Abstract
Background
Haematological malignancies and their treatments are likely to affect SARS-CoV-2 vaccine efficacy. We aimed to evaluate serological response to BNT162b2 vaccine in patients with haematological malignancies by type of treatment.
Methods
Our national prospective cohort study was done in Lithuania and assessed serological response to one and two BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine doses in healthy health-care workers and in patients with haematological malignancies. Eligible participants were aged 18 years or older, had received both vaccine doses, and had available biobanked blood samples from before vaccination and after the second dose. Biobanked samples and health data were obtained from Vilnius University Hospital Santaros Klinikos Biobank. Abbott Architect SARS-CoV-2 IgG Quant II chemiluminescent microparticle assay was used to quantify serum anti-SARS-CoV-2-S1 IgG antibody (anti-S1 IgG antibody) concentrations 0–10 days before the first BNT162b2 vaccine, on the day of second immunisation (around day 21), and 7 to 21 days after the second immunisation. Adverse events were assessed by a standardised questionnaire. Breakthrough infections were characterised clinically and by SARS-CoV-2 genotyping whenever possible. This study is registered with ClinicalTrials.gov, NCT04871165.
Findings
Between Jan 8 and April 21, 2021, 885 participants with haematological malignancies were included in the study. 857 patients were anti-S1 IgG seronegative at timepoint 0 and constituted the main analysis cohort. The age-matched comparison was made between 315 patients with haematological malignancies who were aged 18–60 years and 67 healthy health-care workers in the same age group. Patients aged 18–60 years with haematological malignancies had lower median anti-S1 IgG antibody responses after two BNT162b2 vaccine doses than did health-care workers of the same age group (median 6961 AU/mL [IQR 1292–20 672] vs 21 395 AU/mL [14 831–33 553]; p<0·0001). Compared with untreated patients with haematological malignancies (n=53; median 5761 AU/mL [629–16 141]), patients actively treated with Bruton tyrosine kinase inhibitors (BTKIs; n=44; 0 AU/mL [0–7]; p<0·0001), ruxolitinib (n=16; 10 AU/mL [0–45]; p<0·0001), venetoclax (n=10; 4 AU/mL [0–1218]; p=0·0005), or anti-CD20 antibody therapy (n=87; 17 AU/mL [1–2319]; p<0·0001) showed particularly poor anti-S1 IgG antibody responses following two BNT162b2 doses. Patients being treated with tyrosine kinase inhibitors (n=41; 10 537 AU/mL [IQR 2335–19 388]) or patients who received autologous haematopoietic stem-cell transplantation (HSCT; n=192; 6203 AU/mL [1451–16 834]) or allogeneic HSCT (n=122; 6304 AU/mL [1120–16 913]) were among the subgroups with the highest numerical responses. Nine SARS-CoV-2 infections and three COVID-19 deaths were observed among fully vaccinated patients with haematological malignancies.
Interpretation
Patients with haematological malignancies mount blunted and heterogeneous antibody responses to the full course of BNT162b2 mRNA vaccination. Patients who are actively treated with BTKIs, ruxolitinib, venetoclax, or anti-CD20 antibody therapies seem to be the most negatively affected and might be left unprotected from SARS-CoV-2 infection. Breakthrough severe SARS-CoV-2 infections in fully vaccinated patients with haematological malignancies emphasise the importance of ongoing strict adherence to non-pharmacological interventions and household vaccination while SARS-CoV-2 is circulating in the community.
Funding
Vilnius University Hospital Santaros Klinikos.
Translation
For the Lithuanian translation of the abstract see Supplementary Materials section.
Introduction
Patients with haematological malignancies have a very high COVID-19 case fatality rate, reaching as high as 48% in some cohorts.1, 2, 3 Therefore, protecting this group of people from COVID-19 is of particular importance. In clinical trials, vaccines against SARS-CoV-2 have been shown to induce efficient antibody and T-cell responses and to protect from symptomatic COVID-19 disease.4, 5 Immunological response to these vaccines might be reduced by the immunosuppressive nature of haematological malignancies themselves and their treatments. However, patients with active or recently treated haematological malignancies were not included in these pivotal trials, and vaccine efficacy in this clinically vulnerable patient group remains poorly characterised. We report an assessment of antibody response after one and two BNT162b2 vaccine doses by the type of treatment and latency from treatment in patients with different haematological malignancies as well as clinical outcomes of breakthrough infections.
Research in context.
Evidence before this study
We searched PubMed with the terms (“COVID-19” OR “SARS-CoV-2”) AND (“cancer” or “malignancy”) AND (“vaccination” OR “immunisation”) for articles published in English between Dec 1, 2020, and May 25, 2021. The search retrieved five peer-reviewed studies reporting immune responses to vaccination in patients with haematological malignancies. Two studies examined the efficacy of mRNA vaccines in patients with chronic lymphocytic leukaemia and suggested that patients treated with Bruton tyrosine kinase inhibitors (BTKIs) or anti-CD20 antibody therapies rarely seroconvert after full vaccination. One study focused on patients with multiple myeloma and showed that 56% of patients seroconvert after a single dose of ChAdOx1 or BNT162b2 vaccine, which correlated with quiescent disease, good baseline immunoglobulin levels, and being off treatment. One early report showed that serological responses after one BNT162b2 vaccine dose in patients with haematological malignancies were lower than in healthy individuals or patients with solid cancers. T-cell responses were detected in nine of 18 assessed patients with haematological malignancies. One cohort of 21 patients with myeloproliferative neoplasms showed qualitative antibody responses in 75% of patients and T-cell responses in 80%. None of the studies reported clinical outcomes.
Added value of this study
We report quantitative serological responses and early clinical outcomes in a cohort of 885 patients with haematological malignancies, including patients who have received a haematopoietic stem-cell transplantation (HSCT) and one and two doses of BNT162b2 mRNA vaccine. Our study stratifies patients by current or most recent treatment, treatment latency, and anti-SARS-CoV-2-S1 IgG antibody serological status before the first vaccine dose. We show that patients with haematological malignancies mount severely dampened antibody responses compared with healthy individuals. In addition to BTKI and anti-CD20 antibody therapies, our findings suggest that ruxolitinib and venetoclax treatments are associated with almost absent antibody responses. Patients on hydroxycarbamide for myeloproliferative neoplasms and patients on immunomodulators, proteosome inhibitors, or both mount poorer antibody responses than do untreated patients with haematological malignancies. We show that patients on tyrosine kinase treatments or having received an HSCT or systemic chemotherapy more than 6 months before the first dose of vaccine can expect good antibody responses to BNT162b2. Previously seroconverted patients with a haematological malignancy elicit a potent antibody response to one dose of BNT162b2. Finally, our study suggests that fully vaccinated patients with haematological malignancies can develop severe and often fatal COVID-19 disease, even with a SARS-CoV-2 strain not associated with substantial immune evasion.
Implications of all the available evidence
Our study suggests that in seronegative patients with haematological malignancies, the second dose of SARS-CoV-2 vaccination should not be delayed. Lower overall serological response and severe breakthrough infections among patients with haematological malignancies calls for continuous use of non-vaccine protection in these patients, including personal protection, periodic pre-emptive testing, and household vaccination programmes, as well as novel pharmacological pre-emptive postexposure interventions when these become available. These measures particularly apply to patients treated with BTKIs, venetoclax, ruxolitinib, or anti-CD20 antibody therapies. Patients without active treatment for more than 6 months after HSCT or systemic chemotherapy, excluding anti-CD20 antibody therapies, might develop a protective immune response with two vaccine doses 3 weeks apart. Observed vigorous antibody responses to a single dose of vaccine in previously seroconverted individuals might inform serological testing and booster campaigns for patients with haematological malignancies in the later stage of the pandemic.
Methods
Study design and participants
This national Lithuanian prospective cohort study evaluated the humoral response to BNT162b2 vaccine in patients with haematological malignancies and compared it with the response in healthy health-care workers. In Lithuania, the adult patient population with haematological malignancies were prioritised for early vaccination as per the Lithuanian Government COVID-19 vaccination strategy. Study participants had been vaccinated according to the vaccination schedule specified in the BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine summary of product characteristics.6
Study inclusion criteria for the cohort with haematological malignancies were current or past diagnosis of a haematological malignancy regardless of treatment status; signed written, informed consent for biobanking as well as study-specific written, informed consent; aged 18 years and older; having received both BNT162b2 vaccine doses; and having available biobanked blood samples from before vaccination and after the second BNT162b2 vaccine dose. Health-care workers with written, informed consent for study participation were recruited from a single centre, Vilnius University Hospital Santaros Klinikos (Vilnius, Lithuania), and constituted a healthy control group. The inclusion criteria for the control group were the same as for the patient group, other than the diagnosis of a haematological malignancy. Participants with positive anti-SARS-CoV-2 antibody status at baseline were included in the study but were analysed separately.
The biological samples and health data were obtained from Vilnius University Hospital Santaros Klinikos Biobank (Vilnius, Lithuania). The data collection cutoff date was May 17, 2021. The study was approved by Vilnius Regional Bioethics Committee (approval number 2021/3-1331-803).
Procedures
We compared serological responses between the two cohorts, and between subgroups within the haematological malignancy cohort, to examine the effect of haematological malignancies and treatment on response to two doses of BNT162b2 vaccine. The primary endpoint was SARS-CoV-2 spike protein subunit 1 binding IgG antibody (anti-S1 IgG antibody) concentration at timepoint 2 (7–21 days after the second vaccine dose) and timepoint 1 (on the day of administration of the second dose), which were compared with timepoint 0 (up to 10 days before the administration of the first vaccine dose). Antibody responses in participants with haematological malignancies (stratified by current or most recent treatment) were tested at timepoint 0, timepoint 1, and timepoint 2, whereas responses in health-care workers were tested at timepoint 0 and timepoint 2 only. Full blood counts for participants with haematological malignancies were evaluated at timepoint 0.
We used Abbott Architect SARS-CoV-2 IgG Quant II chemiluminescent microparticle immunoassay (Abbott, Sligo, Ireland) to detect IgG antibodies to the receptor binding domain of the S1 subunit of the spike protein of SARS-CoV-2 as per the manufacturer's instructions. Anti-S1 IgG antibody concentrations are shown as absolute or log10 converted values in arbitrary units per mL (AU/mL) at all timepoints. The analytical measurement range of the assay was 0−40 000 AU/mL and within the laboratory, coefficient of variation was reported to be between 4·2% and 5·1%.7 Per the manufacturer's instructions, a threshold of anti-S1 IgG antibody concentrations more than 50 AU/mL was used to classify participants as seropositive against SARS-CoV-2 spike protein. Abbott SARS-CoV-2 IgG chemiluminescent microparticle immunoassay (Abbott, Libertyville Township, IL, USA; sensitivity 93·9% [95% CI 86·3–98·0]) was used for qualitative detection of SARS-CoV-2 anti-nucleocapsid IgG antibodies (anti-N IgG antibodies) in participants with seroconversion to spike protein at timepoint 0.8 Participants with positive anti-S1 IgG at timepoint 0 were classified as anti-S1 seropositive before vaccination. They were further subdivided into anti-N seropositive and anti-N seronegative. Anti-N IgG antibodies are known to have a shorter half-life than anti-S1 IgG antibodies, therefore individuals who were anti-S1 IgG-positive but anti-N IgG-negative might have had an older exposure to SARS-CoV-2 (preprint).9 Anti-S1 IgG antibody concentrations were compared between timepoint 1 and timepoint 2 within seropositive and seronegative groups.
Between May 15 and May 17, 2021, the electronic medical records of participants with haematological malignancies were reviewed for evidence of positive SARS-CoV-2 PCR test results since vaccination, and all PCR confirmed SARS-CoV-2 infections since the first dose of vaccination were classified as breakthrough infections. For patients with breakthrough infections, demographics (age, gender), haematological malignancy and treatment, comorbidities, SARS-CoV-2 cycle threshold values, COVID-19 severity and treatment, disease course details, and outcomes were collected. In some centres, all SARS-CoV-2 positive samples had PCR-based screening for the spike protein-encoding gene mutations of interest: 23063A→U (Asn501Tyr) and 23012G→A (Glu484Lys) mutations, and the deletion of codons 69 and 70. All vaccinated patients were asked to complete an adverse event questionnaire after the first and second immunisations, which we adapted from the original phase 3 BNT162b2 trial.5 Adverse event grading is shown in appendix 2 (p 12).
Statistical analysis
Study sample size was not based on statistical hypothesis testing. Here we report an early interim analysis of the data. To obtain a broader qualitative picture of immunological responses and their persistence in this diverse group of patients, over 3 years the study aims to recruit at least 1200 patients with haematological malignancies who received COVID-19 vaccines. Patient recruitment is ongoing and in the future we plan to report results of longer follow-up of the expanded patient cohort.
Anti-S1 IgG antibody titres, medians, IQRs, and minimal–maximal value ranges are shown. Antibody titres had non-normal distribution and frequent outliers, therefore comparisons between groups were done and reported using the Mood's median test. To assess the effect of different therapies on anti-S1 IgG antibody responses after two doses of BNT162b2 vaccine, the group of untreated patients with haematological malignancies was chosen as a control group and treatment groups with at least five patients were included. Patients with haematological malignancies who had never received specific treatment for the haematological malignancy were assigned to the untreated group. We also compared responses between patients receiving active treatment at the time of vaccination, and patients receiving their last treatment less than 6 months before vaccination, 6–12 months before vaccination, and 12 months or longer before vaccination.
The statistical significance of all comparisons was additionally confirmed with the Mann-Whitney U test, when each of the two groups had 20 or more data points, or the Kruskal-Wallis test in the remaining instances. We did paired sample comparisons using the Wilcoxon test.
We applied Spearman's correlation to assess the relationship between anti-S1 IgG antibody concentrations and age, sex, and white blood cell counts. A p value of less than 0·05 was regarded as statistically significant. Statistical analyses were done using Python 3.7.6 with NumPy 1.19.1, Pandas 1.1.2, and SciPy 1.4.1 packages. This study is registered with ClinicalTrials.gov, NCT04871165.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
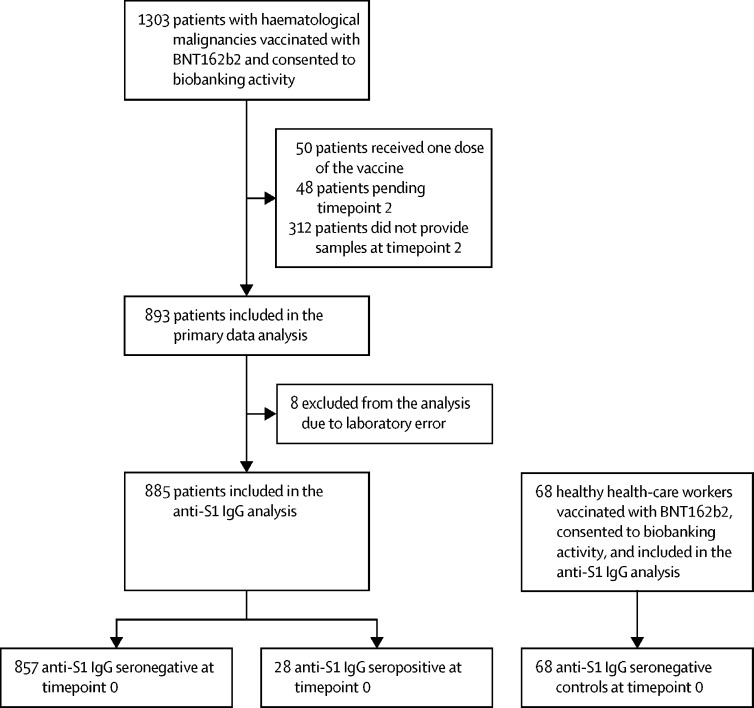
1303 patients with haematological malignancies who were vaccinated with BNT162b2 consented to biobanking activity, 885 of whom received both vaccine doses between Jan 8 and April 21, 2021, and were included in the final analysis (figure 1 ). 68 health-care workers received both BNT162b2 vaccine doses in the same period, consented to biobanking activity, and were also included in the final analysis. 857 patients with haematological malignancies who were seronegative for anti-S1 IgG antibodies at timepoint 0 constituted the main analysis cohort. Data from 28 patients with haematological malignancies who were seropositive at timepoint 0, indicating previous SARS-CoV-2 exposure, were analysed separately. All participants were White European, except one patient of East Asian descent. Median interval between two vaccine doses was 21 days (IQR 21–21). One individual received the second dose on day 17, one received the second dose on day 20, and 112 participants received their second dose between day 22 and 37. The median age of the seronegative patients with a haematological malignancy was 65 years (IQR 54–72) and this varied by both treatment and underlying disease (table ). The median age of healthy health-care workers was 40 years (IQR 32–53). Detailed characteristics of the study participants and their treatments are shown in the table and appendix 2 pp 5–8.
Figure 1.
Study flow-chart
Table.
Baseline characteristics by current or most recent treatment
| N | Female | Male | Age, years | Receiving treatment at the time of vaccination | Last treatment less than 6 months ago | Last treatment 6–12 months ago | Last treatment more than 12 months ago | |
|---|---|---|---|---|---|---|---|---|
| Healthy health-care workers | 68 | 56 (82%) | 12 (18%) | 40 (32–53) | 0 | 0 | 0 | 0 |
| Haematological malignancy and anti-S1 IgG seronegative at timepoint 0 | 857 | 453 (53%) | 404 (47%) | 65 (54–72) | 344 (40%) | 47 (5%) | 62 (7%) | 351 (41%) |
| Autologous HSCT | 192 | 105 (55%) | 87 (45%) | 63 (54–69) | 0 | 7 (4%) | 20 (10%) | 165 (86%) |
| Allogeneic HSCT | 122 | 61 (50%) | 61 (50%) | 55 (43–65) | 0 | 5 (4%) | 13 (11%) | 104 (85%) |
| Myeloablative conditioning | 48 | 25 (52%) | 23 (48%) | 43 (35–51) | 0 | 1 (2%) | 3 (6%) | 44 (92%) |
| Reduced-intensity conditioning | 74 | 36 (49%) | 38 (51%) | 62 (53–70) | 0 | 4 (5%) | 10 (14%) | 60 (81%) |
| IMiDs, proteasome inhibitor, or both | 76 | 49 (64%) | 27 (36%) | 70 (65–75) | 49 (64%) | 8 (11%) | 3 (4%) | 16 (21%) |
| IMiDs no proteasome inhibitors* | 24 | 12 (50%) | 12 (50%) | 69 (65–74) | 16 (67%) | 4 (17%) | 0 | 4 (17%) |
| Proteasome inhibitors no IMiDs† | 19 | 13 (68%) | 6 (32%) | 79 (73–81) | 9 (47%) | 3 (16%) | 1 (5%) | 6 (32%) |
| IMiDs plus proteasome inhibitors‡ | 33 | 24 (73%) | 9 (27%) | 69 (62–73) | 24 (73%) | 1 (3%) | 2 (6%) | 6 (18%) |
| Hydroxycarbamide | 146 | 94 (64%) | 52 (26%) | 70 (65–75) | 144 (99%) | 0 | 1 (1%) | 1 (1%) |
| Anti-CD20 antibodies with or without chemotherapy | 87 | 39 (45%) | 48 (55%) | 67 (59–72) | 4 (5%) | 16 (18%) | 19 (22%) | 48 (55%) |
| Anti-CD20 monotherapy | 12 | 4 (33%) | 8 (67%) | 65 (59–70) | 3 (25%) | 4 (33%) | 1 (8%) | 4 (33%) |
| Anti-CD20 plus CHOP§ | 31 | 17 (55%) | 14 (45%) | 61 (49–72) | 0 | 3 (10%) | 6 (19%) | 22 (71%) |
| Anti-CD20 plus bendamustine, cladribine, fludarabine | 27 | 10 (37%) | 17 (63%) | 67 (64–72) | 1 (4%) | 6 (22%) | 8 (30%) | 12 (44%) |
| Other anti-CD20 antibody therapy¶ | 17 | 8 (47%) | 9 (53%) | 72 (67–77) | 0 | 3 (18%) | 4 (24%) | 10 (59%) |
| Other systemic therapy‖ | 44 | 26 (59%) | 18 (41%) | 51 (33–66) | 16 (36%) | 8 (18%) | 6 (14%) | 14 (32%) |
| Bruton tyrosine kinase inhibitors (ibrutinib or acalabrutinib) | 44 | 19 (43%) | 25 (57%) | 76 (66–79) | 42 (95%) | 2 (5%) | 0 | 0 |
| Tyrosine kinase inhibitors** | 41 | 10 (24%) | 31 (76%) | 54 (41–62) | 39 (95%) | 0 | 0 | 2 (5%) |
| Ruxolitinib | 16 | 8 (50%) | 8 (50%) | 66 (61–71) | 16 (100%) | 0 | 0 | 0 |
| Immunosuppressants††‡‡ | 8 | 4 (50%) | 4 (50%) | 58 (50–66) | 7 (88%) | 1 (13%) | 0 | 0 |
| Venetoclax with or without other§§ | 10 | 3 (30%) | 7 (70%) | 60 (58–70) | 10 (100%) | 0 | 0 | 0 |
| Anagrelide or interferon | 16 | 12 (75%) | 4 (25%) | 60 (44–71) | 16 (100%) | 0 | 0 | 0 |
| Nivolumab | 2 | 2 (100%) | 0 | 38 (35–40) | 1 (50%) | 0 | 0 | 1 (50%) |
| Untreated | 53 | 21 (40%) | 32 (60%) | 62 (51–73) | 0 | 0 | 0 | 0 |
| Haematological malignancy and anti-S1 IgG seropositive at timepoint 0 | 28 | 15 (54%) | 13 (46%) | 60 (44–65) | 9 (32%) | 1 (4%) | 2 (7%) | 16 (57%) |
Data are N, n (%), or median (IQR). Percentages may not total 100 due to rounding. BEACOPP=bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone. CHOP=cyclophosphamide, doxorubicin, vincristine, prednisolone. escBEACOPP=escalated doses of BEACOPP. HiCHOP=escalated doses of CHOP. HSCT=haematopoietic stem-cell transplantation. IMiDs=immunomodulatory imide drugs. miniCHOP=reduced doses of CHOP.
IMiDs no proteasome inhibitors=cyclophosphamide, thalidomide, dexamethasone; lenalidomide, dexamethasone; pomalidomide, dexamethasone; thalidomide, prednisolone; lenalidomide.
Proteasome inhibitors no IMiDs=carfilzomib, dexamethasone; cyclophosphamide, bortezomib, dexamethasone.
IMiDs plus proteasome inhibitors=bortezomib, thalidomide, dexamethasone; carfilzomib, lenalidomide, dexamethasone; ixazomib, lenalidomide, dexamethasone.
Anti-CD20 plus CHOP=rituximab plus CHOP; rituximab plus miniCHOP; rituximab plus HiCHOP.
Other anti-CD20=rituximab, dexamethasone, cytarabine, cisplatin; rituximab, temozolomide, etoposide, liposomal doxorubicin, dexamethasone, ibrutinib; rituximab, methotrexate; rituximab, fludarabine, cyclophosphamide; rituximab, cyclophosphamide; rituximab, cyclophosphamide, dexamethasone; rituximab, chlorambucil; Obinutuzumab, chlorambucil.
Other systemic therapy=cladribine; bendamustine; vinblastine; cyclophosphamide, prednisolone; vincristine, dexamethasone; bendamustine, dexamethasone; busulfan; 6-mercaptopurine, methotrexate; methotrexate; high-dose methotrexate; CHOP; carmustine, etoposidecytarabine, melphalan; BEACOPP; escBEACOPP; doxorubicin, bleomycin, vinblastine, dacarbazine; doxorubicin, vinblastine, dacarbazine; high-dose cytarabine; decitabine; arsenic trioxide, all-trans retinoic acid; glasdegib, low-dose cytarabine; ivosidenib or placebo.
Tyrosine kinase inhibitors=imatinib; dasatinib; nilotinib; gilteritinib.
Patients receiving standard immunosuppression after allogeneic HSCT were included in the allogeneic HSCT group; patients receiving immunosuppressive therapy for chronic graft-versus-host disease or other diseases were included in the immunosuppressants group.
Immunosuppressants=methylprednisolone; prednisolone; dexamethasone; budesonide; mycophenolate mofetil; mycophenolate mofetil, methylprednisolone.
Other=decitabine; azacytidine; low-dose cytarabine; low-dose cytarabine, gilteritinib; ibrutinib.
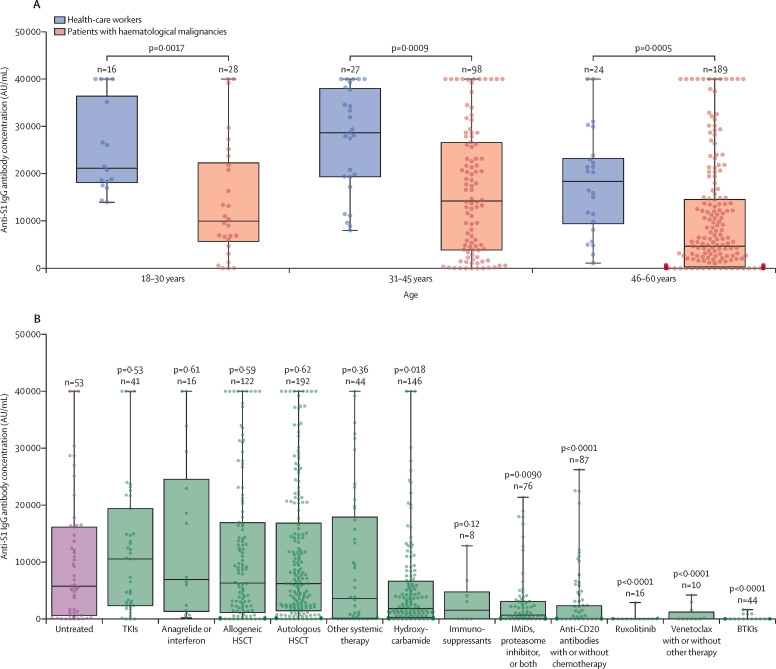
The anti-S1 IgG antibody concentration after two immunisations showed a strong correlation with age in the cohort of patients with haematological malignancies (p<0·0001 for both males and females; appendix 2 p 14). Results of the analysis of the relationship between anti-S1 IgG antibody concentrations and sex and white blood cell counts are shown in appendix 2 (p 14). We limited our comparative analysis to 315 (37%) patients aged 18–60 years because we did not have a healthy control cohort for patients aged 61 years and older (67 health-care workers were aged 18–60 years and included in the age-matched comparison). In the 18–60 year age group, patients in the haematological malignancy cohort had lower anti-S1 IgG antibody response after two BNT162b2 doses (timepoint 2) than did healthy health-care workers (median 6961 AU/mL [IQR 1292–20 672] vs 21 395 AU/mL [14 831–33 553]; p<0·0001). The effect was consistent across three age subgroups (figure 2A ). The median anti-S1 IgG antibody response in patients older than 60 years with haematological malignancies was 1140 AU/mL (IQR 34–6029) after two vaccine doses (timepoint 2).
Figure 2.
Serological response to two doses of BNT162b2 mRNA vaccine
The boxes show IQR, centre line shows the median, and whiskers show maximum and minimum values; the dots show individual participants. (A) Serological response to two doses of BNT162b2 in healthy individuals and in individuals with haematological malignancies grouped by age. (B) Serological response to two doses of BNT162b2 in treated patients compared with untreated patients with haematological malignancies; p values are for the comparison between the median anti-S1 IgG antibody concentration of each treatment group and the untreated group; the treatment regimens of each group are shown in the table. BTKIs=Bruton tyrosine kinase inhibitors. HSCT=haematopoietic stem-cell transplantation. IMiDs=immunomodulatory imide drugs. TKIs=tyrosine kinase inhibitors.
All participants in the cohort of healthy health-care workers mounted a robust anti-S1 IgG antibody response after the second dose of BNT162b2 vaccine (from timepoint 0 to timepoint 2) with a relatively narrow IQR. However, responses (from timepoint 0 to timepoint 1 to timepoint 2) in patients with haematological malignancies were heterogeneous, as shown by median responses varying by as much as three orders of magnitude at timepoint 2 between the different treatment groups, with wide intragroup IQRs (appendix 2 p 15).
Untreated patients with haematological malignancies had a median age of 62 years [IQR 51–73]). Underlying diseases in the untreated patient group included myelodysplastic syndrome, chronic lymphocytic leukaemia, multiple myeloma, non-Hodgkin lymphoma, myeloproliferative neoplasms, and Hodgkin lymphoma (appendix 2 p 5–9). Reasons for no treatment included new diagnosis, not meeting treatment criteria, or being unfit for active treatment (received supportive treatment only). The median anti-S1 IgG antibody concentration after two doses (timepoint 2) in the untreated cohort was 5761 AU/mL (IQR 629–16 141) compared with the response in the groups who received treatment with ruxolitinib (10 AU/mL [IQR 0–45]; p<0·0001), Bruton tyrosine kinase inhibitors (BTKIs; 0 AU/mL [0–7]; p<0·0001), venetoclax (4 AU/mL [0–1218]; p=0·0005), or anti-CD20 antibodies (17 AU/mL [1–2319]; p<0·0001), which all resulted in an almost absent serological response (figure 2B), with the median anti-S1 IgG antibody concentrations two orders of magnitude lower than those in the untreated patient group (appendix 2 p 15). Anti-S1 IgG antibody concentrations in the groups who were treated with hydroxycarbamide (1825 AU/mL [234–6622]; p=0·018) or with an immunomodulator with or without a proteasome inhibitor (679 AU/mL [45–3090]; p=0·0090) were also significantly lower than in the untreated patient group (figure 2B). Serological responses in patients treated with tyrosine kinase inhibitors (TKIs; 10 537 AU/mL [IQR 2335–19 388]; p=0·53), anagrelide or interferon (6927 AU/mL [1339–24 541]; p=0·61), autologous haematopoietic stem-cell transplantation (HSCT; 6203 AU/mL [1451–16 834]; p=0·62), allogeneic HSCT (6304 AU/mL [1120–16 913]; p=0·59), other systemic therapies (3591 AU/mL [124–17 898]; p=0·36), or immunosuppressants (1543 AU/mL [9–4764]; p=0·12) did not differ from the untreated patients with haematological malignancies (figure 2B). Four (80%) of five patients with previous splenectomy and with a systemic treatment-free period of 12 months or longer also mounted anti-S1 IgG antibody responses (appendix 2 p 10).
Generally, patients with haematological malignancies who had seroconverted by timepoint 1 showed a large increase in anti-S1 IgG antibody concentrations following the second immunisation (by timepoint 2): a median increase of 18·2 times (IQR 7·4–38·3) between timepoint 1 and timepoint 2 in patients who had allogeneic HSCT, 21·8 times (9·6–39·7) in patients who had autologous HSCT, 20·9 times (4·5–49·9) in patients who received other systemic therapies, 24·4 times (9·3–44·3) in patients who received TKIs, 16·1 times (3·4–32·8) in patients who received hydroxycarbamide, and 23·6 times (4·9–68·4) in patients who received an immunomodulator with or without a proteasome inhibitor. Responses at timepoint 1 and timepoint 2 for all patient groups are shown in appendix 2 (p 15). By contrast, most patients with a haematological malignancy who did not seroconvert after the first vaccine dose had low anti-S1 IgG antibody concentrations following the second vaccine dose (median 38 AU/mL [IQR 1–517]) with only a few mounting robust anti-S1 IgG antibody responses at timepoint 2 (appendix 2 p 16).
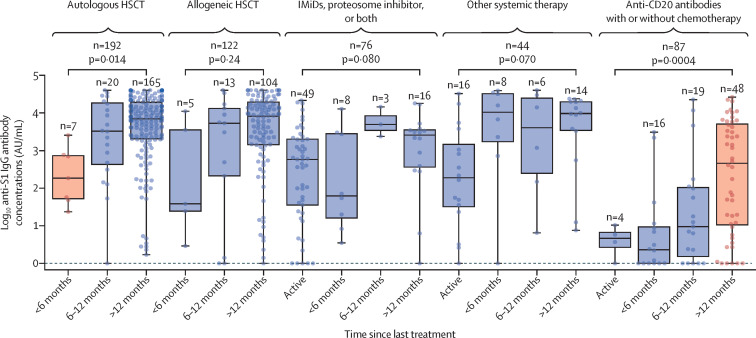
Almost all patients on hydroxycardamide, TKIs, BTKIs, ruxolitinib, anagrelide or interferon, immunosuppressant, or venetoclax were receiving treatment at the time of vaccination (table). In patients having had either allogeneic HSCT or autologous HSCT, serological responses were low within the first 6 months of HSCT but improved afterwards (figure 3 ). The anti-S1 IgG antibody responses were numerically lower in patients actively treated with other systemic treatment compared with the subgroups at 6 months and later, although the differences between subgroups were not statistically significant (figure 3). The responses in patients actively treated with immunomodulators with or without proteasome inhibitors were particularly heterogeneous (figure 3). Patients treated with an immunomodulator with or without a proteasome inhibitor with a treatment-free period of 6 months or longer, which reflected disease remission, had numerically higher serological responses than did other patients in the same treatment group, but this difference was not significant. Responses in the anti-CD20 antibody group were low within the first 12 months since last treatment; beyond 12 months after last treatment, serological responses improved but remained heterogeneous.
Figure 3.
Serological response after the second dose of vaccine stratified by time since treatment
The boxes show IQR, centre line shows the median, and whiskers show maximum and minimum values; the dots show individual participants; and p values are for the comparisons of anti-S1 IgG antibody median values within each treatment group. The subgroup differing significantly from others within the treatment group is shown in orange. The treatment regimens of each group are shown in the table. HSCT=haematopoietic stem-cell transplantation. IMiDs=immunomodulatory imide drugs.
28 patients with haematological malignancies were seropositive for anti-S1 IgG antibodies (median 571 AU/mL [IQR 114–3193]) before the first vaccination (appendix 2 p 11). 18 (64%) of 28 were also positive for anti-N IgG antibodies and had higher anti-S1 IgG antibody concentrations than did the anti-N IgG-negative group (appendix 2 p 17). At timepoint 2, the median anti-S1 IgG antibody concentration in previously seroconverted patients with haematological malignancies was higher (although not statistically significantly) than in the health-care workers who had not seroconverted (30 529 AU/mL [IQR 7573–40 000] vs 21 395 AU/mL [14 831–33 553]; p=0·36; appendix 2 p 15, 18). After one vaccine dose, the previously seroconverted patients with haematological malignancies generated significantly better anti-S1 IgG antibody responses (14 515 AU/mL [4110–36884]) than did seronegative patients with haematological malignancies after two doses (2396 AU/mL [95–11 227]; p=0·0054; appendix 2 p 18).
During follow-up (median 94 days since the second immunisation [IQR 73–102]) until May 17, 2021, nine (1%) of 885 fully vaccinated patients with haematological malignancies were diagnosed with COVID-19 (six required supplemental oxygen and three died from COVID-19 pneumonitis; appendix 2 p 13). Six (67%) patients with breakthrough infection did not seroconvert following the second SARS-CoV-2 immunisation. Notably, a 60 year-old patient with untreated amyloid light-chain amyloidosis, who had an anti-S1 IgG antibody concentration of 1138 AU/mL at timepoint 2, contracted SARS-CoV-2 32 days after the second vaccination with nadir SARS-CoV-2 PCR cycle threshold value of 8, and died from COVID-19, despite dexamethasone and remdesivir treatment. Seven SARS-CoV-2 samples from seven patients with haematological malignancies were screened for mutations of interest. All seven had a 23063A→U (spike protein Asn501Tyr) mutation and deletion of codons 69 and 70 in the spike protein, whereas no cases of 23012G→A (spike protein Gly484Lys) mutation were detected. This is consistent with the B.1.1.7 strain that has been dominant in Lithuania since February, 2021.
Adverse event questionnaires were returned by 662 (77%) of the 857 participants with haematological malignancies after the first immunisation and by 575 (67%) after the second immunisation. Adverse events were more common after the second dose, with fatigue being the most prevalent symptom (72 [13%] of 575 participants; appendix 2 p 19). No grade 4 adverse events were reported.
Discussion
Our study shows that seronegative patients with haematological malignancies mount heterogeneous and markedly blunted serological responses to two doses of BNT162b2 compared with healthy individuals, regardless of age or treatment. Patients treated with BTKIs, ruxolitinib, or venetoclax, or who have received anti-CD20 antibodies less than 12 months before vaccination, might not mount any meaningful antibody response to BNT162b2 vaccination and might be rendered unprotected from COVID-19, as suggested by severe breakthrough infections. By contrast, patients actively treated with TKIs or having received autologous or allogeneic HSCT or systemic chemotherapy more than 6 months before vaccination show marked serological responses to BNT162b2 immunisation.
Interpretation of immunological response to vaccination is complex and requires accounting for underlying pathology, disease status, current or past treatment, interval between treatment and vaccination, age, type of vaccine, and correlation between readouts of immune system function and clinical protection. We chose to report the immune response to SARS-CoV-2 vaccination by the type of current or most recent treatment for haematological malignancies, which might be more applicable to patient care. We used quantitative rather than qualitative seroconversion reporting because correlations between the antibody concentrations and the protective immunity against SARS-CoV-2 infection have not been established yet.
In an early report by Monin and colleagues,10 eight (18%) of 44 patients with haematological malignancies mounted antibody responses and nine (50%) of 18 patients mounted T-cell responses to a single BNT162b2 dose—both antibody and T cell concentrations in patients with haematological malignancies were lower than in healthy participants or patients with solid cancers. Two reports focusing on chronic lymphocytic leukaemia showed that patients actively treated with BTKIs or anti-CD20 antibodies had particularly poor responses to two doses of an mRNA vaccine.11, 12 We observed similar results in our cohort in patients with chronic lymphocytic leukaemia and other B-cell malignancies. It takes around 9–12 months after anti-CD20 antibody treatment for B-cell reconstitution to be observed, but the serological response often remains blunted even beyond this period.13 In our study, venetoclax in combination with other therapies, excluding anti-CD20 antibodies, was associated with absent immunological response to vaccination. In the study by Herishanu and colleagues,11 only two of five patients with chronic lymphocytic leukaemia who were treated with venetoclax monotherapy mounted any serological response to BNT162b2 immunisation.
In another previous study, only 4% of patients with chronic lymphocytic leukaemia treated with BTKIs mounted a serological response when exposed to the de-novo antigen in the hepatitis B vaccine.14 However, when challenged with anamnestic antigens in varicella zoster virus vaccine, patients treated with BTKIs mounted a better serological response.14, 15 Building on varicella zoster virus vaccine studies, we could speculate that people who have seroconverted against the SARS-CoV-2 spike protein before initiation of BTKI treatment might have greater benefit from SARS-CoV-2 vaccination.
Suppression of de-novo immunological response to BNT162b2 in patients treated with ruxolitinib is a novel finding of our study.16 Ruxolitinib, a JAK 1/2 inhibitor, blocks a common signalling pathway used by multiple cytokine receptors and has been shown to almost completely block both dendritic cell activation and the potentiation of CD8 T cells.17 The immunosuppressive effect of JAK1/2 inhibitors has been applied in the treatment of graft-versus-host disease (GVHD) and is being investigated for severe forms of COVID-19.18 Discontinuation of ruxolitinib during SARS-CoV-2 infection for patients with myelofibrosis was associated with an increase in mortality (60 day survival: 68% in patients continuing ruxolitinib, 11% in patients discontinuing ruxolitinib; p<0·001), which might suggest a beneficial role of ruxolitinib in reducing the effect of cytokine storm.3 However, the immunosuppressive effect of ruxolitinib might become disadvantageous when an immune response to SARS-CoV-2 vaccine is needed.
In a cohort of 22 patients with myeloproliferative neoplasms, there were neutralising antibody responses in 85% and T-cell responses in 80% of participants after a single dose of BNT162b2.19 However, interpretation of these results is hindered by multiple treatment modalities in a small number of patients and inclusion of patients with previous SARS-CoV-2 exposure. In our cohort, patients treated with hydroxycarbamide for myeloproliferative neoplasms generally had only modest serological responses, significantly lower than untreated patients. The older patient population in the hydroxycarbamide group might at least partially explain this difference, because children receiving hydroxycarbamide for sickle cell disease have similar antibody responses to pneumococcal vaccines compared with children not receiving this treatment.20 Patients with chronic myeloid leukaemia treated with TKIs have shown good responses to seasonal influenza vaccines and had a limited benefit from the booster vaccine.21 Likewise, in our study, patients treated with TKIs mounted a good serological response following the BNT162b2 vaccine, albeit lower than in the healthy population.
The efficacy and longevity of vaccine-induced immunity correlates with the time since HSCT.22, 23 The type of conditioning, presence of GVHD, GVHD prophylaxis and treatment, donor match, and other factors might influence the immune response, but these detailed subgroup analyses were beyond the scope of this study. Our data suggest that many patients who had HSCT more than 6 months ago and are not on active treatment or immunosuppression can expect a reasonable serological response to the BNT162b2 vaccine. This might reflect effectively treated underlying haematological malignancy, younger patient population, and immune reconstitution in patients who are post-transplantation.
A study by Bird and colleagues24 showed that patients with multiple myeloma with active disease, active treatment, or immunoparesis had inferior serological responses to a single dose of ChAdOx1 or BNT162b2 vaccines; however, no associations between serological response and multiple myeloma treatment type were detected. Treatment and disease-related immunoparesis in patients with multiple myeloma are difficult to disentangle because treatment regimens include combinations of drugs from different classes administered in multiple treatment lines. In our study, patients receiving immunomodulators, proteasome inhibitors, or both had generally blunted antibody responses compared with untreated patients with haematological malignancies, with numerically improved responses in those with a treatment-free period of more than 6 months. This result might be attributed to sustained multiple myeloma disease control as maintenance therapies were only rarely used in the patients included in our study.
Neutralising antibodies have been shown to prevent SARS-CoV-2 infection in primates, with similar results of passive vaccination being seen in humans.25, 26 Meanwhile, previous experience with SARS-CoV suggests that T-cell immunity is important in preventing a severe course of COVID-19.27 Qualitatively, the presence of anti-spike IgG antibodies appears to provide around 90% protection for 6 months in healthy convalescent individuals.28 Good correlation between anti-S1 IgG antibody titre and virus neutralisation has been established in both convalescent and vaccinated individuals.4 A recent study suggests that approximately 50% neutralisation would be provided by 20% of the mean convalescent level, while BNT162b2 vaccination yields neutralising antibody titres that are 2–3 times higher than in convalescent individuals.4, 29 However, our study shows that many of the patients with haematological malignancies yield anti-spike IgG antibody titres that often are orders of magnitude lower than those seen healthy individuals, which might not result in a meaningful virus neutralisation and protection from SARS-CoV-2 infection. In our population of patients with haematological malignancies, the self-reported adverse events to vaccination were markedly less common compared with the original phase 3 BNT162b2 trial data, and probably reflect a lower immunological response in these patients.5
In this study, there were severe breakthrough SARS-CoV-2 infections with a high viral load in fully vaccinated patients with haematological malignancies, three of which were fatal. The genotyping suggests that the infections were caused by the B.1.1.7 lineage, showing that fully vaccinated patients with haematological malignancies might develop severe COVID-19, even with strains not associated with substantial BNT162b2-induced immunity evasion. Although most of the infections happened in patients with low anti-S IgG antibody titres, severe COVID-19 was also seen in a patient with a reasonable antibody response to vaccination. The number of breakthrough infections we observed is too low to draw any conclusions about the protective antibody concentrations, which are currently unknown.
Our study has several limitations. We focused on the effect of current or most recent treatments, but factors such as disease duration and status, previous therapies, and patient age are likely to influence serological response to vaccination. These complex associations should be addressed in disease-specific studies with adequate patient numbers. We also did not assess the serum of vaccinated participants for neutralisation, but a strong correlation between anti-S1 IgG antibody concentrations and the SARS-CoV-2 neutralisation potential has already been established.4 Our study did not evaluate T-cell responses to vaccination in patients with haematological malignancies. Although assessment of the in-vitro T-cell activity in serological non-responders would be of interest, currently there are no clinical correlates between T-cell response to vaccine and COVID-19 protection. Our study provides a point assessment of serological responses as well as the clinical outcomes temporally close to vaccination. The persistence of antibody response is of importance for continuous protection against COVID-19, and data from further timepoints would be instrumental in informing revaccination strategies. Some subgroups included in our analyses also contained relatively few patients, making it difficult to draw firm conclusions. Finally, our patients received the BNT162b2 mRNA vaccine only, and the immunological response to adenoviral vector, protein, virus inactivated, or other vaccines in patients with haematological malignancies might be different.
Our findings suggest that in seronegative patients with haematological malignancies, the second dose of BNT162b2 should not be delayed. In previously seroconverted patients, the second dose is less urgent. Breakthrough severe SARS-CoV-2 infections in fully vaccinated patients with haematological malignancies emphasise the importance of ongoing strict adherence to non-pharmacological interventions, including personal protection, periodic pre-emptive testing, and household vaccination programmes, as well as novel pharmacological pre-emptive postexposure interventions when these become available. Prospective studies to develop optimal vaccination strategies in patients with haematological malignancies are warranted.
Data sharing
Data collected for the study, including de-identified participant data, and data dictionary or other related documents (eg, study protocol, statistical analysis plan, informed consent form) will be made available to others upon request. These data will be shared with researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. The data will be available beginning 3 months and ending 36 months following publication. Proposals should be directed to kazimieras.maneikis@santa.lt; data requestors will need to sign a data access agreement.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
The study was funded by Vilnius University Hospital Santaros Klinikos. We thank Vilnius University Hospital Santaros Klinikos Biobank for providing biological samples and health information and Andrius Žučenka for proofreading the manuscript.
Contributors
KM, DN, LK, VP, RC, and LG designed the study. KM, KS, UR, VV, and VB contributed to data collection. KS, KM, TB, and LG analysed the data. KM, KS, and TB accessed and verified the data. TB, LG, KM, and KS drafted the initial manuscript. All authors contributed to interpretation of the data, critically reviewed and approved the final draft of the manuscript, and made the decision to submit for publication. All authors had full access to all the data in the study and all authors accept responsibility to submit for publication. KM had full access to all the data in the study after its completion and had final responsibility for the decision to submit for publication.
Supplementary Materials
References
- 1.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chari A, Samur MK, Martinez-Lopez J, et al. Clinical features associated with COVID-19 outcome in multiple myeloma: first results from the International Myeloma Society data set. Blood. 2020;136:3033–3040. doi: 10.1182/blood.2020008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbui T, Vannucchi AM, Alvarez-Larran A, et al. High mortality rate in COVID-19 patients with myeloproliferative neoplasms after abrupt withdrawal of ruxolitinib. Leukemia. 2021;35:485–493. doi: 10.1038/s41375-020-01107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Medicines Agency Comirnaty summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf
- 7.Abbott SARS-CoV-2 IgG II Quant. https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2
- 8.Public Health England Evaluation of the Abbott SARS-CoV-2 IgG for the detection of anti-SARS-CoV-2 antibodies. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/890566/Evaluation_of_Abbott_SARS_CoV_2_IgG_PHE.pdf
- 9.Collier DA, Ferreira IATM, Datir R, et al. Age-related heterogeneity in neutralising antibody responses to SARS-CoV-2 following BNT162b2 vaccination. medRxiv. 2021 doi: 10.1101/2021.02.03.21251054. published online March 7. (preprint, version 4). [DOI] [Google Scholar]
- 10.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021 doi: 10.1182/blood.2021011568. published online April 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roeker LE, Knorr DA, Pessin MS, et al. Anti-SARS-CoV-2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34:3047–3049. doi: 10.1038/s41375-020-01030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anolik JH, Friedberg JW, Zheng B, et al. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007;122:139–145. doi: 10.1016/j.clim.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137:185–189. doi: 10.1182/blood.2020008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zent CS, Brady MT, Delage C, et al. Short term results of vaccination with adjuvanted recombinant varicella zoster glycoprotein E during initial BTK inhibitor therapy for CLL or lymphoplasmacytic lymphoma. Leukemia. 2021;35:1788–1791. doi: 10.1038/s41375-020-01074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikulska M, Cesaro S, de Lavallade H, et al. Vaccination of patients with haematological malignancies who did not have transplantations: guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7) Lancet Infect Dis. 2019;19:e188–e199. doi: 10.1016/S1473-3099(18)30601-7. [DOI] [PubMed] [Google Scholar]
- 17.Heine A, Held SAE, Daecke SN, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122:1192–1202. doi: 10.1182/blood-2013-03-484642. [DOI] [PubMed] [Google Scholar]
- 18.Elli EM, Baratè C, Mendicino F, Palandri F, Palumbo GA. Mechanisms underlying the anti-inflammatory and immunosuppressive activity of ruxolitinib. Front Oncol. 2019;9 doi: 10.3389/fonc.2019.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington P, de Lavallade H, Doores KJ, et al. Single dose of BNT162b2 mRNA vaccine against SARS-CoV-2 induces high frequency of neutralising antibody and polyfunctional T-cell responses in patients with myeloproliferative neoplasms. Leukemia. 2021 doi: 10.1038/s41375-021-01300-7. published online May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lederman HM, Connolly MA, Kalpatthi R, et al. Immunologic effects of hydroxyurea in sickle cell anaemia. Pediatrics. 2014;134:686–695. doi: 10.1542/peds.2014-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Lavallade H, Garland P, Sekine T, et al. Repeated vaccination is required to optimize seroprotection against H1N1 in the immunocompromised host. Haematologica. 2011;96:307–314. doi: 10.3324/haematol.2010.032664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelhard D, Nagler A, Hardan I, et al. Antibody response to a two-dose regimen of influenza vaccine in allogeneic T cell-depleted and autologous BMT recipients. Bone Marrow Transplant. 1993;11:1–5. [PubMed] [Google Scholar]
- 23.Cordonnier C, Labopin M, Robin C, et al. Long-term persistence of the immune response to antipneumococcal vaccines after allo-SCT: 10-year follow-up of the EBMT-IDWP01 trial. Bone Marrow Transplant. 2015;50:978–983. doi: 10.1038/bmt.2015.42. [DOI] [PubMed] [Google Scholar]
- 24.Bird S, Panopoulou A, Shea RL, et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8:e389–e392. doi: 10.1016/S2352-3026(21)00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.REGENERON REGENERON reports positive interim data with REGEN-COV™ antibody cocktail used as passive vaccine to prevent COVID-19. Jan 26, 2021. https://investor.regeneron.com/news-releases/news-release-details/regeneron-reports-positive-interim-data-regen-covtm-antibody
- 27.Cameron MJ, Bermejo-Martin JF, Danesh A, Muller MP, Kelvin DJ. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133:13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021 doi: 10.1038/s41591-021-01377-8. published online May 17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for the study, including de-identified participant data, and data dictionary or other related documents (eg, study protocol, statistical analysis plan, informed consent form) will be made available to others upon request. These data will be shared with researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. The data will be available beginning 3 months and ending 36 months following publication. Proposals should be directed to kazimieras.maneikis@santa.lt; data requestors will need to sign a data access agreement.