CIM-plates was seeded by a total of 2X104 cells in each well containing Serum Free Media (SFM). Fresh 10% FBS-DMEM Media was added to each well of the lower chamber (LC). The CIM-plates were left in a 37°C incubator for 1 hour to allow cell attachment. For the cell invasion, cells were starved for 24 hours. Matrigel (Corning; Cat Number #354234) was diluted with cold SFM to a concentration of 800 μg/mL solution and it was used to coat wells in the UC of the CIM plates. The coated UC was incubated in a 37°C incubator for 4 hours. Subsequently, wells at the UC received a total of 2X104 cells (C4-2 NS and C4-2 Snail shRNA) in SFM. A fresh 10% FBS-DMEM media was used for the LC. Similar to migration, the CIM-plates seeded by cells were left in in a 37°C incubator for 1 hour for cell attachment. After several time optimizations, we allowed the xCELLigence system to automatically continue measuring the impedance value of each well every 15 minutes for a duration of 120 hours which are expressed as a CI value for both migration and invasion. We used plots to illustrate the Cell index value for over time-points for migration and invasion. A GraphPad Prism (version 8) was used to analyze the rate of migration and invasion (slope) during a critical time window for this specific cell line. The critical time-point for these cells were approximately between 60 hours to 80 hours after starting the experiments.
2.5. Boyden Chamber Cell Migration Assay
2x104 NS20Y, 5x104 or 10x104 sNF96.2 nerve cells in 5% DCC (for NS20Y) or DMEM media (for sNF96.2) were seeded on the bottom well of a trans-well plate and allowed to attach overnight. As a control, we included wells with 5% DCC media or DMEM containing 10% FBS. Meanwhile, Inserts were coated with 4.46 ug/ul rat tail collagen overnight at 4°C. The next day, 5x104 LNCaP Neo control, LNCaP Snail, C4-2 NS or C4-2 Snail shRNA PCa cells were seeded on the top insert coated with collagen. Cells were incubated and allowed to migrate for over a period of 48 hours. Subsequently, PCa cells that had migrated to the underside of the insert were stained with crystal violet, and counted to obtain relative migration levels.
2.6. Cell Adhesion Assay
2x103 or 6x103 NS20Y nerve cells were plated in 96-well plate overnight. 5X103 LNCaP Neo/Snail (overexpression) or C4-2 NS/ Snail shRNA (knockdown) cells were added to nerve cells for 20 minutes. Unattached cells were washed off with percoll flotation medium. Attached cells were fixed with percoll fixative, stained with crystal violet and quantitated. The cancer cells are easily identified by their extended morphology, since the NS20Y remain small and rounded before prolonged stimulation.
2.7. Western Blot Analysis
To confirm Snail expression in LNCaP cells overexpressing Snail and C4-2 cells with endogenous Snail knockdown, cell lysates from LNCaP Neo/Snail and C4-2 parental, NS, and Snail shRNA were collected using lysis buffer (1X modified RIPA buffer, 1X Protease inhibitor, 1mM PMSF, and 1mM sodium orthovanadate). Supernatants were collected and quantified using a BCA assay (Thermo Scientific, Waltham, MA). 30 μg of cell lysate was resolved using 10% sodium dodecyl sulfate-polyacrylamide (SDS) gel electrophoresis followed by transblotting onto nitrocellulose membrane (Bio-Rad Labratories, Hercules, CA). Membranes were incubated with primary monoclonal Snail antibody and secondary Mouse antibody followed by visualization using Luminata Forte ECL reagent. The blots were subsequently stripped using Restore western blot stripping buffer (Pierce Biotechnology, Rockford, IL) and reprobed with Actin antibody.
2.8. Conditioned Media
2x106 Cells (LNCaP Neo/Snail, and C4-2 NS/Snail shRNA,) were plated in p100 dishes in complete RPMI media overnight. Subsequently, media was removed and phenol-red free RPMI media containing 5% dextrose charcoal-stripped serum (DCC) was added and incubated at 37°C for 48 hours. After 48 hours, the supernatant was collected, spun down for 5 minutes, and utilized as conditioned media for experiments.
2.9. Neurite Outgrowth Assay
This assay was performed using either a semi-permeable cell culture insert system or a quantitative neurite outgrowth assay kit according to manufacter’s instructions. Briefly for the quantitative neurite outgrowth assay, NS20Y nerve cells (6x103 cells/well) were plated in 96-well dishes in DMEM media (100 μl) overnight. After attachment, DMEM media was removed and conditioned media (100 μl) derived from prostate cancer cells was added or control DMEM or 5% DCC media. The co-culture was incubated for 48 hours after which the conditioned media was removed, and the nerve cells incubated with fix/stain solution for viability assessment and membrane staining that stains the neurite outgrowth. After 20 minutes, the fix/stain solution was removed from the assay and Background Suppression dye was added prior to plate reading using Elisa plate reader (Excitation/Emission (nm): 495/515 (Cell Viability), 555/564 (Cell Membrane Stain). The data was graphed using Graph Pad Prism. Images of viability and neurite outgrowth staining were captured using ZEISS confocal microscope.
For neurite outgrowth assay using the cell culture insert system, LNCaP prostate cancer cells expressing Snail or empty vector Neo control were plated on the top insert in RPMI media with a semipermeable membrane separating it from the bottom well where PC-12 nerve cells were plated. Cells were incubated for 48 hours and the top insert containing the LNCaP cells was removed and PC-12 nerve cells were then analyzed using a bright-field microscopy in a Zeiss inverted microscope. The microscope settings were kept unchanged for all related samples. The ImageJ software was used to quantitate neurite outgrowth of cells. Results were calculated by averaging data from 6 individual fields per experiment. To measure the length (micrometer-μM)) of neurites in PC-12 cells co-cultured with LNCaP prostate cancer cells, we used a modified method previously described [18]. The co-cultured PC-12 cells were stained with a MaP2 antibody, a dendritic marker followed by a confocal fluorescence microscopy. We used ImageJ to measure neurites developed in PC-12 cells in 5 individual fields per experiment. Subsequently, we used Prism GraphPad software to calculate average sizes and generate a bar graph to illustrate significant differences between groups.
2.10. Real Time-Lapse Movie
7X103 NS20Y nerve cells were plated in a 16 well slide in their normal DMEM media overnight. The following day, DMEM media was removed from the nerve cells and CM from C4-2 NS or C4-2 Snail shRNA cells were added to the NS20Y nerve cells. In addition to the experimental nerve cells a negative control well of nerve cells was cultured with 5% DCC only. The experimental and negative control nerve cells were placed in a 37°C, 5% CO2 chamber and photographed using time-lapse microscopy on a Zeiss LSM 700 with differential interference contrast (DIC) for 8 hours. The incubated nerve cells’ behavior was recorded using ZEN Black software. Experimental and control cells were analyzed for cell behavior, mobility, cell to cell interaction and neuronal extensions.
2.11. Statistical analysis
Data were analyzed by a paired student's t-test or ANOVA using GraphPad Prism software. For all experiments ns means non-significant, * means 0.05 > p value > 0.01, ** means 0.01 > p value > 0.001, *** means p value < 0.001, and **** means p value < 0.0001.
3 ∣. RESULTS
3.1. Prostate cancer cells proliferate, migrate and invade significantly slower in the absence of Snail.
We have already shown that Snail as a transcription factor can increase cell migration and invasion in prostate cancer cells [17]. To confirm whether the absence of Snail can lead to suppression of cell adherence/proliferation, migration and invasion, we decided to monitor these three events in a label-free realtime cell analysis platform called xCELLigence system [19-21]. We used C4-2 cells with stable Snail knockdown (C4-2 Snail shRNA) or non-silencing control (C4-2 NS) for these experiments. Fig. 1 illustrates both cell index and calculated slopes for above three cell events. Fig. 1A and 1B indicate that the absence of Snail has no significant effect on cell adherence occurring in the first 40 minutes of recording at two critical time points (60 hours to 80 hours). However, Fig. 1B shows the absence of Snail significantly decreases the cell proliferation at two critical time points (60 hours to 80 hours). More importantly, we found the absence of Snail in C4-2 Snail shRNA cells significantly decreases both cell migration and invasion (Fig. 1 Panels C-F). These results further confirmed the tumorigenic function of Snail transcriptional factor in prostate cancer cells.
FIGURE 1. Silencing of Snail protein significantly reduces cell proliferation, migration and invasion in prostate cancer cells.
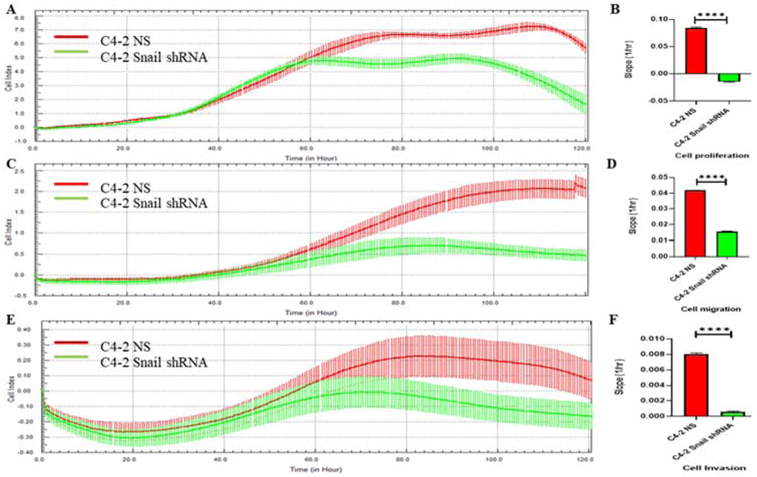
C4-2 cells with stable Snail knockdown (C4-2 Snail shRNA) or non-silencing control (C4-2 NS) were plated were monitored in real time for cell proliferation, migration and invasion for 120 hours using the xCELLigence system. Panel A, C, E are representative graphs comparing the rate of proliferation, migration or invasion using the calculated cell index, respectively. Panels B, D and F show calculated slopes for these three events during a critical time-points (60 hours to 80 hours). Experiments were repeated two times with N of 4 per cell line per experiment and analyzed by GraphPad Prism 8 (****P<0.0001).
3.2. Snail promotes cancer cell migration towards nerve cells in vitro
The purpose of this experiment was to assess how cancer cells expressing Snail promote migration towards neuronal cells. LNCaP cells overexpressing Snail and C4-2 cells with stable Snail knockdown were first analyzed for their expression of Snail. LNCaP Neo (empty vector) showed very faint to almost no expression of Snail by western blot analysis, while LNCaP Snail 18 clone showed higher levels of Snail as we have previously published (Fig. 2A, Supplemental Fig. 1A)[17]. Conversely, we have previously shown that transduction of C4-2 cells with 3 shRNA clones (A12, B9 and E8) decreases Snail expression, with the largest decrease with the E8 clone [22], which we subsequently named C4-2 Snail shRNA and confirmed knockdown by western blot analysis (Fig. 2B, Supplemental Fig. 1B). Subsequently, we chose LNCaP Snail 18 clone or Neo (empty vector) and C4-2 NS (expressing Snail) and E8 (Snail shRNA) clones as representatives for our study. These cells were utilized in a trans-well boyden chamber migration assay with sNF96.2 or NS20Y nerve cells (Fig. 2C, D). sNF96.2 or NS20Y nerve cells were plated on the bottom well overnight to seed. The following day, prostate cancer cells were plated on a top insert and allowed to migrate for two days. When migration levels were analyzed, LNCaP Snail and C4-2 NS cells showed significantly higher migration toward nerve cells compared to LNCaP Neo and C4-2 Snail shRNA, respectively (Fig. 2C, D). These results give indication that expression of Snail plays a role in cancer cell migration towards nerve cells.
FIGURE 2. Snail promotes cancer cell migration towards nerve cells in vitro.
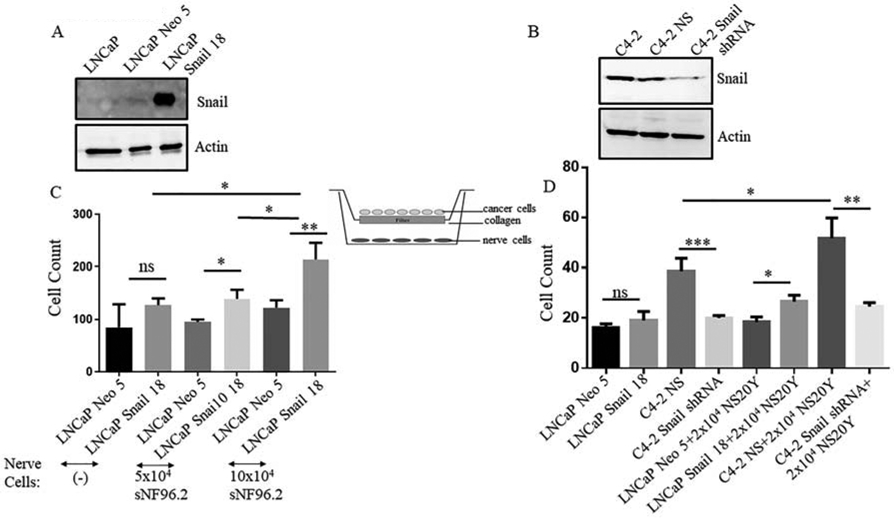
Western blot analysis was utilized to analyze Snail expression in (A) clone 18 of LNCaP Snail (stably overexpressing Snail) or LNCaP Neo (empty vector) cDNA, and (B) parental C4-2, C4-2 NS (non-silencing control) and C4-2 Snail shRNA (stable Snail knockdown with shRNA). Actin was utilized as a loading control. These same cells were utilized in different trans-well migration assays with (C) sNF96 or (D) NS20Y nerve cells plated on the bottom well overnight to seed, or media with no nerve cells. The following day, prostate cancer cells were plated on a top insert and allowed to migrate for two days. Migration levels quantitated are representative of triplicate experiments done twice independently. Statistical analysis was done using GraphPad Prism. (n = 6, *p< 0.05,**p< 0.01,***p< 0.001; ns, non-significant).
3.3. Snail promotes cancer cell adhesion to nerve cells in vitro
Most scientific literature focusing on cancer invasion of the peripheral nerve has suggested that cancer cells have the ability to travel along the nerve sheath. We further investigated if cancer cells had the ability to attach to nerve cells in vitro by performing a cell adhesion assay. The hypothesis was that cancer cells with high expression of Snail would display higher adhesion to nerve cells compared to cells with low Snail expression. NS20Y nerve cells were plated in a 96 well plate and incubated overnight. LNCaP Neo (empty vector), LNCaP Snail (high Snail), C4-2 NS (high Snail) or C4-2 Snail shRNA (Snail knockdown) were cultured with nerve cells. Cancer cells were analyzed for adhesion to nerve cells. The results showed that LNCaP Snail and C4-2 NS (high Snail expressing) displayed significantly higher adhesion to nerve cells compared to LNCaP Neo and C4-2 Snail shRNA cancer cells, respectively (Fig. 3).
FIGURE 3. Snail promotes cancer cell adhesion to nerve cells in vitro.
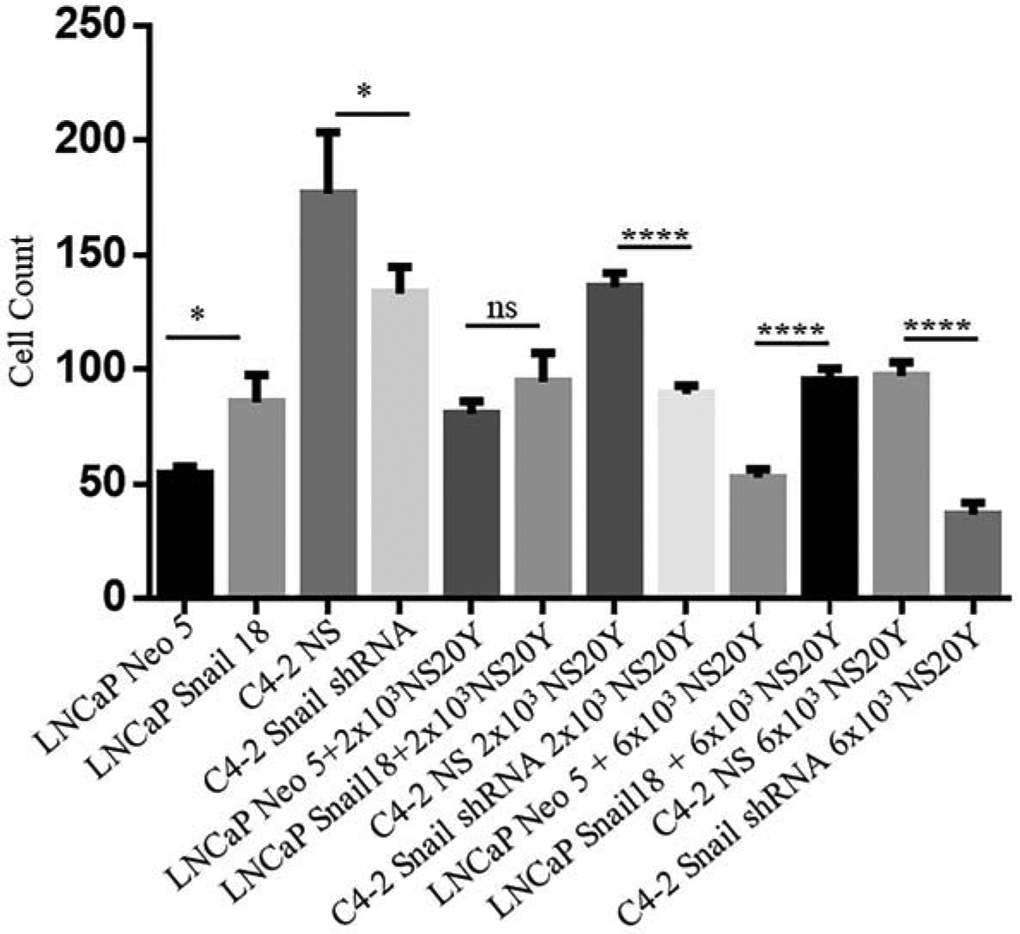
NS20Y nerve cells were plated in a 96 well plate and incubated overnight. LNCaP Neo (empty vector) or LNCaP Snail 18 (clone overexpressing Snail) and C4-2 NS (control) or C4-2 Snail shRNA (knockdown) were cultured with nerve cells for 30 minutes, and cells which were attached were fixed with percoll fixative, stained with crystal violet. The cancer cells were then counted; they are easily identified by their extended morphology, since the NS20Y remain small and rounded before prolonged stimulation. Cancer adhesion levels quantitated are representative of triplicate experiments done twice independently (n = 6, *p< 0.05, ****p< 0.0001; ns, non-significant). Statistical analysis was done using GraphPad Prism.
3.4. Stable overexpression of Snail in LNCaP cells promotes neuronal outgrowth in PC-12 nerve cells
Next, we wanted to determine whether Snail expressed in prostate cancer cells plays a role in neurite outgrowth of nerve cells. LNCaP prostate cancer cells stably expressing empty vector (Neo) or Snail cDNA were cultured on 12-well cell culture inserts with semipermeable support membranes pre-coated with collagen type I (Fig. 4A). We have already shown that PC-12 cells have essential receptors to respond to nerve growth factor (NGF) by differentiating into sympathetic ganglion neurons [23]. PC-12 cells were cultured on the bottom of these wells (Fig. 4A). Neurite outgrowth was quantified by the percentage of neuronal cells which showed a phenotype of axonal extensions compared to those that showed no elongations. Our results show that PC-12 cells which were co-cultured with LNCaP prostate cancer cells expressing Snail promoted significantly higher neuronal outgrowth compared to PC-12 neuronal cells that were cultured with LNCaP cells expressing empty vector (Fig. 4B versus 4C). Nerve growth factor (NGF, 100 ng/mL) was utilized as a positive control (Fig. 4D,G). In another set of experiments, co-cultured PC-12 cells were stained by a MaP2 antibody, a dendritic marker followed by a confocal fluorescence microscopy. After visualization of neurites with MaP2 (Fig. 4E-G), we used ImageJ software to measure the length of neurites in 5 individual microscopic field as previously done [18]. The averages of neurite length were calculated by Prism GraphPad (Fig. 4I). These results suggest that the presence of overexpressed Snail enhances both number of PC-12 cells with distinct neurites (Fig. 4H) as well as the length of individual neurites (Fig. 4I).
FIGURE 4. Stable overexpression of Snail in LNCaP cells promotes neuronal outgrowth in PC-12 nerve cells.
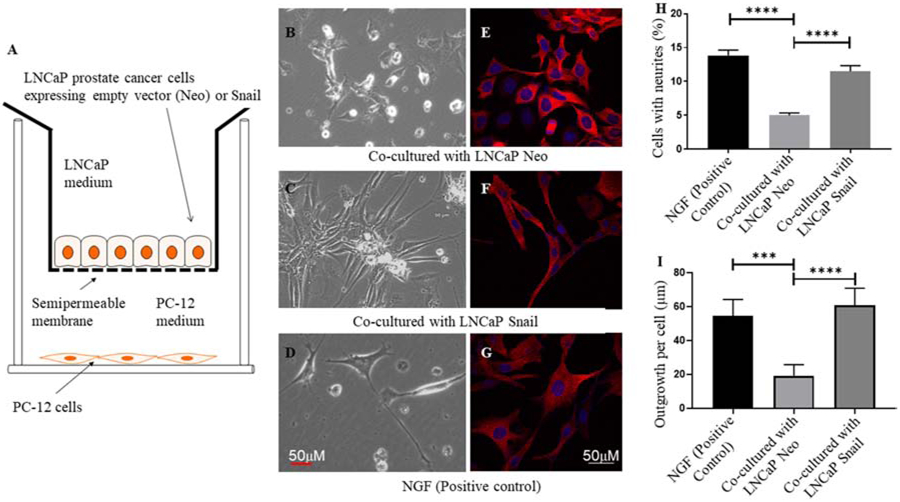
(A) LNCaP prostate cancer cells stably expressing empty vector (Neo) or Snail cDNA (LNCaP Snail) were cultured on 12-well cell culture inserts with semipermeable support membranes pre-coated with collagen type I. PC-12 cells were cultured on the bottom of these wells. Co-cultured PC-12 cells with neurite outgrowth, visualized by light microscopy (Panels B-D) were quantified based on the percentage of neuronal cells which showed a phenotype of axonal extensions. In another set of experiments, co-cultured PC-12 cells were stained with a MaP2 antibody, a dendritic marker followed by a confocal fluorescence microscopy (Panels E-G), and measurement of neurite length per cell (Panel I). Quantitated data in panels H and I are representative of 6 and 5 microscopic fields per experiment, respectively (***p< 0.001, ****p< 0.0001). Statistical analysis was done using GraphPad Prism. Nerve growth factor (NGF, 100 ng/mL) was utilized as a positive control. Scale bars are 50 μm.
3.5. Snail in C4-2 prostate cancer cells increases neurite outgrowth in NS20Y neuronal cells.
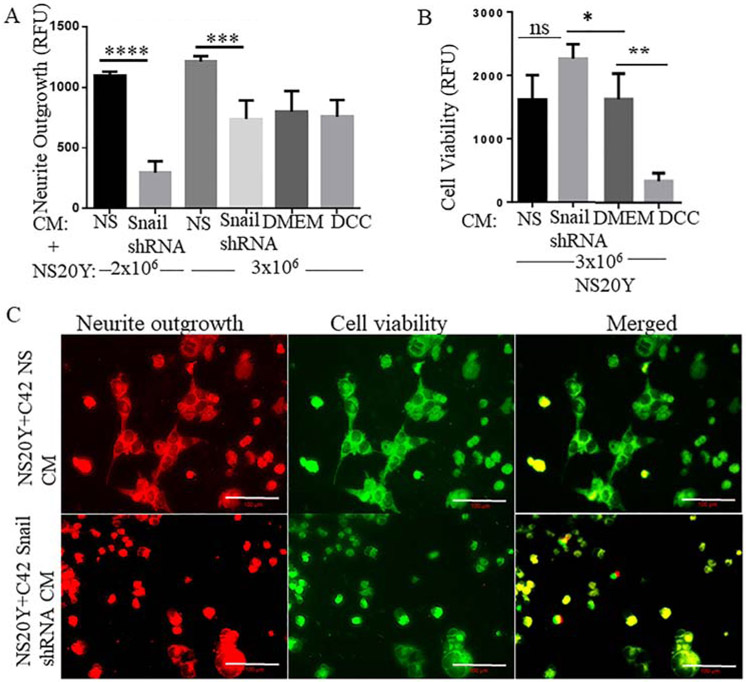
Our previous results supported the hypothesis that Snail promotes neurite outgrowth, therefore we further confirmed these findings utilizing C4-2 prostate cancer cell line with knockdown of endogenous Snail. Conditioned media (CM) from C4-2 NS or C4-2 Snail shRNA were added to NS20Y neuronal cells for 48 hours followed by a quantitative neurite outgrowth assay that measures both cell viability to assess neuronal cell health and neurite outgrowth. Our results indicated that NS20Y neuronal cells treated with C4-2 NS (Snail high) CM displayed higher neurite outgrowth levels compared to the neuronal cells which were treated with C4-2 Snail shRNA (Snail low) CM (Fig. 5A). Interestingly, NS20Y nerve cells treated with C4-2 Snail shRNA (Snail low) CM maintained significantly higher cell health compared to when nerve cells were treated with C4-2 NS (Snail high) condition media (Fig. 5B). We also utilized fluorescent imaging to visualize the neurite outgrowth and cell viability staining (Fig. 5C). Therefore, Snail-expressing cells promote neurite outgrowth of NS20Y cells independent of cell viability.
FIGURE 5. Depletion of Snail in C4-2 prostate cancer cells inhibits neurite outgrowth in NS20Y neuronal cells.
C4-2 NS or Snail shRNA cells were utilized to make conditioned media (CM). (A) Subsequently, NS20Y neuronal cells were cultured for 48 hours with CM followed by a quantitative neurite outgrowth assay that stains and quantifies neurite outgrowth. (B) Neuronal cells simultaneously stained for cell viability were quantified. (C) These same cells were also imaged using Zeiss Confocal microscope in 20X magnification. Data are the average + SD of three independent measurements. Data was analyzed by GraphPad Prism, *p< 0.05, **p< 0.01, ***p< 0.001,****p< 0.0001. Scale bars are 100 μm.
3.6. Time-lapse microscopy shows that Snail-expressing cancer cells stimulate neuronal cell mobility, communication, and axonal branching in vitro
In order to directly visualize the behavior of neuronal cells under various conditions based on differential expression of Snail, we performed live imaging and real time movie recording on the Zeiss microscope. We observed that NS20Y nerve cells cultured with C4-2 NS (high Snail) CM displayed more cell to cell interaction, the nerve endings (axons) appeared to be elongated extending outward from the perimeter of the nerve cell body and more cell movement, especially when compared to NS20Y nerve cells which were cultured with C4-2 Snail shRNA CM, or 5% DCC media (Fig. 6).
Figure 6. Visualization of neurite outgrowth in response to Snail-expressing cancer cells.
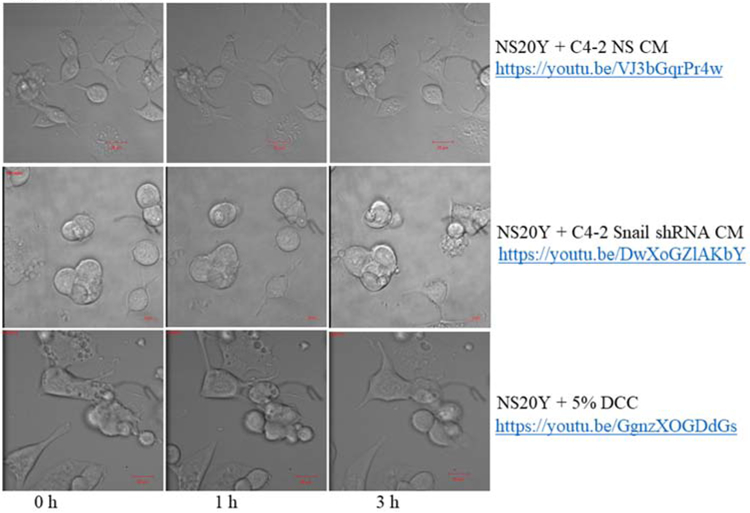
NS20Y neuronal cells were treated with conditioned media (CM) from C4-2 NS or C4-2 Snail shRNA cells for 4-6 hours. Treatment with 5% DCC was included as a negative control. The experimental and negative control nerve cells were photographed using time-lapse microscopy on a Zeiss LSM. The incubated nerve cells behavior was recorded using ZEN Black software. Images of nerve cells were taken every 60 seconds. Representative images are also shown at 0 h, 1 h, and 3 h. We observed that NS20Y nerve cells treated with CM from C4-2 NS prostate cancer cells (https://youtu.be/VJ3bGqrPr4w), displayed the highest cell to cell interaction, axonal elongation, and neurite formation compared to C4-2 Snail shRNA CM (https://youtu.be/DwXoGZlAKbY) or 5% DCC (https://youtu.be/GgnzXOGDdGs). Scale bars are 20 μm. All real time movies were recorded using 40X magnification.
4 ∣. DISCUSSION
Cellular invasion, one of the hallmarks of cancer, is characterized by the movement of cells through a three-dimensional matrix, resulting in remodeling of the cellular environment [24]. Cellular invasion requires adhesion, proteolysis of the extracellular matrix, and migration of cells. Snail is mainly associated with inducing the epithelial mesenchymal transition and enabling cells to migrate to other organs [15]. Migration of cancer cells is a prerequisite for metastasis [25]. The neurotransmitter/receptor system is also involved in cancer cell migration [25]. Netrin 1, a classical neurotransmitter, has a stimulatory effect on the migration of colon carcinoma cells and breast cancer cells [25]. Interestingly, this study proves that Snail-expressing cancer cells display increased migration towards sNF96.2 and NS20Ynerve cells. This gives us strong implications that when cancer cells express high levels of Snail, they are prompted to move towards nerves within close proximity. Literature and research have suggested that several types of tumors cells invade adjacent tissues by migrating along the resident nerves of the tumor microenvironment [26]. This process, called perineural invasion (PNI), typically occurs along extrinsic nerves, with Schwann cells providing physical guidance for the tumor cells [26]. Other studies have examined the molecular mechanism of the NGF signaling pathway in PNI in pancreatic cancer [27]. They revealed that knocking down NGF or its receptors, TRKA and p75NTR, or treatment with GW441756, a TRKA kinase inhibitor, reduces the proliferation and migration of pancreatic cancer cells in vitro [27]. However, in our studies we did not focus on the role of neurotrophic factors in mediating cancer cell migration towards nerves but sought to define a critical and overlooked function of the key EMT and migratory inducer such as Snail within this process.
We also made novel efforts to identify a role for Snail in prostate tumor cell adhesion to nerve cells. We discovered that when Snail expression was increased, adhesion levels of prostate cancer cells to nerves were significantly higher compared to when Snail was knocked down. Recent work identified the role of the transmembrane chemokine CX3CL1 and its receptor CX3CR1 in the adhesion and migration of pancreatic tumor cells on nerves during pancreatic cancer PNI [28]. In this study, activation of β1 integrins and focal adhesion kinase (FAK) was necessary for CX3CR1-mediated pancreatic tumor cell neurotropism [28]. Continuing studies determined the role of the glial cell-derived neurotrophic factor (GDNF), a chemoattractant secreted by nerves, in regulating pancreatic tumor cell adherence and invasion on nerves [29]. Future studies will need to determine putative cell adhesion molecules released by Snail-expressing prostate cancer cells that may mediate prostate cancer cell adhesion to nerve cells.
Additionally, we looked at the relationship between cancer cells expressing Snail and neurite outgrowth. Neurite outgrowth is an elaborate biological process that has been thought to involve a cross talk and reciprocal communication between nerve fibers and tumors [10, 25]. A diverse number of proteins and growth factors originally derived and expressed in the nervous system have been connected to the initiation of these axonal or dendritic projections [2, 10, 25]. In our study, we have discovered that prostate cancer cells expressing Snail may secrete factors that stimulate neurite outgrowth in PC-12 and NS20Y nerve cells. We demonstrated this utilizing CM from LNCaP cell line which were engineered to stably overexpress Snail, or CM from C4-2 cell line with stable Snail knockdown. Currently, no research has been reported correlating Snail from cancer cells to induction of neurite outgrowth in nerve cells. Due to the fact that Snail is a transcription factor, the fact that it can promote neurite outgrowth suggests it may regulate upregulation and/or secretion of other factors that subsequently increase neurite outgrowth.
Neurotrophic factors such as Brain Derived Neurotrophic Factor (BDNF) and NGF, which specifically promote neurite outgrowth, support axonal regeneration and can also be secreted by tumor cells [10]. For sufficient axonal growth and elongation, neuronal cells are dependent upon activation of signaling pathways and secretion of various proteins [25]. Previous studies have shown that the Snail and Twist axis is required for BDNF-induced EMT and metastasis in malignancies such as breast, colon, and ovarian cancer [30]. It is plausible that Snail may increase secretion of neurotrophic factors such as these that act in a paracrine manner to increase neurite outgrowth of nerve cells. Another study, examined how blocking NGF signaling by NGF neutralizing antibodies inhibited the neurite formation in PC-12 cells in response to CM from pancreatic cancer cells [27]. This indicated a reciprocal signaling pathway between the pancreatic cancer cells and nerves [27]. Similarly, our study indicates a mechanism involving neurite formation in response to CM from prostate cancer cells, but our study is unique in showing this response can be attributed to high Snail expression.
Further studies have utilized Salivary Adenoid Cancer Cells (SACC) and dorsal root ganglia in an in vitro matrigel co-culture system and discovered that the mouse dorsal root ganglia formed branch-like extensions towards the salivary adenoid cancer cells [31]. Other studies have utilized PC-12 nerve cells and metastatic HPV16 mEERL cells and discovered that exosomes from these cancer cells stimulate neurite outgrowth [32]. They noticed that the PC-12 nerve cells which were not treated with exosomes from HPV16 tumors did not display formation of neurite branching or extensions [32]. Our future studies will consist of examining the factors within the CM released from prostate cancer cells that may regulate neurite outgrowth in nerve cells.
5 ∣. CONCLUSIONS
Overall, we have provided sufficient findings that give rise to the claim that Snail-expressing prostate cancer cells promote migration and adhesion of cancer cells to nerve cells, as well as neurite outgrowth. Our studies and other research suggest that cancer cells may release molecules or proteins in vitro which stimulate this activity in nerve cells. This suggests that Snail can mediate prostate cancer cell interactions with the nervous system to possibly promote metastasis, thus, therapeutic targeting of Snail may inhibit the tumor-micro-environmental interactions.
Supplementary Material
Highlights.
Prostate cancer cells expressing Snail transcription factor migrate towards nerve cells
Snail transcription factor promotes adhesion of prostate cancer cells to nerve cells
Conditioned media from Snail-expressing prostate cancer cells promotes neurite outgrowth
Acknowledgement
The authors would like to thank Marisha Morris for her assistance with the migration assays.
Funding
This work was supported by: National Institutes of Health (NIH)/National Institute on Minority Health & Health Disparities (NIMHD)/Research Centers in Minority Institutions Program (RCMI) Grant No. 2U54MD007590-32; NIH/National Institute of General Medical Sciences (NIGMS) Research Initiative for Scientific Enhancement (RISE) Program Grant No. 5R25GM060414-16. KR is supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number U54GM128729.
Abbreviations:
- PCa
prostate cancer
- CM
conditioned media
- EMT
epithelial mesenchymal transition
- PNI
perineural invasion
- NGF
nerve growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that there are no competing interests, financial and non-financial, in relation to the work described.
REFERENCES:
- 1.Carragher NO, Fonseca BD, and Frame MC, Calpain activity is generally elevated during transformation but has oncogene-specific biological functions. Neoplasia, 2004. 6(1): p. 53–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zareba P, et al. , Perineural Invasion and Risk of Lethal Prostate Cancer. Cancer Epidemiol Biomarkers Prev, 2017. 26(5): p. 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuol N, et al. , Role of the nervous system in cancer metastasis. J Exp Clin Cancer Res, 2018. 37(1): p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chelmicka-Szorc E and Arnason BG, Effect of 6-hydroxydopamine on tumor growth. Cancer Res, 1976. 36(7 PT 1): p. 2382–4. [PubMed] [Google Scholar]
- 5.Seifert P and Spitznas M, Tumours may be innervated. Virchows Arch, 2001. 438(3): p. 228–31. [DOI] [PubMed] [Google Scholar]
- 6.Li JH, et al. , Stimulation of dorsal root ganglion neurons activity by pancreatic cancer cell lines. Cell Biol Int, 2008. 32(12): p. 1530–5. [DOI] [PubMed] [Google Scholar]
- 7.Takano T, et al. , Discovery of long-range inhibitory signaling to ensure single axon formation. Nat Commun, 2017. 8(1): p. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arimura N and Kaibuchi K, Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci, 2007. 8(3): p. 194–205. [DOI] [PubMed] [Google Scholar]
- 9.Miller KE and Suter DM, An Integrated Cytoskeletal Model of Neurite Outgrowth. Front Cell Neurosci, 2018. 12: p. 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancino M, et al. , The neuronal influence on tumor progression. Biochim Biophys Acta, 2011. 1816(2): p. 105–18. [DOI] [PubMed] [Google Scholar]
- 11.Venkatesh HS, et al. , Electrical and synaptic integration of glioma into neural circuits. Nature, 2019. 573(7775): p. 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Silva B, et al. , Chemically induced neurite-like outgrowth reveals a multicellular network function in patient-derived glioblastoma cells. J Cell Sci, 2019. 132(19). [DOI] [PubMed] [Google Scholar]
- 13.McKeithen D, et al. , Snail transcription factor regulates neuroendocrine differentiation in LNCaP prostate cancer cells. Prostate, 2010. 70(9): p. 982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cano A, et al. , The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol, 2000. 2(2): p. 76–83. [DOI] [PubMed] [Google Scholar]
- 15.Smith BN and Odero-Marah VA, The role of Snail in prostate cancer. Cell Adh Migr, 2012. 6(5): p. 433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poblete CE, et al. , Increased SNAIL expression and low syndecan levels are associated with high Gleason grade in prostate cancer. Int J Oncol, 2014. 44(3): p. 647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neal CL, et al. , Snail transcription factor negatively regulates maspin tumor suppressor in human prostate cancer cells. BMC Cancer, 2012. 12: p. 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pemberton K, Mersman B, and Xu F, Using ImageJ to Assess Neurite Outgrowth in Mammalian Cell Cultures: Research Data Quantification Exercises in Undergraduate Neuroscience Lab. J Undergrad Neurosci Educ, 2018. 16(2): p. A186–A194. [PMC free article] [PubMed] [Google Scholar]
- 19.Dowling CM, Herranz Ors C, and Kiely PA, Using real-time impedance-based assays to monitor the effects of fibroblast-derived media on the adhesion, proliferation, migration and invasion of colon cancer cells. Biosci Rep, 2014. 34(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley CA, et al. , Transcriptional upregulation of c-MET is associated with invasion and tumor budding in colorectal cancer. Oncotarget, 2016. 7(48): p. 78932–78945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez JA, et al. , Diminished expression of CRHR2 in human colon cancer promotes tumor growth and EMT via persistent IL-6/Stat3 signaling. Cell Mol Gastroenterol Hepatol, 2015. 1(6): p. 610–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neal CL, McKeithen D, and Odero-Marah VA, Snail negatively regulates cell adhesion to extracellular matrix and integrin expression via the MAPK pathway in prostate cancer cells. Cell Adh Migr, 2011. 5(3): p. 249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezvani K, Teng Y, and De Biasi M, The ubiquitin-proteasome system regulates the stability of neuronal nicotinic acetylcholine receptors. J Mol Neurosci, 2010. 40(1-2): p. 177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Merbel AF, et al. , Protocols for Migration and Invasion Studies in Prostate Cancer. Methods Mol Biol, 2018. 1786: p. 67–79. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Sun Y, and Gao D, Role of the nervous system in cancer metastasis. Oncol Lett, 2013. 5(4): p. 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duchalais E, et al. , Colorectal Cancer Cells Adhere to and Migrate Along the Neurons of the Enteric Nervous System. Cell Mol Gastroenterol Hepatol, 2018. 5(1): p. 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bapat AA, et al. , Blocking Nerve Growth Factor Signaling Reduces the Neural Invasion Potential of Pancreatic Cancer Cells. PLoS One, 2016. 11(10): p. e0165586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchesi F, et al. , The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res, 2008. 68(21): p. 9060–9. [DOI] [PubMed] [Google Scholar]
- 29.Gil Z, et al. , Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst, 2010. 102(2): p. 107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smit MA, et al. , A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol, 2009. 29(13): p. 3722–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao T, et al. , The CCL5/CCR5 Chemotactic Pathway Promotes Perineural Invasion in Salivary Adenoid Cystic Carcinoma. J Oral Maxillofac Surg, 2018. 76(8): p. 1708–1718. [DOI] [PubMed] [Google Scholar]
- 32.Madeo M, et al. , Cancer exosomes induce tumor innervation. Nat Commun, 2018. 9(1): p. 4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.