Abstract
Background
Although COVID-19 has greatly affected many low-income and middle-income countries, detailed information about patients admitted to the intensive care unit (ICU) is still scarce. Our aim was to examine ventilation characteristics and outcomes in invasively ventilated patients with COVID-19 in Argentina, an upper middle-income country.
Methods
In this prospective, multicentre cohort study (SATICOVID), we enrolled patients aged 18 years or older with RT-PCR-confirmed COVID-19 who were on invasive mechanical ventilation and admitted to one of 63 ICUs in Argentina. Patient demographics and clinical, laboratory, and general management variables were collected on day 1 (ICU admission); physiological respiratory and ventilation variables were collected on days 1, 3, and 7. The primary outcome was all-cause in-hospital mortality. All patients were followed until death in hospital or hospital discharge, whichever occurred first. Secondary outcomes were ICU mortality, identification of independent predictors of mortality, duration of invasive mechanical ventilation, and patterns of change in physiological respiratory and mechanical ventilation variables. The study is registered with ClinicalTrials.gov, NCT04611269, and is complete.
Findings
Between March 20, 2020, and Oct 31, 2020, we enrolled 1909 invasively ventilated patients with COVID-19, with a median age of 62 years [IQR 52–70]. 1294 (67·8%) were men, hypertension and obesity were the main comorbidities, and 939 (49·2%) patients required vasopressors. Lung-protective ventilation was widely used and median duration of ventilation was 13 days (IQR 7–22). Median tidal volume was 6·1 mL/kg predicted bodyweight (IQR 6·0–7·0) on day 1, and the value increased significantly up to day 7; positive end-expiratory pressure was 10 cm H2O (8–12) on day 1, with a slight but significant decrease to day 7. Ratio of partial pressure of arterial oxygen (PaO2) to fractional inspired oxygen (FiO2) was 160 (IQR 111–218), respiratory system compliance 36 mL/cm H2O (29–44), driving pressure 12 cm H2O (10–14), and FiO2 0·60 (0·45–0·80) on day 1. Acute respiratory distress syndrome developed in 1672 (87·6%) of patients; 1176 (61·6%) received prone positioning. In-hospital mortality was 57·7% (1101/1909 patients) and ICU mortality was 57·0% (1088/1909 patients); 462 (43·8%) patients died of refractory hypoxaemia, frequently overlapping with septic shock (n=174). Cox regression identified age (hazard ratio 1·02 [95% CI 1·01–1·03]), Charlson score (1·16 [1·11–1·23]), endotracheal intubation outside of the ICU (ie, before ICU admission; 1·37 [1·10–1·71]), vasopressor use on day 1 (1·29 [1·07–1·55]), D-dimer concentration (1·02 [1·01–1·03]), PaO2/FiO2 on day 1 (0·998 [0·997–0·999]), arterial pH on day 1 (1·01 [1·00–1·01]), driving pressure on day 1 (1·05 [1·03–1·08]), acute kidney injury (1·66 [1·36–2·03]), and month of admission (1·10 [1·03–1·18]) as independent predictors of mortality.
Interpretation
In patients with COVID-19 who required invasive mechanical ventilation, lung-protective ventilation was widely used but mortality was high. Predictors of mortality in our study broadly agreed with those identified in studies of invasively ventilated patients in high-income countries. The sustained burden of COVID-19 on scarce health-care personnel might have contributed to high mortality over the course of our study in Argentina. These data might help to identify points for improvement in the management of patients in middle-income countries and elsewhere.
Funding
None.
Translation
For the Spanish translation of the Summary see Supplementary Materials section.
Introduction
Since the first case of pneumonia related to SARS-CoV-2 was reported in 2019, COVID-19 has spread relentlessly across the world. On March 11, 2020, WHO declared COVID-19 a pandemic; as of May 1, 2021, 153 480 005 cases of COVID-19 had been confirmed, with 3 206 117 deaths.1
From the beginning of the pandemic, there was great concern in the clinical and research communities about its potential impact on low-income and middle-income countries (LMICs), given their profound, long-lasting economic and educational inequities, social turbulence, and fragile health systems.2, 3, 4 Epidemiological information about critically ill patients with COVID-19 in LMICs has been scarce, although some countries with nationwide databases have reported worthwhile information.5, 6, 7 In Argentina, an upper middle-income country (defined by the World Bank as economies with a gross national income per capita of between US$4046 and US$12 535), information provided by the Ministry of Health is fragmented owing to the absence of an integrated health-care system, and available data about private health subsectors is deficient. In this context, the Argentine Society of Intensive Care (Sociedad Argentina de Terapia Intensiva or SATI) launched a prospective cohort study with the aim of describing epidemiological, clinical, and physiological characteristics, ventilation settings and received treatments, and outcomes in patients with laboratory-confirmed COVID-19 who required invasive mechanical ventilation.
Research in context.
Evidence before this study
Although the impact of COVID-19 on low-income and middle-income countries (LMICs) is widely recognised, little is known about outcomes for patients receiving invasive mechanical ventilation in these regions. We searched PubMed on Feb 12, 2021, for studies of adults patients (≥18 years) using the terms “coronavirus” OR “COVID-19” AND “mechanical ventilation” AND “ICU” AND “mortality”. We included articles that had at least an abstract written in English. Our search identified 258 articles, many of which were single-centre studies with few patients. We identified nationwide, retrospective cohort studies that reported epidemiological characteristics and outcome measures from Germany, Mexico, Brazil, Iran, the UK, and the USA. The first three of these studies reported the number of patients admitted to the intensive care unit (ICU), the number of patients who required mechanical ventilation, and mortality; but there was no mention of mechanical ventilation variables. Mortality was greater than 55% in these studies. We also identified another four multicentre cohort studies that included patients on mechanical ventilation, which provide detailed data analysis of mechanical ventilation variables, and the association of these variables with outcomes. These studies were from the Netherlands (553 patients from 18 ICUs); Italy (1591 patients from 72 ICUs); France, Belgium, and Switzerland (the REVA cohort, 3376 patients from 138 ICUs); and Spain (742 patients from 36 ICUs, all with acute respiratory distress syndrome). Only the REVA cohort and the study from Spain were prospective studies. None of them originated in a LMIC.
Added value of this study
To our knowledge, SATICOVID is the first prospective, multicentre cohort study carried out in a LMIC during the pandemic; it includes 1909 patients with RT-PCR-confirmed COVID-19 from 63 ICUs in Argentina. The study provides a detailed analysis of patient epidemiological characteristics, laboratory findings, symptoms, respiratory physiological parameters, and mechanical ventilation variables over time, and causes of death. The entire cohort of patients was followed until death or hospital discharge.
Implications of all the available evidence
Lung-protective ventilation was widely used in Argentina, as elsewhere. Overall in-hospital mortality for invasively ventilated patients with COVID-19 was high, as has been reported in other regions (eg, Mexico and Brazil). Mortality was related to age, comorbidities, acute cardiovascular and kidney dysfunction, and compromised oxygenation; we also found an association with driving pressure, a variable of respiratory mechanics. Although the health system in Argentina was well resourced in terms of equipment and consumables during periods of high demand, mortality increased throughout the study period, from April to October, 2020, perhaps as a result of the sustained burden on scarce health-care personnel. Our findings add to the existing body of knowledge about COVID-19 epidemiological aspects and outcomes, and also about the current practice of mechanical ventilation.
Our primary aim was to determine in-hospital mortality. Given that hospital mortality for patients with critical conditions such as sepsis, and for mechanically ventilated patients with respiratory failure due to 2009 H1N1 influenza, was reported to be higher in Argentina than in high-income countries,3, 8 we hypothesised that in invasively ventilated patients with confirmed COVID-19, hospital mortality in our cohort would be higher than the 26% reported for the Lombardy Region, Italy,9 at the time of study initiation.
Methods
Study design and population
SATICOVID was a prospective, multicentre cohort study that enrolled patients aged 18 years and older with RT-PCR-confirmed SARS-CoV-2 infection who required invasive mechanical ventilation and were admitted to 63 ICUs in Argentina (appendix 2, pp 5–6). As specified in the protocol, patients were excluded from the analysis if SARS-CoV-2 infection was not confirmed, according to WHO guidance, or if they had a severe respiratory infection or pneumonia proven to be due to another cause. Patients were also excluded if no baseline data were recorded or if no details of ventilatory parameters were available. Patients were followed until death in hospital or hospital discharge, whichever occurred first, allowing a complete case analysis.
SATI announced the study on its website and via emails to all society members to invite them to participate in the study. Electronic forms for reporting of hospital and ICU data, case characteristics, and ventilation parameters were designed and distributed by the investigators via email (appendix 2, pp 48–64). Paper forms for case reporting were also available to local researchers to complete, scan, and send to a specific email address. These forms were provided by the principal investigator (EE). The email was monitored by EE and VSKE. A trained data-entry specialist (who was not involved in any other part of the study) added the data collected from these forms to a central database (Excel), which was then was exported into a Stata dta. Only EE, AD, and CIL had access to the database. Individual patient data were anonymised by assigning a numerical code to each case. Code numbers were assigned in order of admission.
Each local institutional review board approved the study and defined the requirement for informed consent. The SATICOVID study protocol is available in appendix 2 (p 26).
Recorded variables
On day 1, at ICU admission, we recorded patient demographics and characteristics, including date of admission; age (as a continuous variable and in 10-year categories: <40 years, 40–49 years, 50–59 years, and so on, up to ≥80 years); sex; body-mass index (BMI); comorbidities and Charlson score; Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores; need for vasopressors; laboratory variables; smoking status; and any alcohol-related problem (appendix 2, p 34). We also registered patient signs and symptoms of infection before hospital admission; number of days from symptom onset to hospital admission; number of days from hospital admission to initiation of invasive mechanical ventilation; preintubation use of high-flow nasal cannula (HFNC) and non-invasive mechanical ventilation (NIV); and the site of endotracheal intubation (outside or inside the ICU).
Physiological respiratory and mechanical ventilation variables were collected on admission to the ICU (day 1) and on days 3 and 7: blood gas analysis (arterial pH, partial pressure of arterial carbon dioxide (PaCO2), partial pressure of arterial oxygen (PaO2), and arterial oxygen saturation; plasma bicarbonate and base excess were then calculated); proportion of patients with lung infiltrates involving 3–4 quadrants on chest x-ray; ratio of PaO2 to fractional inspired oxygen (FiO2); tidal volume in mL/kg predicted bodyweight; FiO2; respiratory rate; positive end-expiratory pressure (PEEP); plateau pressure; respiratory system compliance; and driving pressure. Score on the Richmond Agitation-Sedation Scale (RASS) was recorded, with values of –5 and –4 points considered as deep sedation.
We also registered development of acute respiratory distress syndrome (ARDS);10 use of prone positioning, including number and duration of sessions (in hours); acute kidney failure and requirement for renal replacement therapy; septic shock; maximum fever value; development of bacteraemia; ventilation-associated pneumonia; thromboembolic events and their localisation; and use of specific treatments. Corticosteroid use was analysed before and after the publication of the RECOVERY trial, which showed a benefit of dexamethasone in hospitalised patients with COVID-19.11 Causes of death were selected from a list of nine predetermined possibilities: refractory hypoxaemia, septic shock, multiorgan dysfunction syndrome, acute myocardial infarction, acute heart failure, stroke, do-not-resuscitate order, pulmonary thromboembolism, and other; more than one cause of death could be considered. Duration of mechanical ventilation and length of ICU and hospital stay, in days, were also recorded.
Site investigators collected worst values for each variable daily, from admission to the ICU to ICU discharge. Definitions of comorbidities, physiological and mechanical ventilation variables, complications, and causes of death are provided in appendix 2 (pp 22–25).
Outcomes
The primary outcome measure was all-cause in-hospital mortality. All patients were followed until death or discharge, whichever occurred first. When the study was designed and the protocol prepared (appendix 2, p 26) at the beginning of the pandemic, we selected all-cause ICU mortality as the primary outcome, similar to most epidemiological studies. On Nov 15, 2020, we changed the outcome to in-hospital mortality, including patients who died in the ICU and after discharge from the ICU, because it more comprehensively reflects the full course of the disease. An additional, exploratory outcome was mortality and acuity over the duration of the study, from April to October, 2020, according to the month of hospital admission.12
Secondary outcomes were ICU mortality; independent predictors of mortality; duration of invasive mechanical ventilation (in days); and patterns of change in physiological respiratory and mechanical ventilation variables on days 1 (ICU admission), 3, and 7 for the entire cohort, and in survivor and non-survivor subgroups. 28-day mortality was added as a modification and was included as secondary outcome on Nov 15, 2020, for comparison with other reports.
Statistical analysis
Before data analysis, two investigators (EE and FGR) screened the database for errors against standardised ranges in each hospital. Investigators were contacted (by EE and VSKE) with queries and to address inconsistencies. Validated or corrected data were then entered into the database. Missing data were not imputed. Given that this was an observational study and there was no risk to patients, we sought to include as many patients as possible, with no predefined sample size.
Variables are reported as absolute numbers and percentages, or medians and IQRs. Differences between survivors and non-survivors in recorded variables were analysed with the χ2 test or Fisher's exact test, or the t test or Wilcoxon rank-sum test, as appropriate. All tests were two-sided, and a p value of <0·05 was considered to be statistically significant.
Generalised estimating equations were used to account for correlations between respiratory variables in the entire cohort over time, and between subgroups of survivors and non-survivors. An unstructured correlation matrix was selected. p values for time-effect for the entire cohort and for time-by-subgroup interaction were calculated and a Bonferroni correction used to adjust for multiple comparisons.
Mortality at 28 days and 90 days was plotted as time-to-event curves using the Kaplan-Meier method. The Kaplan-Meier analysis was cut at 90 days for simplicity, although some patients did not have an outcome (death or discharge) at this point. Kaplan-Meier curves were also constructed to compare time-to-event differences in patients according to comorbidities (Charlson score <2 and ≥2 points) and 10-year age category. Differences in each case were analysed with the log-rank test.
Cox regression analysis was used to determine independent predictors of hospital mortality. Variables differing between survivors and non-survivors with a p value <0·20, according to the χ2 test or Fisher's exact test, or t test or Wilcoxon rank-sum test, were entered into the multivariable regression model. Harrell's C index was calculated to test the predictive capacity of the model. The proportional hazard assumption was tested by visual inspection of Schoenfeld residuals and by testing predicted versus observed values of model variables.
For the post-hoc analysis of mortality over the months of the study (April to October), a multiple χ2 test was used. Age and acuity scores, such as APACHE II and SOFA, were compared by means of one-way ANOVA.
Missing data for each variable at each timepoint (day 1, 3, and 7) are shown in appendix 2 (pp 15–17). Data were analysed with Stata 14.0 (StataCorp LP, College Station, TX, USA). The trial is registered with ClinicalTrials.gov, NCT04611269.
Role of the funding source
There was no funding source for this study.
Results
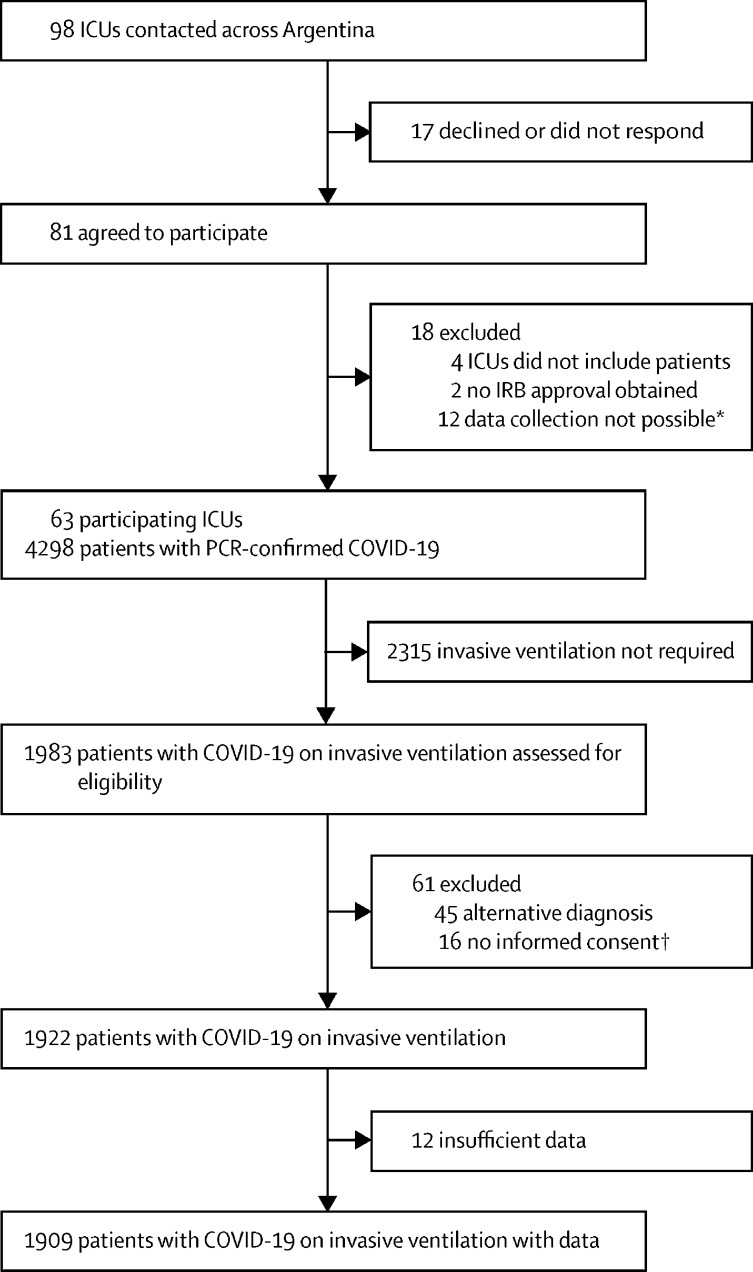
Between March 20, 2020, and Oct 31, 2020, we enrolled 1909 invasively ventilated patients with RT-PCR-confirmed COVID-19, admitted to 63 ICUs (figure 1 ). Patients had a median age of 62 years (IQR 52–70), were predominantly male (1294 [67·8%]), and 1750 (91·7%) had comorbidities, of which arterial hypertension (897 [46·9%]), obesity (847 [44·4%]), and diabetes (553 [29·0%]) were the most frequent (table 1 ). Patients stayed in hospital after admission for a median of 1 day (IQR 0–4) before being admitted to the ICU. 144 (7·5%) patients were on HFNC for a median of 1 day (0–2) and 73 (3·8%) patients were on NIV for a median of 1 day (1–2). 416 (22·2%) of 1872 patients underwent endotracheal intubation outside the ICU, 283 (15·1%) in the emergency department and 133 (7·1%) in the general ward (table 1). Symptoms lasted for a median of 5 days (3–7) before hospital admission, the most common of which were dyspnoea (1443 [75·6%]), fever (1424 [74·6%]), and cough (1188 [62·2%]; appendix 2, p 8).
Figure 1.
Study profile
IRB=institutional review board. *Limited capacity to enroll patients because of insufficient staff resources during a time of high COVID-19 activity. †Each local IRB defined the requirement for informed consent.
Table 1.
Epidemiological variables and risk factors in invasively ventilated patients with COVID-19
| All patients (n=1909) | Survivors (n=808) | Non-survivors (n=1101) | p value | ||
|---|---|---|---|---|---|
| Age (years) | 62 (52 to 70) | 58 (49 to 68) | 64 (55 to 72) | <0·0001 | |
| Sex | |||||
| Male | 1294 (67·8%) | 537 (66·5%) | 757 (68·8%) | 0·289 | |
| Female | 615 (32·2%) | 271 (33·5%) | 344 (31·2%) | 0·289 | |
| Weight (kg) | 85 (75 to 100) | 85 (75 to 100) | 85 (75 to 98) | 0·930 | |
| BMI (kg/m2) | 29 (26 to 34) | 29 (27 to 34) | 29 (26 to 34) | 0·564 | |
| Comorbidities | |||||
| Arterial hypertension | 897/1909 (46·9%) | 326/808 (40·3%) | 571/1101 (51·9%) | <0·0001 | |
| Obesity (BMI ≥30) | 847/1909 (44·4%) | 374/808 (46·3%) | 473/1101 (43·0%) | 0·148 | |
| Morbid obesity (BMI >40 kg/m2) | 290/1909 (15·2%) | 123/808 (15·2%) | 167/1101 (15·2%) | 0·974 | |
| Diabetes | 553/1909 (29·0%) | 204/808 (25·2%) | 349/1101 (31·7%) | 0·002 | |
| Respiratory disease | 263/1909 (13·8%) | 98/808 (12·1%) | 165/1101 (15·0%) | 0·070 | |
| Ischaemic heart disease | 123/1909 (6·4%) | 34/808 (4·2%) | 89/1101 (8·1%) | 0·001 | |
| Chronic kidney disease | 118/1909 (6·2%) | 33/808 (4·1%) | 85/1101 (7·7%) | 0·001 | |
| Chronic heart failure | 100/1909 (5·2%) | 20/808 (2·4%) | 80/1101 (7·3%) | <0·0001 | |
| Immunosuppression | 92/1909 (4·8%) | 28/808 (3·5%) | 64/1101 (5·8%) | 0·018 | |
| Oncohaematological disease | 54/1909 (2·8%) | 11/808 (1·4%) | 43/1101 (3·9%) | 0·001 | |
| Chemotherapy in the previous 6 months | 36/1909 (1·9%) | 7/808 (0·1%) | 29/1101 (2·6%) | 0·005 | |
| Chronic liver disease | 34/1909 (1·8%) | 7/808 (0·9%) | 27/1101 (2·4%) | 0·010 | |
| Solid organ transplantation | 17/1909 (0·9%) | 5/808 (1·0%) | 12/1101 (1·1%) | 0·279 | |
| Bone marrow transplantation | 3/1909 (0·2%) | 1/808 (0·1%) | 20/1101 (1·8%) | 1·000 | |
| Pregnant or post-partum | 4/1909 (0·2%) | 3/808 (0·4%) | 1/1101 (0·1%) | 0·317 | |
| Presence of cardiovascular disease* | 944/1909 (49·4%) | 336/808 (41·6%) | 608/1101 (55·2%) | <0·0001 | |
| Charlson comorbidity score | 1 (1 to 2) | 1 (0 to 2) | 2 (1 to 3) | <0·0001 | |
| No comorbidities | 159/1909 (8·3%) | 106/808 (13·1%) | 53/1101 (4·8%) | <0·0001 | |
| Habits and drug use | |||||
| ACE inhibitors or AII receptor blockers | 352/1909 (18·4%) | 141/808 (17·5%) | 211/1101 (19·2%) | 0·373 | |
| Current smoker | 267/1909 (14·0%) | 94/808 (11·6%) | 173/1101 (15·7%) | 0·011 | |
| Statins | 137/1909 (7·1%) | 58/808 (7·2%) | 79/1101 (7·2%) | 0·998 | |
| β blockers | 135/1909 (7·1%) | 48/808 (5·3%) | 87/1101 (7·9%) | 0·090 | |
| Self-reported alcohol-related problem | 52/1909 (2·7%) | 19/808 (2·4%) | 33/1101 (3·0%) | 0·394 | |
| Duration of symptoms before admission to hospital (days) | 5 (3 to 7) | 5 (3 to 7) | 5 (3 to 7) | 0·266 | |
| Period between hospital and ICU admission (days) | 1 (0 to 4) | 1 (0 to 4) | 1 (0 to 4) | 0·881 | |
| Respiratory management before ICU admission | |||||
| Prior use of non-invasive mechanical ventilation | 73/1909 (3·8%) | 24/808 (3·0%) | 49/1101 (4·4%) | 0·096 | |
| Duration of non-invasive mechanical ventilation (days) | 1 (1 to 2) | 1 (1 to 2) | 1 (1 to 2) | 0·37 | |
| Prior use of high-flow nasal cannula | 144/1909 (7·5%) | 58/808 (7·2%) | 86/1101 (7·8%) | 0·61 | |
| Duration of high-flow nasal cannula use (days) | 1 (0 to 2) | 1 (0 to 2) | 1 (0 to 2) | 0·14 | |
| Requirement for invasive mechanical ventilation before ICU admission | 129/1909 (6·8%) | 40/808 (5·0%) | 89/1101 (8·1%) | 0·01 | |
| Duration of invasive mechanical ventilation before ICU admission (days) | 1 (1 to 2) | 1 (1 to 2) | 1 (1 to 2) | 0·460 | |
| Endotracheal intubation outside the ICU | 416/1872 (22·2%) | 150/789 (19·0%) | 266/1083 (24·6%) | 0·004 | |
| Variables of disease severity† | |||||
| APACHE II | 15 (10 to 20) | 13 (9 to 18) | 16 (12 to 22) | <0·0001 | |
| SOFA24-h | 5 (3 to 8) | 4 (3 to 7) | 6 (4 to 8) | <0·0001 | |
| Pre-intubation respiratory rate | 32 (28 to 36) | 32 (28 to 36) | 32 (28 to 36) | 0·530 | |
| Oxygen saturation by pulse oximetry at admission | 89 (86 to 94) | 91 (88 to 94) | 89 (85 to 93) | <0·0001 | |
| Extension of lung infiltrates over 3–4 quadrants on chest x-ray or CT scan | 1324/1650 (80·2%) | 553/701 (78·9%) | 771/949 (81·2%) | 0·235 | |
| Requirement for vasopressors | 939/1909 (49·2%) | 345/808 (42·7%) | 594/1101 (53·9%) | <0·0001 | |
| Fluid balance on day 1 (mL) | 716 (to 100 to 1700) | 650 (to 53 to 1600) | 787 (to 154 to 1779) | 0·310 | |
Data are n/N (%) or median (IQR). Percentages were calculated according to the data recorded for each variable. Missing data corresponding to each variable are shown in appendix 2 (pp 15–16). ACE=angiotensin-converting enzyme. AII=angiotensin II. APACHE II=Acute Physiology and Chronic Health Evaluation. BMI=body-mass index. ICU=intensive care unit. SOFA=Sequential Organ Failure Assessment.
Includes any cardiovascular disease: arterial hypertension, ischaemic heart disease, chronic heart failure.
Calculated within the first 24 h of ICU admission.
In patients admitted to the ICU, 1456 (79·3%) with spontaneous breathing had tachypnoea, median oxygen saturation was 89% (IQR 86–94), 1324 (80·2%) had lung infiltrates involving 3–4 quadrants on chest x-ray, and these 1456 patients underwent endotracheal intubation within 0 days (0–1) from admission to the ICU. Requirement for vasopressors was common (939 [49·2%]). The most frequent laboratory alterations were mild leucocytosis with lymphopenia, and increased lactate dehydrogenase and markers of inflammation, such as D-dimer, ferritin, and arterial lactate concentrations (table 2 ).
Table 2.
Laboratory findings
| All patients (n=1909) | Survivors (n=808) | Non-survivors (n=1101) | p value | |
|---|---|---|---|---|
| Haemoglobin (g/L) | 13 (11–14) | 13 (12–14) | 13 (11–14) | 0·001 |
| White blood cell count (× 109 per L) | 11·0 (7·6–15·0) | 10·4 (7·2–14·2) | 11·4 (7·9–15·5) | <0·0001 |
| Lymphocyte count (× 109 per L) | 0·8 (0·5–1·1) | 0·8 (0·5–1·1) | 0·7 (0·5–1·1) | 0·110 |
| Platelet count (× 109 per L) | 224·0 (168·0–299·0) | 224·0 (171·0–302·0) | 224·0 (166·0–294·0) | 0·190 |
| Aspartate aminotransferase (U/L) | 42 (29–65) | 41 (29–65) | 43 (28–66) | 0·505 |
| Alanine aminotransferase (U/L) | 39 (26–64) | 40 (27–69) | 39 (24–63) | 0·009 |
| Total bilirubin (μmol/L) | 10·3 (6·8–15·4) | 9·4 (6·8–15·4) | 10·3 (6·8–15·7) | 0·006 |
| Lactate dehydrogenase (U/L) | 512 (355–750) | 453 (326–653) | 558 (383–829) | <0·0001 |
| Blood urea nitrogen (mmol/L) | 3·42 (2·41–5·28) | 2·95 (2·17–4·20) | 4·04 (2·72–5·98) | <0·0001 |
| Creatinine (μmol/L) | 79·6 (61·9–114·9) | 70·7 (61·9–97·2) | 88·4 (61·9–132·6) | <0·0001 |
| D-dimer (mg/L) | 1·13 (0·56–3·08) | 0·90 (0·50–2·21) | 1·44 (0·70–3·83) | <0·0001 |
| Ferritin (ng/mL) | 1063 (545–1775) | 1031 (525–1885) | 1108 (580–1700) | 0·493 |
| Arterial lactate (mmol/L) | 1·8 (1·4–2·4) | 1·7 (1·3–2·3) | 1·9 (1·5–2·6) | <0·0001 |
Data are expressed as median (IQR). Numbers and percentages of missing data, and their distribution across survivors and non-survivors, are shown in appendix 2 (pp 15–16).
Median duration of invasive mechanical ventilation was 13 days (IQR 7–22). Physiological respiratory and mechanical ventilation variables in the entire cohort on days 1, 3, and 7 are shown in appendix 2 (pp 9–10). Median tidal volume administered was 6·1 mL/kg predicted bodyweight (IQR 6·0–7·0) on day 1, and the value increased significantly up to day 7; applied PEEP levels were intermediate at 10 cm H2O (8–12) on day 1, with a slight but significant decrease to day 7. PaO2/FiO2 was 160 (111–218) on day 1, increased to day 3, and stabilised by day 7. Median respiratory system compliance (36 mL/cm H2O [29–44] on day 1), plateau pressure (23 cm H2O [20–26]), and FiO2 (0·60 [0·45–0·80]) improved slightly but significantly over time; similar trends were noted for blood pH, bicarbonate, and base excess. Hypercapnia was present in all patients from day 1 and remained stable. 1779 (97·4%) of 1827 patients were receiving deep sedation (RASS of –4 or –5 points) on day 1, but this proportion decreased over time.
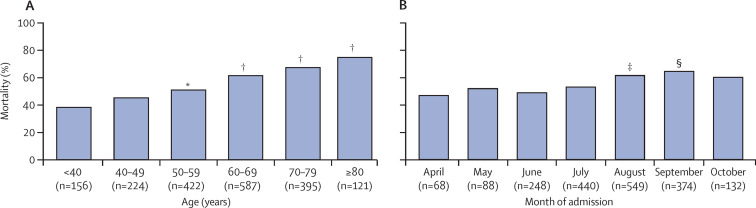
1101 (57·7%) of 1909 patients died in hospital (primary outcome). 28-day mortality was 50·6% (966 of 1909 patients) and ICU mortality was 57·0% (1088 of 1909 patients). Kaplan-Meier survival estimates for the entire group, and according to Charlson score (<2 and ≥2 points) and age category, are shown in appendix 2 (pp 19–21). Mortality increased with age category (figure 2A ) and over the study period, from April to October (except for a small, non-significant decrease observed in June; figure 2B). Specifically, in an exploratory, post-hoc analysis, mortality was significantly higher for patients admitted during the period from August to October, the second half of the study, compared with April to July (figure 2B). However, patient acuity was higher during the first month of the study than in the subsequent months of May to October (appendix 2, p 11).
Figure 2.
All-cause in-hospital mortality according to age category (A) and month of hospital admission (B)
Number of patients in each category included on x axis. *Indicates p=0·007 vs age <40 years. †p<0·0001 vs age <40 years. ‡p=0·018 vs April. §p=0·005 vs April.
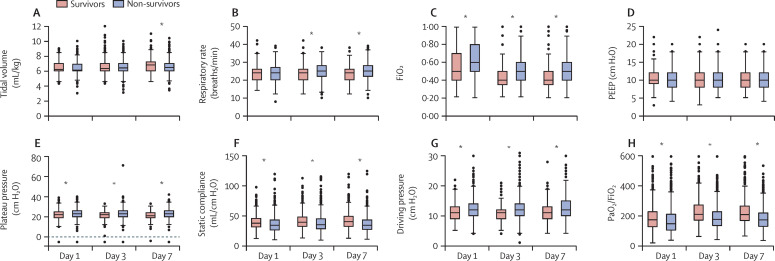
Older age, higher Charlson score, cardiovascular disease, chronic kidney disease, immunosuppression, smoking, arterial hypertension, diabetes, ischaemic heart disease, chronic heart failure, oncohaematological disease, and having received chemotherapy were significantly more common in non-survivors (n=1101) than in survivors (n=808; table 1). Non-survivors were sicker on admission than survivors, with significantly higher APACHE II and SOFA scores, and higher use of vasopressors, and they were more likely to receive invasive mechanical ventilation before ICU admission (table 1). Similarly, most laboratory values differed significantly between non-survivors and survivors at admission—even non-survivors with laboratory variables within the healthy range were still significantly different to survivors (table 2). Differences in physiological respiratory and mechanical ventilation variables between survivors and non-survivors on days 1, 3, and 7 are shown in figure 3 and in appendix 2 (pp 9, 18). Throughout the study period (on days 1, 3, and 7), PaO2/FiO2, blood pH, and base excess were significantly higher, and PaCO2 and lactate concentrations were significantly lower, in survivors than in non-survivors. Tidal volume increased significantly over time in both groups (from day 1 to day 7), although the increase was less in non-survivors than survivors. PEEP levels were similar in both subgroups. At all timepoints, variables for respiratory mechanics, such as respiratory system compliance, plateau pressure, driving pressure, and FiO2, showed significant differences between survivors and non-survivors. The proportion of patients requiring deep sedation decreased in survivors after day 1; it also decreased in non-survivors but to a lesser extent.
Figure 3.
Lung mechanics, mechanical ventilation, and PaO2/FiO2 in survivors and non-survivors, at days 1, 3, and 7
A time-by-group interaction is present for all variables (p<0·001) except for PEEP levels. FiO2=fractional concentration of oxygen in inspired air. PaO2/FiO2=ratio of partial pressure of arterial oxygen to fractional inspired oxygen. PEEP=positive end-expiratory pressure. *Differences between survivors and non-survivors, when present (p<0·01), are given for each timepoint, corrected for multiple comparisons.
Complications in all patients were frequent. ARDS developed in 1672 (87·6%) patients. Prone positioning was used in 1176 (61·6 %) patients, most frequently in non-survivors, who received more sessions (table 3 ). Extracorporeal membrane oxygenation was seldom used (n=1, survivor). Other complications, such as septic shock, acute kidney injury, and requirement for renal replacement therapy were common, while thrombotic episodes developed in only 170 (8·9%) patients; all were significantly more frequent in non-survivors than survivors (table 3). Corticosteroid administration did not differ between subgroups, but its use increased significantly from 576 (68·2%) of 844 patients in the April–July period to 1006 (96·1%) of 1047 patients in the August–October period. Infectious complications were similar in both subgroups. One-quarter of patients underwent tracheostomy, which was more frequent in survivors. Duration of invasive mechanical ventilation, as well as ICU and hospital stays, were prolonged, although shorter in non-survivors (table 3). Refractory hypoxaemia was the single most common cause of mortality in the entire cohort, followed by septic shock and multiorgan dysfunction syndrome, and patients often had more than one cause of death (appendix 2, p 13).
Table 3.
Disease evolution, therapeutic modalities, and complications during ICU stay
| All patients (n=1909) | Survivors (n=808) | Non-survivors (n=1101) | p value | ||
|---|---|---|---|---|---|
| ARDS development | 1672/1909 (87·6%) | 658/808 (81·4%) | 1014/1101 (92·1%) | <0·0001 | |
| Prone positioning | 1176/1909 (61·6%) | 430/808 (53·2%) | 746/1101 (67·8%) | <0·0001 | |
| Number of sessions | 2 (2–4) | 2 (1–4) | 3 (2–4) | 0·041 | |
| Duration of sessions (h) | 24 (21–36) | 24 (20–35) | 24 (22–36) | 0·051 | |
| Septic shock | 1513/1909 (79·3%) | 539/808 (63·7%) | 974/1101 (88·5%) | <0·0001 | |
| Acute kidney injury | 997/1909 (52·2%) | 272/808 (32·7%) | 725/1101 (65·8%) | <0·0001 | |
| Renal replacement therapy | 373/1909 (19·5%) | 91/808 (11·3%) | 282/1101 (25·6%) | <0·0001 | |
| Ventilator-associated pneumonia | 617/1909 (32·3%) | 267/808 (33·0%) | 350/1101 (31·8%) | 0·54 | |
| Bacteraemia (all microorganisms) | 446/1909 (23·4%) | 192/808 (23·8%) | 254/1101 (23·1%) | 0·72 | |
| Bacteraemia (Gram-negative bacilli) | 191/1909 (10·0%) | 81/808 (10·0%) | 110/1101 (10·0%) | 0·98 | |
| Maximum fever (°C) | 38·5 (38·0–39·0) | 38·5 (38·0–39·0) | 38·6 (38·0–39·0) | 0·20 | |
| Maximum fever ≥39°C | 419/1832 (22·9%) | 162/599 (32·1%) | 257/783 (32·8%) | 0.02 | |
| Thromboembolic complications | 170/1909 (8·9%) | 57/808 (7·1%) | 113/1101 (10·3%) | 0·02 | |
| Deep vein thrombosis | 57/1909 (3·0%) | 28/808 (3·4%) | 29/1101 (2·6%) | 0·29 | |
| Pulmonary embolism | 62/1909 (3·2%) | 24/808 (3·0%) | 38/1101 (3·5%) | 0·25 | |
| Ischaemic stroke | 17/1909 (0·9%) | 3/808 (1·0%) | 14/1101 (1·3%) | 0·03 | |
| Ischaemia of the extremities | 14/1909 (0·7%) | 2/808 (0·2%) | 12/1101 (1·1%) | 0·03 | |
| Dexamethasone use | 1612/1909 (84·4%) | 662/800 (82·8%) | 950/1101 (86·3%) | 0·09 | |
| Convalescent plasma use | 605/1909 (31·7%) | 305/808 (37·8%) | 300/1101 (27·3%) | <0·0001 | |
| Tracheostomy | 464/1909 (24·3%) | 293/808 (36·2%) | 171/1101 (15·5%) | <0·0001 | |
| Length of invasive mechanical ventilation (days) | 13 (7–22) | 14 (8–28) | 11 (6–19) | <0·0001 | |
| Length of ICU stay (days) | 16 (10–27) | 23 (14–37) | 13 (8–21) | <0·0001 | |
| Length of hospital stay (days) | 22 (13–35) | 34 (23–51) | 16 (10–24) | <0·0001 | |
Data are n/N (%) or median (IQR). Numbers and percentages of missing data, and their distribution across survivors and non-survivors, are shown in appendix 2 (pp 15–16). ARDS=acute respiratory distress syndrome. ICU=intensive care unit.
Cox regression identified age (hazard ratio [HR] 1·02 [95% CI 1·01–1·03]), Charlson score (1·16 [1·11–1·23]), endotracheal intubation outside the ICU (1·37 [1·10–1·71]), vasopressor use on day 1 (1·29 [1·07–1·55]), D-dimer concentration (1·02 [1·01–1·03]), PaO2/FiO2on day 1 (0·998 [0·997–0·999]), arterial pH on day 1 (1·01 [1·00–1·01]), driving pressure on day 1 (1·05 [1·03–1·08]), acute kidney injury (1·66 [1·36–2·03]), and month of admission (1·10 [1·03–1·18]) as independent predictors of mortality (appendix 2, p 14).
Discussion
SATICOVID was a large, prospective cohort study of 1909 patients with COVID-19 requiring invasive mechanical ventilation and ICU admission in Argentina, an upper middle-income country. Patients were predominantly older and male, had symptoms for a median of 5 days before admission to hospital, and stayed a median of 1 day in hospital before being admitted to the ICU. 11·4% (217 of 1909 patients) of patients had not improved on HFNC or NIV, and 22·2% underwent endotracheal intubation outside the ICU.
Overall in-hospital mortality was high, at 57·7%. High mortality for invasively ventilated patients has been seen in studies from China (49%), Lombardy, Italy (53%), and Germany (55%).7, 9, 13, 14 Patients in the German study7 were about 6 years older than those in our study, while patients from Italy were of a similar age to ours (63 years);9 in the Chinese study,13 the median age of critical cases was not shown. However, in patients 70 years and older, mortality was similar in Germany and in our study; for example, in patients aged 70–79 years, mortality was 63% in the German study7 and 68% in ours, and in those aged 80 years and older, mortality was 72% and 75%, respectively. Conversely, other cohorts have shown lower mortality rates in patients receiving invasive mechanical ventilation, such as 28% in New York, 31% in France, 32% in Spain, 35% in the Netherlands, and 43% in the UK.12, 15, 16, 17, 18 In two large, retrospective population studies in Mexico and Brazil, in-hospital mortality of patients with COVID-19 on mechanical ventilation was high (76% and 80%, respectively).5, 6 These differences mirror findings in the ICON and LUNG SAFE studies, in which LMICs showed higher mortality for sepsis and ARDS than did high-income countries.19, 20 Complex economic and organisational factors in LMICs explain worse outcomes for ICU patients. Deep inequities, defined as systematic, unjust, and preventable differences in determinants of health, such as socioeconomic status, demographics, and geography, might generate differences in access to health services in different population subgroups, which affect health-related outcomes.3 Furthermore, in LMICs, health systems are usually fragmented in public, private, and social security sectors, which maintain the differences according to socioeconomic status and affect the provision of health care, particularly critical care.21
Identifying independent determinants of prognosis in critically ill, mechanically ventilated patients with COVID-19 is key to optimising use of ICU resources. As in other cohorts of patients with COVID-19, increasing age was an independent predictor of mortality. Risk factors for mortality were similar to those identified in other studies, in general, but the presence of at least one comorbidity in 92% of our patients is, to our knowledge, the highest recorded.5, 6, 9, 12, 18 Obesity was highly prevalent in our cohort (44·4%) but, surprisingly, it was not associated with increased mortality, as reported in other studies.22 However, another study found that mortality did not differ between BMI categories.23 Other conditions, such as arterial hypertension, diabetes, chronic kidney failure, cardiovascular disease, and immunosuppression were more frequent in non-survivors. No single comorbidity was independently associated with mortality in the Cox regression analysis (data not shown), but Charlson score, a validated comorbidity index, was independently associated with mortality.
Although the effect of older age and pre-existing conditions on mortality is clear, it is worth noting that other factors, such as profound physiological derangements on day 1 (eg, decreased oxygenation, increased driving pressure, requirement for vasopressors, acidosis, activation of coagulation), intubation outside the ICU, and admission during the second half of the study (August–October), were also independently associated with mortality.
Intubation of patients outside the ICU might reflect severity of hypoxaemia on admission to hospital or rapid deterioration on the general ward. It might also indicate insufficient number of ICU beds; however, patients were on invasive mechanical ventilation outside the ICU for only a short duration before ICU admission. Conversely, intensivists' experience with timely management of severe respiratory failure might have contributed to decreased mortality in patients intubated in the ICU compared with those intubated before ICU admission.
The reduction in PaO2/FiO2 ratio was similar to that seen in studies of patients with COVID-19 from Italy, France, Spain, and the Netherlands.9, 12, 16, 17 As in these reports, compliance with lung-protective ventilation was high. Tidal volumes used were between 6·1 and 6·5 mL/kg predicted bodyweight, except in survivors at day 7; plateau pressures were less than 30 cm H2O, and driving pressures were less than 15 cm H2O at day 1, 3, and 7.9, 12, 16, 17 FiO2 remained between 0·45 and 0·60; however, PEEP values (approximately 10 cm H2O) were slightly lower than those previously reported for patients with COVID-19.9, 12, 16, 17 A similar use of protective mechanical ventilation has been reported from Asian middle-income countries.24 In accordance with existing information for ARDS not related to COVID-19,25 driving pressure was strongly associated with mortality, which is a novel finding.
Oxygenation and mechanical ventilation variables differed consistently between survivors and non-survivors over time. Of note, tidal volume and PEEP were similar in both subgroups at all time points (with the exception of tidal volume at day 7). Since refractory hypoxaemia was the most common cause of death, there is probably some room for improvement in the FiO2 and PEEP settings used for patients. Prone positioning, which is associated with better outcomes in ARDS,26 was used in most patients, as in other COVID-19 studies.9, 15, 27 This practice was more frequent in non-survivors, probably reflecting its application in the case of the most severely affected patients.
With respect to haemodynamic alterations, our study highlights the relevance of cardiovascular dysfunction. The requirement of vasopressors by patients on day 1 was frequent and was independently associated with hospital mortality, even with lactate levels of 2·0 mmol/L or less. Accordingly, reports have identified cardiovascular SOFA score and the lowest systolic blood pressure recorded for a patient as predictors of mortality.12, 28
Renal dysfunction in COVID-19 is common and can occur via various mechanisms, but its development in patients on invasive mechanical ventilation implies a worse prognosis.29, 30 In our study, blood urea nitrogen and serum creatinine concentrations were already significantly different between survivors and non-survivors on admission at day 1, and acute kidney injury during the ICU stay was a strong, independent predictor of mortality. The activation of both thrombotic and fibrinolytic pathways, reflected by increased D-dimer values in patients admitted to hospital with COVID-19, was reported early in the pandemic and has been independently associated with mortality.31 Our study confirms these findings, which are also in line with the findings of a meta-analysis.32
The month of hospital admission was independently associated with mortality, but whereas mortality was reported to improve over time in France and the UK, we found that mortality was higher among patients admitted in the later months compared with April, in Argentina.12, 18 This increase in mortality cannot be ascribed to differences in age or in the severity of disease, because patients were more severely ill on admission to hospital during the first month of the pandemic. Nor can it be attributed to low adherence to the only therapeutic measure proven to be effective (dexamethasone); on the contrary, administration of corticosteroids increased after RECOVERY trial results were published in July, 2020.11 We believe that the increase in mortality over time might reflect the profound stress placed on the health system by the pandemic, counteracting the benefits of learning related to the management of COVID-19 over the study period. In Argentina, ICU beds, ventilators, and personal protective equipment were widely available in periods of increased ICU demand due to timely acquisition and distribution by the government and by private and non-profit organisations. However, ICU personnel became scarce. The number of intensivists was already low before the pandemic, and many contracted COVID-19 or even died as the peak of cases approached.33 Although the health system was not overwhelmed in terms of insufficient equipment, denial of care, or a lack of beds, lower quality of care might have occurred because of the high and sustained burden on health-care personnel. Evidence for an effect of increased ICU strain on health-care workers on mortality has been reported in a retrospective analysis of 8516 patients with COVID-19 admitted to 88 US Veterans Affairs hospitals.34 Patients who were treated during periods of peak ICU demand had nearly twice the risk of mortality compared with patients treated during periods of low demand. Moreover, the duration of mechanical ventilation in the ICU and ICU and hospital stays were prolonged over the course of the study, as described in other cohorts, which certainly contributed to the burden on the health-care system.6, 12, 17
This study has several strengths. It was conducted prospectively, and it is one of the largest cohorts of patients with COVID-19 requiring invasive mechanical ventilation. It provides a comprehensive evaluation of risk factors, markers of disease severity, patterns of change in respiratory variables, use of lung-protective strategies, complications, causes of death, and prognostic factors. It is, to our knowledge, the first exhaustive Latin American study in a setting of scarce information about the most severely affected patients with COVID-19 in LMICs. RT-PCR testing is standardised in Argentina, which makes diagnosis homogeneous. All patients in the study completed the course of disease to death or hospital discharge.
Nevertheless, this study has some limitations. First, since participation in the study was voluntary, ICUs with higher or lower mortality might be under-represented, and the final figure we report for in-hospital mortality might be different to that of unselected, nationwide cohorts in Latin America. Second, admission policies and patient management might have differed between the centres in our study. Third, non-ventilated patients with COVID-19 in the ICU were not included, so the full spectrum of disease was not characterised. Fourth, notwithstanding the prospective nature of the study, some variables have missing data due to the high burden of work during the pandemic and the scarce time personnel had available to collect data (as reported in other studies). Nevertheless, in the case of most variables, data were missing for less than 5% of cases. Exceptions were D-dimer, lactate, and ferritin concentrations due to lack of laboratory capacity for their measurement in some centres. Fifth, to minimise the workload for health-care workers in the ICU, data registration beyond the date of admission to the ICU (ie, day 1), or beyond day 7 for ventilation management, was not performed. Therefore, we cannot exclude an effect of these unrecorded variables on mortality. Sixth, five centres recruited fewer than five patients and some patients with COVID-19 might have been missed due to the lack of personnel. Finally, data collected in Argentina might not be representative of other LMICs and other regions.
To conclude, in SATICOVID, in-hospital mortality was high in patients with COVID-19 requiring invasive mechanical ventilation. Pre-existing conditions, such as age and Charlson index, together with physiological impairments (alterations in oxygenation, presence of hypotension, acidosis, acute kidney injury, and activation of coagulation) and mechanical ventilation variables, were independent predictors of in-hospital mortality. Thus, signs of early organ dysfunction (ie, alterations in oxygenation, presence of hypotension, acidosis, acute kidney injury, and activation of coagulation) appear to be a prognostic factor in severe COVID-19. We also found a paradoxical increase in mortality throughout the first wave of the pandemic, possibly reflecting increasing strain on the health-care system. Long duration of mechanical ventilation and prolonged ICU stay contributed to the pressure on ICU capacity. We believe that the information provided here will help to improve health-care management in the second wave of the pandemic and beyond.
Data sharing
De-identified individual participant data that underlie the results reported in this Article (text, tables, figures, and appendices), data dictionaries, and study protocol will be available from 9 months to 36 months after Article publication to researchers who provide a methodologically sound proposal, for any purpose of analysis. Proposals should be directed to estenssoro.elisa@gmail.com; to gain access, data requestors will need to sign a data access agreement.
Declaration of interests
The authors declare no competing interests.
Contributors
EE, GP, RR, FGR, and VSKE conceived and designed the study. EE, CIL, and AD analysed the data. EE and AD drafted the manuscript. FGR, GP, and VSKE were in charge of the project administration. AD designed the figures. EE, AD, and CIL verified the data. EE, GP, RR, FGR, VSKE, CIL, AD, MA, IR, DP, MB, VM, CG, ST, CO, PNRB, MFV, EC, MGS, NT, and VA contributed to the acquisition and interpretation of data. All authors had full access to all the data and had responsibility for the decision to submit for publication. All authors have seen and approved the final version of the manuscript.
Contributor Information
SATI-COVID-19 Study Group:
E Estenssoro, A Dubin, C I Loudet, F Ríos, V S Kanoore Edul, G Plotnikow, R Reina, M Andrian, J Ivacachi, I Romero, C Garay, D Piezny, J Sagardía, M Bezzi, S Borello, V Mandich, D Chiacchiara, C Groer, C García Almirón, A Kovac, S Torres, C Cesio, C Orlandi, R Hernández, P N Rubatto Birri, M Mugno, M F Valenti, R A Gómez, E Cunto, V Chediack, M G Sáenz, C Marchena, N Tiribelli, M Guaymas, V Aphalo, D Vázquez, Y Saad, D Sánchez, F Iglesias, P Casteluccio, B Lattanzio, S Eiguren, D Noval, S Fredes, G C Izzo, H Cabrera, M O Pozo, S Sac, N Tornatore, J Sakugawa, C Villafañe, A Di Sibio, P Maskin, P Rodríguez, N Nihany, M Mogadouro, F Pálizas (h), E Cornú, M Esperatti, J M Pintos, G Badariotti, G Echevarría, A M Mazzola, C Giuggia, N Dargains, A Turano, F Pugliese, M J Zec Baskarad, M Chamadoira, J C Medina, M Búsico, F Villarejo, H Collazos, T Huanca, J C Pendino, L Talamonti, F Skrzypiec, C Tascón, G Genovese, H Alul, A Zavattieri, A J Herrera, N Rosales, M G Quintana, A Risso Vazquez, M Lugaro, E Díaz Rousseaux, M Falcone, F Kurban, M Cini, G Zakalik, C Pellegrini, G Fernández, J P Sottile, S Barrios, O Hamada, V Mendiluce, D Villalba, F Sacco, V Mezzina, C Servin, M Quinteros, H Nuñez, M L Campassi, D Banegas, C Balasini, V Leiva, F Maicol, G Domeniconi, V Vilaseca, A Barrientos, F Larocca, L Kumar, R Luna, M Deheza Lonardi, A Oholeguy, J Carnero Echegaray, C Marazzi, P Helca Regis, F Rópolo, A Bobadilla, V Thomas, N Funes Nelson, C Villavicencio, P Machare, N Aramayo, C González, M Ferriccioni, and J Bergesio
Supplementary Materials
References
- 1.WHO WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/
- 2.Kirby T. South America prepares for the impact of COVID-19. Lancet Respir Med. 2020;8:551–552. doi: 10.1016/S2213-2600(20)30218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estenssoro E, Loudet CI, Edul VSK. Health inequities in the diagnosis and outcome of sepsis in Argentina: a prospective cohort study. Crit Care. 2019;23:250. doi: 10.1186/s13054-019-2522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Lancet COVID-19 in Latin America: a humanitarian crisis. Lancet. 2020;396 doi: 10.1016/S0140-6736(20)32328-X. [DOI] [PubMed] [Google Scholar]
- 5.Ñamendys-Silva SA, Gutiérrez-Villaseñor A, Romero-González JP. Hospital mortality in mechanically ventilated COVID-19 patients in Mexico. Intensive Care Med. 2020;46:2086–2088. doi: 10.1007/s00134-020-06256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranzani OT, Bastos LSL, Gelli JGM. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021:407–418. doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karagiannidis C, Mostert C, Hentschker C. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estenssoro E, Ríos FG, Apezteguía C. Pandemic 2009 influenza A in Argentina: a study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. 2010;182:41–48. doi: 10.1164/201001-0037OC. [DOI] [PubMed] [Google Scholar]
- 9.Grasselli G, Zangrillo A, Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranieri VM, Rubenfeld GD, Thompson BT. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 11.Horby P, Lim WS, Emberson JR. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 14.Grasselli G, Greco M, Zanella A. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson S, Hirsch JS, Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46:2200–2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botta M, Tsonas AM, Pillay J. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med. 2021;9:139–148. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doidge JC, Gould DW, Ferrando-Vivas P. Trends in intensive care for patients with COVID-19 in England, Wales and Northern Ireland. Am J Respir Crit Care Med. 2021;203:565–574. doi: 10.1164/rccm.202008-3212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent JL, Marshall JC, Namendys-Silva SA. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 20.Laffey JG, Madotto F, Bellani G. Geo-economic variations in epidemiology, patterns of care, and outcomes in patients with acute respiratory distress syndrome: insights from the LUNG SAFE prospective cohort study. Lancet Respir Med. 2017;5:627–638. doi: 10.1016/S2213-2600(17)30213-8. [DOI] [PubMed] [Google Scholar]
- 21.Estenssoro E, Alegria L, Murias G. Organizational issues, structure and processes of care in 257 ICUs in Latin America: a study of the Latin America Intensive Care Network. Crit Care Med. 2017;45:1325–1336. doi: 10.1097/CCM.0000000000002413. [DOI] [PubMed] [Google Scholar]
- 22.Popkin BM, Du S, Green WD. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21 doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schavemaker R, Schultz MJ, Lagrand WK, van Slobbe-Bijlsma ER, Serpa Neto A, Paulus F. Associations of body mass index with ventilation management and clinical outcomes in invasively ventilated patients with ARDS related to COVID-19-insights from the PRoVENT-COVID Study. J Clin Med. 2021;10 doi: 10.3390/jcm10061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pisani L, Algera AG, Serpa Neto A. Epidemiological characteristics, ventilator management, and clinical outcome in patients receiving invasive ventilation in intensive care units from 10 Asian middle-income countries (PRoVENT-iMiC): an international, multicenter, prospective study. Am J Trop Med Hyg. 2021;104:1022–1033. doi: 10.4269/ajtmh.20-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellani G, Laffey JG, Pham T. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 26.Guérin C, Reignier J, Richard JC. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 27.Ferrando-Vivas P, Doidge J, Thomas K. Prognostic factors for 30-day mortality in critically ill patients with coronavirus disease 2019: an observational cohort study. Crit Care Med. 2021;49:102–111. doi: 10.1097/CCM.0000000000004740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez-Solem E, Petersen TS, Hansen C. Developing and validating COVID-19 adverse outcome risk prediction models from a bi-national European cohort of 5594 patients. Sci Rep. 2021;11 doi: 10.1038/s41598-021-81844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng Y, Luo R, Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaibi K, Dao M, Pham T. Severe acute kidney injury in patients with COVID-19 and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202:1299–1301. doi: 10.1164/rccm.202005-1524LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gungor B, Atici A, Baycan OF. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: a systematic review and meta-analysis. Am J Emerg Med. 2021;39:173–179. doi: 10.1016/j.ajem.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ministry of Health of Argentina Analysis of health-care personnel affected by COVID-19. https://www.argentina.gob.ar/noticias/analisis-de-la-situacion-del-personal-de-salud-afectado-por-covid-19
- 34.Bravata DM, Perkins AJ, Myers LJ. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs Hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.34266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual participant data that underlie the results reported in this Article (text, tables, figures, and appendices), data dictionaries, and study protocol will be available from 9 months to 36 months after Article publication to researchers who provide a methodologically sound proposal, for any purpose of analysis. Proposals should be directed to estenssoro.elisa@gmail.com; to gain access, data requestors will need to sign a data access agreement.