Abstract
Background
Concurrent with the Pfizer–BioNTech BNT162b2 COVID-19 vaccine roll-out in Israel initiated on Dec 19, 2020, we assessed the early antibody responses and antibody kinetics after each vaccine dose in health-care workers of different ages and sexes, and with different comorbidities.
Methods
We did a prospective, single-centre, longitudinal cohort study at the Sheba Medical Centre (Tel-Hashomer, Israel). Eligible participants were health-care workers at the centre who had a negative anti-SARS-CoV-2 IgG assay before receiving the first dose of the intramuscular vaccine, and at least one serological antibody test after the first dose of the vaccine. Health-care workers with a positive SARS-CoV-2 PCR test before vaccination, a positive anti-SARS-CoV-2 IgG serology test before vaccination, or infection with COVID-19 after vaccination were excluded from the study. Participants were followed up weekly for 5 weeks after the first vaccine dose; a second dose was given at week 3. Serum samples were obtained at baseline and at each weekly follow-up, and antibodies were tested at 1–2 weeks after the first vaccine dose, at week 3 with the administration of the second vaccine dose, and at weeks 4–5 (ie, 1–2 weeks after the second vaccine dose). Participants with comorbidities were approached to participate in an enriched comorbidities subgroup, and at least two neutralising assays were done during the 5 weeks of follow-up in those individuals. IgG assays were done for the entire study population, whereas IgM, IgA, and neutralising antibody assays were done only in the enriched comorbidities subgroup. Concentrations of IgG greater than 0·62 sample-to-cutoff (s/co) ratio and of IgA greater than 1·1 s/co, and titres of neutralising antibodies greater than 10 were considered positive. Scatter plot and correlation analyses, logistic and linear regression analyses, and linear mixed models were used to investigate the longitudinal antibody responses.
Findings
Between Dec 19, 2020, and Jan 30, 2021, we obtained 4026 serum samples from 2607 eligible, vaccinated participants. 342 individuals were included in the enriched comorbidities subgroup. The first vaccine dose elicited positive IgG and neutralising antibody responses at week 3 in 707 (88·0%) of 803 individuals, and 264 (71·0%) of 372 individuals, respectively, which were rapidly increased at week 4 (ie, 1 week after the second vaccine dose) in 1011 (98·4%) of 1027 and 357 (96·5%) of 370 individuals, respectively. Over 4 weeks of follow-up after vaccination, a high correlation (r=0·92) was detected between IgG against the receptor-binding domain and neutralising antibody titres. First-dose induced IgG response was significantly lower in individuals aged 66 years and older (ratio of means 0·25, 95% CI 0·19–0·31) and immunosuppressed individuals (0·21, 0·14–0·31) compared with individuals aged 18·00–45·99 years and individuals with no immunosuppression, respectively. This disparity was partly abrogated following the second dose. Overall, endpoint regression analysis showed that lower antibody concentrations were consistently associated with male sex (ratio of means 0·84, 95% CI 0·80–0·89), older age (ie, ≥66 years; 0·64, 0·58–0·71), immunosuppression (0·44, 0·33–0·58), and other specific comorbidities: diabetes (0·88, 0·79–0·98), hypertension (0·90, 0·82–0·98), heart disease (0·86, 0·75–1·00), and autoimmune diseases (0·82, 0·73–0·92).
Interpretation
BNT162b2 vaccine induces a robust and rapid antibody response. The significant correlation between receptor-binding domain IgG antibodies and neutralisation titres suggests that IgG antibodies might serve as a correlate of neutralisation. The second vaccine dose is particularly important for older and immunosuppressed individuals, highlighting the need for timely second vaccinations and potentially a revaluation of the long gap between doses in some countries. Antibody responses were reduced in susceptible populations and therefore they might be more prone to breakthrough infections.
Funding
Sheba Medical Center, Israel Ministry of Health.
Research in context.
Evidence before this study
The BNT162b2 vaccine was authorised for emergency use against COVID-19 by the US Food and Drug Administration on Dec 11, 2020. We did a literature search through PubMed, and medRxiv and bioRxiv preprint servers, for articles published up to Feb 10, 2021, using the keywords “coronavirus disease 2019”, “COVID-19”, “SARS-CoV-2”, “Pfizer vaccine”, and “BNT162b2”, with no language restrictions. Except for a few clinical phase 1, 2, and 3 studies assessing the vaccine immunogenicity in a very small cohort, no other studies have yet been published. Immunogenicity was reported only in the clinical phase 1 and 2–3 trials, and showed an early IgG antibody response, 21 days after the first dose, and virus neutralisation starting from 7 days after the second dose, in only 12 participants. To date, there has not been any report on immunogenicity in real life, and particularly no data on whether vaccine-induced immune responses vary with age or comorbidities.
Added value of this study
To our knowledge, this is the first longitudinal prospective study across different ages, sexes, and comorbidities that evaluated the antibody response to the Pfizer-BioNTech BNT162b2 vaccine. We established that 99·9% and 96·5% of study participants developed IgG and neutralising antibodies, respectively, rapidly after two vaccine doses and that IgG antibody concentrations were highly correlated with neutralising titres. Additionally, our study identified that older (vs younger) and immunosuppressed (vs immunocompetent) individuals had a significantly lower antibody response after the first. Moreover, although we show that these at-risk populations (in addition to populations with other specific comorbidities) had a substantial antibody response after the second vaccine dose, overall, their IgG and neutralising antibody concentrations by 1–2 weeks after the second dose were statistically significantly lower than younger, healthy populations.
Implications of all the available evidence
The Pfizer-BioNTech BNT162b2 vaccine elicits a significant and robust antibody response. IgG detection by high-throughput serology assays accurately represents the antibody neutralising response. The longer gap between vaccine doses, currently in effect in some countries including the UK, should be re-evaluated for older and immunosuppressed individuals. Antibody responses were reduced in vulnerable populations and therefore they might be more prone to breakthrough infections.
Introduction
The worldwide spread of SARS-CoV-2 has resulted, by the end of January, 2021, in 100 million cases of COVID-19 and more than 2 million deaths. Several biotechnology and pharmaceutical companies have initiated urgent development of COVID-19 vaccines and three, Pfizer–BioNTech, Moderna, and Janssen, were granted emergency use authorisation by the US Food and Drug Administration in December, 2020, and early 2021.
Results from the Pfizer–BioNTech vaccine clinical trials showed 95% efficacy of the vaccine in preventing symptomatic laboratory-confirmed COVID-19 in individuals without evidence of previous SARS-CoV-2 infection.1 However, despite the information acquired about vaccine effectiveness, data on antibody responses following vaccination are based on only a few small-scale studies.2, 3 At 1 month after the second dose, immunogenicity data from 180 clinical trial participants showed a lower virus neutralisation response in older participants aged 65–86 years than in younger participants aged 18–55 years.4 Earlier IgG antibody response data for only 12 participants showed that IgG antibodies against the receptor-binding domain were detected 21 days after the first dose whereas virus neutralisation response was notable starting from 7 days after the second dose.5, 6, 7
The COVID-19 vaccination programme in Israel was initiated on Dec 19, 2020, with the Pfizer–BioNTech vaccine (BNT162b2) being the only administered vaccine. By March 21, 2021, after an estimated 200 000 vaccine administrations per day, 5 185 000 individuals (56% of the 9 300 000 total population) were vaccinated with the first dose; of these, 4 542 000 individuals (49% of the population) have received the second dose.8
A crucial priority for the scientific community is to assess the real-world immunogenicity of the vaccines in a large number of vaccinated individuals. However, data are scarce regarding antibody responses to the first and second vaccine doses in different demographics and susceptible populations, which is challenging for clinicians and policy makers, especially in light of the option raised in some countries to delay the second vaccine administration because of vaccine shortages.
Here we report the first large-scale study to test the antibody response in health-care workers in Israel with different demographic characteristics and comorbidities, and to assess correlates and kinetics of antibody-mediated immunity following vaccination with the Pfizer–BioNTech BNT162b2 vaccine.
Methods
Cohort
We did a prospective longitudinal cohort study in health-care workers of the Sheba Medical Center, the largest tertiary medical centre in Israel, with 1600 beds and 14 719 health-care workers, including employees and temporary personnel, who are mostly retired volunteers (older than 67 years). 18·0% of the workers in the Sheba Medical Center are physicians, 27·0% nurses and nurse aids, 21·3% paramedical personnel, and 33·7% administration and logistic employees. All health-care workers were emailed and offered the chance to join the study and undergo a serological test before receiving the first dose of the vaccine, and then the opportunity to have serological testing weekly for 5 weeks following the first dose. Health-care workers were included in the study if they met the following criteria: a negative anti-SARS-CoV-2 IgG assay before receiving the first dose of the vaccine, and at least one serological test after the first dose of the vaccine. Health-care workers with a positive SARS-CoV-2 PCR test before vaccination, a positive anti-SARS-CoV-2 IgG serology test before vaccination, or infection with COVID-19 following vaccination were excluded from the study. Participants were followed up every 7 days (plus or minus 3 days) for 5 weeks after the first dose of the vaccine for serological testing overall up to five tests at weeks 1, 2, and 3 after the first dose, and weeks 1 and 2 after the second dose. Antibodies were tested at 1–2 weeks after the first vaccine dose, at week 3 with the administration of the second vaccine dose, and at weeks 4–5 (ie, 1–2 weeks after the second vaccine dose). After participants submitted their questionnaire responses, those with comorbidities were specifically approached to participate in an enriched comorbidities subgroup, and at least two neutralising assays were done during the 5 weeks of follow-up in these individuals. IgG assays were done in the entire study population, and IgM, IgA, and neutralising antibody assays were done in individuals with comorbidities. Concentrations of IgG greater than 0·62 sample-to-cutoff (s/co) ratio and of IgA greater than 1·1 s/co, and titres of neutralising antibodies greater than 10 were considered positive. A computer-based questionnaire of demographic characteristics and comorbidities was taken by all health-care workers eligible for this study before receiving the first dose of the vaccine (appendix p 1).
The protocol and informed consent were approved by the institutional review board of the Sheba Medical Center. Written informed consent was obtained from all participants.
Serology assays
Samples from vaccinated health-care workers were tested using the SARS-CoV-2 receptor-binding domain IgG assay (Beckman-Coulter, Brea, CA, USA) commercial test, and an IgM and IgA receptor-binding domain-based ELISA.9, 10 SARS-CoV-2 pseudo-virus neutralisation assay was done using a green fluorescent protein reporter-based pseudotyped virus with a vesicular stomatitis virus backbone coated with SARS-CoV-2 spike protein, which was obtained from Gert Zimmer (Institute of Virology and Immunology, Mittelhäusern, Switzerland). Further details of the assays are in the appendix (p 3).
Statistical analysis
Scatter plots of IgG, IgM, IgA, and neutralising antibodies, and mixed-models figures of log-transformed IgG and neutralising antibodies (in log scale) of experimental groups divided by age, sex, and co-morbidities were done using GraphPad Prism 5.0. The correlation between concentrations of IgG and log-transformed neutralising antibodies was analysed using Spearman's correlation with CIs of 95%.
Log-transformed IgG and neutralising antibody concentrations were analysed as a continuous variable with multivariate linear regression. Additional analysis as a dichotomous variable was done using the IgG and neutralising antibodies cutoff as a dichotomous variable and using multivariate logistic regression. Age, sex, body-mass index (BMI), and comorbidity variables were included in the two models. Participants who did not answer all of the questions from the computer-based questionnaire were excluded from the regression analysis and the mixed models. Statistical analysis was done with SAS version 9.4.
To analyse the changes in IgG and neutralising antibodies over time, we used a linear mixed model with natural log IgG or neutralising antibodies as the dependent variables. Our aim was to examine concentrations at week 3 after the first vaccination (at which time the second vaccination was given) and the change from week 3 to weeks 4–5 (ie, 1–2 weeks after the second vaccination), and to relate these changes to characteristics of the participants. Therefore, we included different sets of independent variables in the model: time since first vaccination with either sex, age, BMI, or disease category and the interaction between time and any of these variables. In this model, each covariate main effect represented its relationship to the IgG concentration or neutralisation titre at week 3, and its interaction with time represented its relationship to the change between week 3 and weeks 4–5. IgG data were examined for interaction of sex with age and several comorbidities including heart disease, hypertension, and diabetes. Because our records included missing data for IgG or neutralising antibodies, we used linear mixed models that are able to handle unequal numbers of repeated observations for individuals as long as they are missing at random. Linear mixed models were also used to isolate the effect of each covariate on the antibody's concentrations. We included in the model a random effect of participant level at week 3, and changes in concentrations from week 3 to week 5. Although the statistical analysis controlled for the potential confounders, we present variables that show statistically significant associations with the humoral responses in the mixed model. The linear mixed models were analysed using R version 3.6.2 statistical software.
Role of the funding source
The funders of the study had no role in the design of the study protocol, data collection, data management, data analysis, data interpretation, or writing of the report.
Results
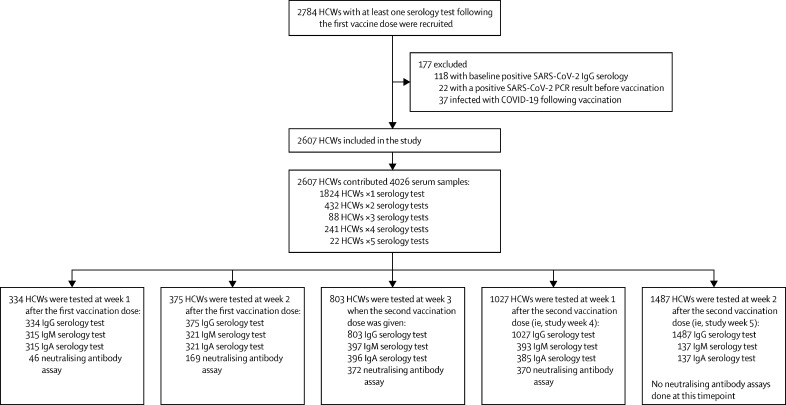
Between Dec 19, 2020, and Jan 30, 2021, 4026 serum samples were collected from 2607 Sheba Medical Centre health-care workers after the first vaccination dose (figure 1 ). From the cohort of 2607 participants in our study, 555 (21·3%) were physicians, 670 (25·7%) nurses and nurse aids, 678 (26·0%) paramedical personnel, and 704 (27·0%) administration and logistic employees. There were no missing data for the independent variables, except for 30 (1·2%) of 2607 participants who didn't answer the BMI questions and were excluded from the regression analysis and the mixed models. Concentrations of IgG antibodies against the receptor-binding domain were evaluated for all study participants at least once during the 5-week timeframe and for 883 participants (33·9%) at least twice. Most of the participants who were tested in week 5 did not have serology tests during the first 4 weeks after the first vaccination dose. IgM, IgA and neutralising antibody assays were done for 397, 396, and 372 individuals respectively. At least two neutralising assays were done during weeks 1–4 of the 5-week follow-up in 342 individuals with comorbidities (thus the enriched subgroup consisted of these 342 individuals). 255 (74·5%), 246 (71·9%), and 240 (70·2%) of these 342 individuals were evaluated weekly during 5 weeks of follow-up for IgG, IgM, and IgA, respectively. Mean age at vaccination was 47·7 years (SD 12·5) for the whole study population and 49·3 years (13·4) for the enriched comorbidities subgroup (table 1 ). Most participants in both the whole study cohort and the subgroup were female (table 1).
Figure 1.
Study profile
Prospective BNT162b2-vaccinated HCW cohort and serology assays following vaccination. HCWs of the Sheba Medical Centre in Israel were followed up weekly for 5 weeks following vaccination between Dec 19, 2020, and Jan 30, 2021. HCW=health-care worker.
Table 1.
Baseline characteristics of the study populations
| Whole study population (n=2607) | Enriched comorbidities subgroup (n=342)* | ||
|---|---|---|---|
| Sex | |||
| Male | 724 (27·8%) | 72 (21·1%) | |
| Female | 1883 (72·2%) | 270 (78·9%) | |
| Age at vaccination (years) | 47·7 (12·5) | 49·3 (13·4) | |
| Range | 18·1–84·9 | 19·5–83·3 | |
| Age group (years) | |||
| <46 | 1204 (46·1%) | 152 (44·4%) | |
| 46–65·99 | 1202 (46·2%) | 141 (41·2%) | |
| ≥66 | 201 (7·7%) | 49 (14·3%) | |
| BMI (kg/m2) | 25·6 (4·8) | 25·1 (4·8) | |
| Range | 11·0–63·5 | 11·0–42·5 | |
| BMI group (kg/m2)† | |||
| <25 | 1343/2577 (52·1%) | 190/338 (56·2%) | |
| 25–29·99 | 816/2577 (31·7%) | 98/338 (29·0%) | |
| ≥30 | 417/2577 (16·2%) | 50/338 (14·8%) | |
| Comorbidities | |||
| Hypertension | 301 (11·55%) | 46 (13·45%) | |
| Dyslipidemia | 184 (7·06%) | 30 (8·77%) | |
| Autoimmune disease | 160 (6·14%) | 39 (11·40%) | |
| Diabetes | 139 (5·33%) | 25 (7·31%) | |
| Heart disease | 79 (3·03%) | 15 (4·39%) | |
| Lung disease | 79 (3·03%) | 18 (5·26%) | |
| Coagulation disorder | 46 (1·76%) | 9 (2·63%) | |
| Immunosuppression‡ | 32 (1·23%) | 12 (3·51%) | |
| Allergic disease§ | 20 (0·77%) | 6 (1·75%) | |
| Liver disease | 18 (0·69%) | 4 (1·17%) | |
| Kidney disease | 16 (0·61%) | 3 (0·88%) | |
| Pregnancy | 8 (0·31%) | 2 (0·58%) | |
Data are n (%), mean (SD), or n/N (%) unless otherwise indicated. BMI=body-mass index.
Subgroup with enriched co-morbidities was established comprising health-care workers who underwent at least two neutralisation assays during the 5 weeks of follow-up.
Denominator shows the number of participants for whom BMI was available.
Included organ transplantation; being on biologic therapy, chemotherapy, or steroids; and splenectomy.
Defined as at least one event of anaphylaxis that required immediate treatment.
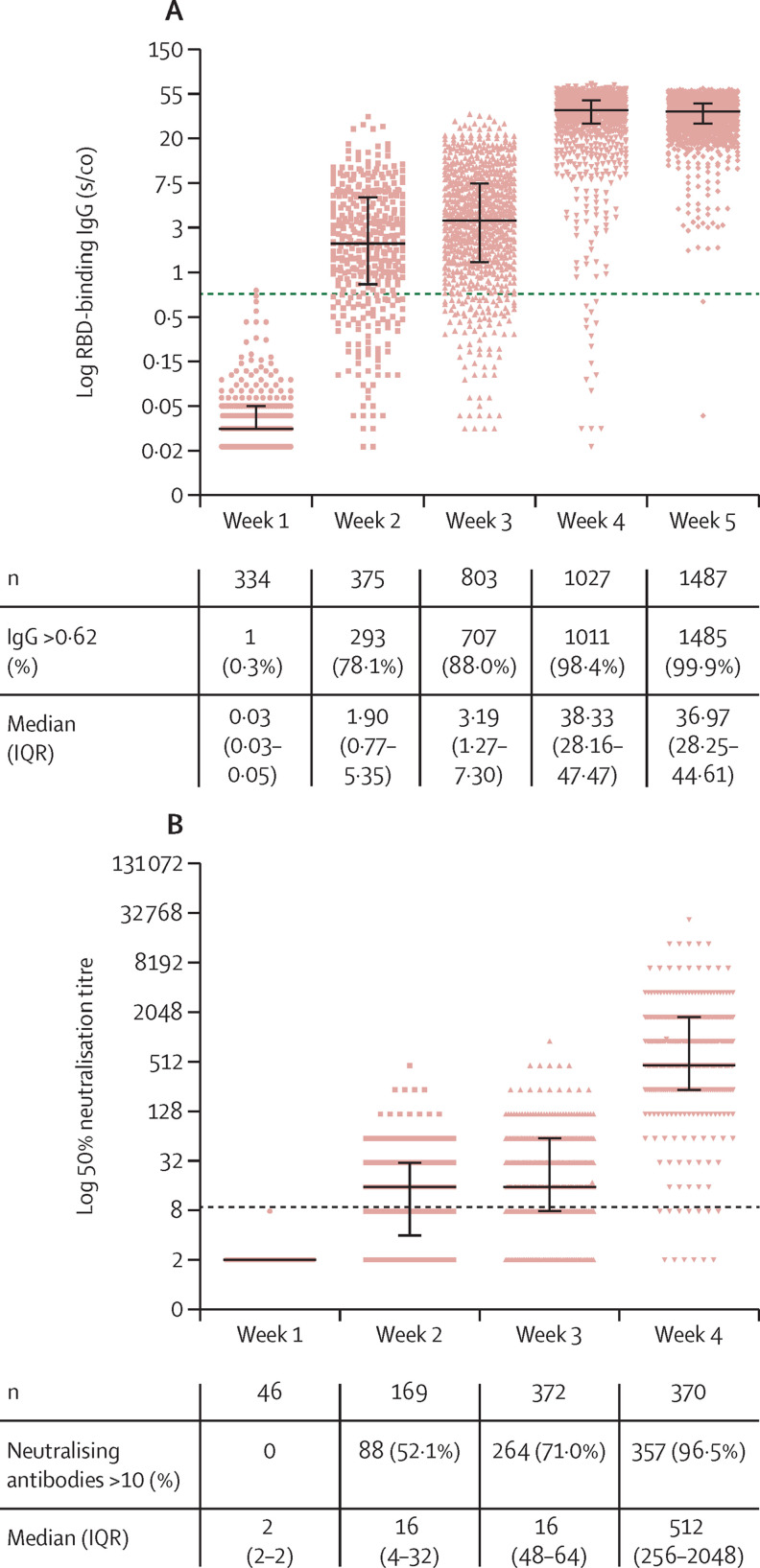
To assess the repertoire and kinetics of the antibody response to vaccination, IgM, IgA and IgG antibodies were evaluated (figure 2 ; appendix p 4). 7 days (plus or minus 3 days) after the first vaccine dose, a small proportion of participants developed IgM, IgA (appendix p 4), and IgG antibodies (12 [4%] of 315, 4 [3%] of 315, and 1 [<1%] of 334, respectively; figure 2). A substantial increase in detectable concentrations of IgA (139 [43%] of 321) and IgG (293 [78%] of 375; figure 2) was noted 14 days (plus or minus 3 days) after the first vaccine dose, whereas IgM was still detected in only a few participants at this timepoint (51 [16%] of 321; appendix p 4). 3 weeks after the first dose, with the administration of the second vaccination dose, IgG antibody titres were still relatively low with only a two-times increase compared with titres at 2 weeks (receptor-binding domain IgG [s/co] 1·9 vs 3·19), despite a consistent rise in individuals with detectable antibodies. The second vaccine dose resulted in a rapid and marked increase in both antibody detection and titres. 7 days after the second dose (week 4) IgG antibodies were detected in most participants with a ten-fold increase in titres compared with that at 3 weeks (receptor-binding domain-binding IgG [s/co] 3·19 vs 38·33), and IgM and IgA antibodies were detected in 109 (28%) of 393 and 326 (85%) of 385 participants, respectively. Importantly, 2 weeks after the second dose (ie, week 5), no statistically significant change in antibody titres was observed relative to week 4; however, nearly all participants had detectable IgG antibodies. Neutralising antibodies developed in around 52% of the enriched subgroup at 2 weeks after the first dose, and in around three-quarters of the subgroup at 3 weeks after the first dose. 7 days after the second dose most of the enriched subgroup had neutralising antibodies and the median titre increased 32 times relative to weeks 2 and 3 (figure 2).
Figure 2.
Quantitation of antibodies following BNT162b2 vaccination
(A) IgG concentrations over 5 weeks following vaccination. (B) Neutralising antibodies over 4 weeks following vaccination; no participants had serum samples assessed for neutralising antibodies at week 5. Antibodies were tested at 1–2 weeks after the first vaccine dose, at week 3 with the administration of the second vaccine dose, and at weeks 4–5, which refer to 1–2 weeks after the second vaccine dose, respectively. The dotted black line indicates the cutoff level of positive antibodies and neutralising concentrations. Solid black lines indicate medians (IQR). Each coloured dot represents one serum sample. RBD=receptor-binding domain. s/co=sample-to-cutoff ratio.
Because neutralisation and IgG antibody concentrations showed a similar trend, we investigated the correlation between these two assays using 953 samples which were tested for both IgG and neutralising antibodies. A significant overall correlation of 0·92 (p<0·0001; appendix p 6) was observed over 4 weeks after vaccination.
To determine whether vaccine-induced antibody responses depended on sex, age, BMI, or specific comorbidities we investigated the induction in IgG and neutralising antibodies 3 weeks after the first vaccine dose in relation to these variables (table 2 ). A multiple logistic regression analysis revealed that a smaller proportion of older participants aged 46 years and older developed reactive IgG and neutralising antibodies compared with those aged 18·00–45·99 years. Immunosuppression was significantly associated with non-reactive response of IgG antibodies.
Table 2.
Predictors of positive IgG, IgA, and neutralising antibodies following vaccination
|
Total positive IgG*(week 3†; n=698) |
Total positive neutralisation*(week 3†; n=261)‡ |
Total positive IgA*(week 4†; n=324)‡ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of individuals assessed (%) | Odds ratio (95% CI) | p value | Number of individuals assessed (%) | Odds ratio (95% CI) | p value | Number of individuals assessed (%) | Odds ratio (95% CI) | p value | ||
| Sex | ||||||||||
| Female | 552 (78%) | 1 (ref) | .. | 215 (79%) | 1 (ref) | .. | 259 (79%) | 1 (ref) | .. | |
| Male | 146 (22%) | 0·65 (0·38–1·11) | 0·115 | 46 (22%) | 0·57 (0·31–1·04) | 0·066 | 65 (21%) | 0·78 (0·38–1·61) | 0·497 | |
| Age (years) | ||||||||||
| 18–45·99 | 324 (43%) | 1 (ref) | <0·0001§ | 133 (44%) | 1 (ref) | <0·0001§ | 150 (42%) | 1 (ref) | 0·0108§ | |
| 46–65·99 | 332 (49%) | 0·17 (0·09–0·34) | <0·0001 | 105 (41%) | 0·47 (0·27–0·83) | 0·009 | 120 (39%) | 0·33 (0·15–0·72) | 0·006 | |
| ≥66 | 42 (8%) | 0·06 (0·02–0·14) | <0·0001 | 23 (15%) | 0·16 (0·08–0·35) | <0·0001 | 54 (19%) | 0·28 (0·11–0·72) | 0·008 | |
| BMI (kg/m2) | ||||||||||
| <25 | 396 (55%) | 1 (ref) | 0·1986§ | 155 (57%) | 1 (ref) | 0·4600§ | 192 (57%) | 1 (ref) | 0·3892§ | |
| 25–29·99 | 195 (30%) | 0·68 (0·34–1·38) | 0·069 | 71 (28%) | 1·17 (0·65–2·11) | 0·59 | 89 (29%) | 0·72 (0·36–1·43) | 0·35 | |
| ≥30 | 107 (15%) | 0·62 (0·37–1·04) | 0·284 | 35 (15%) | 0·71 (0·35–1·44) | 0·348 | 43 (14%) | 0·57 (0·24–1·35) | 0·198 | |
| Comorbidities | ||||||||||
| No hypertension | 623 (88%) | 1 (ref) | .. | 234 (86%) | 1 (ref) | .. | 282 (85%) | 1 (ref) | .. | |
| Hypertension | 75 (12%) | 1·56 (0·79–3·10) | 0·204 | 27 (14%) | 0·73 (0·36–1·47) | 0·379 | 42 (15%) | 1·02 (0·46–2·24) | 0·966 | |
| No autoimmune disease | 650 (93%) | 1 (ref) | .. | 226 (87%) | 1 (ref) | .. | 281 (87%) | 1 (ref) | .. | |
| Autoimmune disease | 48 (7%) | 0·63 (0·27–1·48) | 0·292 | 35 (13%) | 0·67 (0·32–1·38) | 0·278 | 43 (13%) | 0·89 (0·35–2·26) | 0·812 | |
| No diabetes | 663 (95%) | 1 (ref) | .. | 249 (93%) | 1 (ref) | .. | 308 (92%) | 1 (ref) | .. | |
| Diabetes | 35 (6%) | 0·92 (0·39–2·19) | 0·855 | 12 (7%) | 0·53 (0·21–1·30) | 0·166 | 16 (8%) | 0·30 (0·13–0·73) | 0·008 | |
| No lung disease | 672 (96%) | 1 (ref) | .. | 249 (95%) | 1 (ref) | .. | 310 (95%) | 1 (ref) | .. | |
| Lung disease | 26 (4%) | 0·88 (0·28–2·81) | 0·831 | 12 (5%) | 0·89 (0·31–2·60) | 0·838 | 14 (5%) | 0·61 (0·19–1·98) | 0·408 | |
| No heart disease | 680 (97%) | 1 (ref) | .. | 253 (96%) | 1 (ref) | .. | 313 (95%) | 1 (ref) | .. | |
| Heart disease | 18 (3%) | 0·81 (0·30–2·16) | 0·674 | 8 (4%) | 1·42 (0·45–4·49) | 0·553 | 11 (5%) | 0·63 (0·20–1·93) | 0·414 | |
| No immunosuppression | 688 (98%) | 1 (ref) | .. | .. | .. | .. | .. | .. | .. | |
| Immunosuppression¶ | 10 (2%) | 0·07 (0·02–0·24) | <0·0001 | .. | .. | .. | .. | .. | .. | |
Data are from the multivariate logistic regression analysis model. IgM antibodies were detected in very few individuals and for only a short time and are therefore not included in the table. ref=reference value. BMI=body-mass index. ..=data not available.
IgG >0·62, IgA >1·1, and neutralising antibodies >10 were considered positive.
Week 3 refers to the week that the second vaccination dose was administered. Week 4 refers to the week following the second vaccination dose.
IgA and neutralising antibodies were assessed in the enriched subgroup and not the full cohort.
Global p value.
Immunosuppression included organ transplantation; being on biologic therapy, chemotherapy, or steroids; and splenectomy.
We sought to examine which characteristics might be significantly associated with IgA at its peak detection concentration 1 week after the second vaccination (ie, at week 4). Younger age was significantly associated with development of IgA antibodies and diabetes was significantly associated with non-reactive response of IgA antibodies (table 2). High BMI was not significantly associated with an altered IgA response. Since IgM was induced in only a small proportion of the population and decreased rapidly, we did not include it in the analysis.
To determine if the immune responses to vaccination were related to age, sex, or morbidity we took into account the separate effects of each dose as well as the combined effect of the two vaccine doses. We therefore analysed the changes of each vaccine dose separately using a linear mixed model (table 3 ). Figure 3 shows antibody kinetics and mean values for each of the groups found to be significantly different following vaccination in the mixed model. We also evaluated the end-point effects of the full vaccination protocol (the sum of the two doses) by linear regression analysis (appendix p 7).
Table 3.
IgG and neutralising antibody titres (ratio of means) following vaccination
| IgG, weeks 0–3*(n=793) | Neutralisation, weeks 0–3*(n=368) | IgG, weeks 3–5*(n=1395) | Neutralisation, weeks 3–4*(n=419) | |
|---|---|---|---|---|
| Sex | ||||
| Male (vs female) | 0·77 (0·67–0·88) | 0·7 (0·50–0·97) | 1·09 (0·92–1·28) | 0·77 (0·52–1·14) |
| Age (years) | ||||
| 46–65·99 (vs 18–45·99) | 0·52 (0·46–0·58) | 0·57 (0·43–0·75) | 1·66 (1·43–1·93) | 1·04 (0·74–1·46) |
| ≥66 (vs 18–45·99) | 0·25 (0·19–0·31) | 0·28 (0·18–0·42) | 2·66 (2·00–3·53) | 0·93 (0·57–1·51) |
| BMI (kg/m2) | ||||
| 25–29·99 (vs <25) | 0·92 (0·80–1·05) | 0·99 (0·73–1·34) | 1·14 (0·97–1·33) | 0·80 (0·56–1·14) |
| ≥30 (vs <25) | 1·15 (0·97–1·37) | 0·92 (0·62–1·35) | 0·91 (0·74–1·12) | 0·61 (0·39–0·96) |
| Comorbidities | ||||
| Hypertension | 0·99 (0·82–1·20) | 0·89 (0·59–1·34) | 0·89 (0·71–1·13) | 1·72 (1·07–2·79) |
| Autoimmune disease | 0·87 (0·70–1·09) | 0·78 (0·53–1·13) | 0·94 (0·70–1·26) | 0·97 (0·61–1·55) |
| Diabetes | 1·03 (0·80–1·32) | 0·83 (0·50–1·38) | 0·84 (0·62–1·14) | 0·70 (0·38–1·28) |
| Lung disease | 0·74 (0·55–0·99) | 0·64 (0·36–1·14) | 1·56 (1·06–2·29) | 1·21 (0·61–2·40) |
| Heart disease | 0·69 (0·49–0·96) | 0·92 (0·47–1·80) | 1·28 (0·86–1·93) | 0·64 (0·29–1·41) |
| Immunosuppression† | 0·21 (0·14–0·31) | .. | 2·45 (1·35–4·45) | .. |
Data are ratios of means (95% CI) from the linear mixed analysis model. BMI=body-mass index. ..=data not available.
The inductions in IgG and neutralising antibody titres were measured 3 weeks after the first vaccination dose (first-dose effects); and between 3 and 5 weeks for IgG, or between 3 and 4 weeks for neutralising antibodies for the second-dose effects (neutralising antibodies were not assessed at 5 weeks). Week 3 refers to the administration of the second vaccination dose, and weeks 4 and 5 are the first and second weeks following the second vaccination dose, respectively.
Immunosuppression included organ transplantation; being on biologic therapy, chemotherapy, or steroids; and splenectomy.
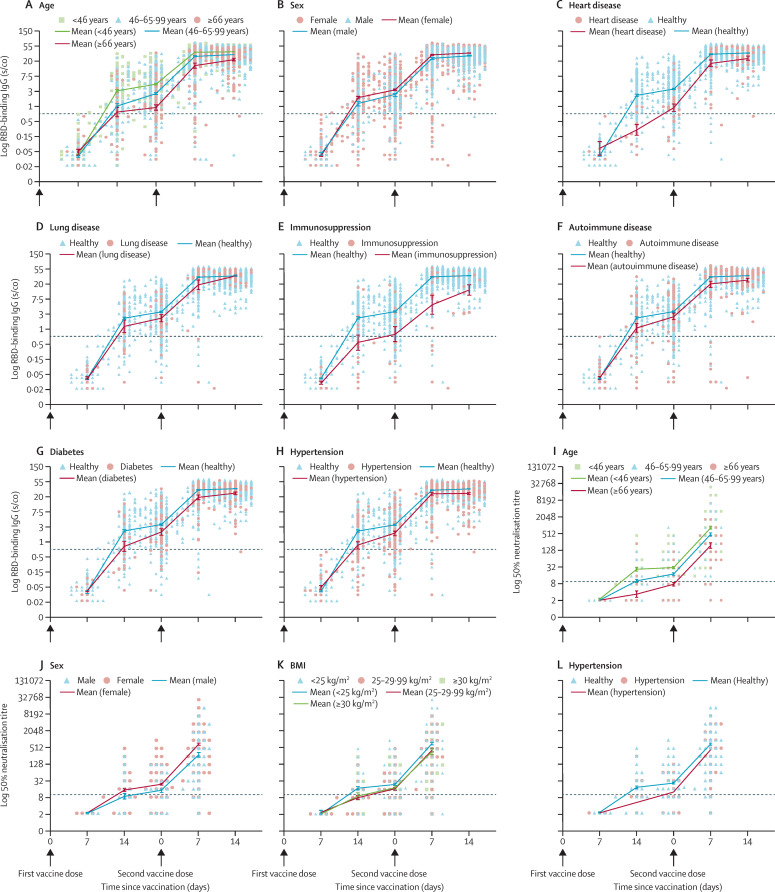
Figure 3.
Kinetics of antibodies following vaccination
(A-H) and (I-L) show the kinetics of IgG and neutralising antibodies within 5 weeks and 4 weeks following vaccination, respectively, in participants with different demographic characteristics and comorbidities. No participants had serum samples assessed for neutralising antibodies at week 5. Only variables showing statistical significance in the linear mixed model are presented. Arrows indicate the days of vaccination. Antibodies were tested at weeks 1–2 after the first vaccination dose, at week 3 with the administration of the second vaccination dose, and at weeks 4–5, which refer to 1–2 weeks after the second vaccination dose. The dotted black line indicates the limit of positive antibodies concentrations. Mean (SEM) are shown. RBD=receptor-binding domain. BMI=body-mass index. s/co=sample/cutoff ratio.
At 3 weeks after the first vaccine dose, we noted significantly smaller IgG titres for males versus females, older individuals (46 years and older versus age 45·99 years and younger), individuals with heart disease (compared with healthy individuals), lung disease (compared with healthy individuals), and immunosuppression (compared with immunocompetent individuals). Intriguingly, these effects were partly or completely abrogated after the second dose, as the fold induction in IgG titres between weeks 3–5 was significantly higher for older individuals aged 46 years and older, lung disease, and immunosuppression, but not significantly higher for heart disease. Additionally, no significant interaction was noted between sex and age or comorbidities. Endpoint regression analysis showed a decrease in IgG concentrations of 16% for males compared with females, 15% for age 46·00–65·99 years compared with 18·00–45·99 years, and 36% for age 66 years and older compared with 18·00–45·99 years (appendix p 7). A 56% decrease in participants with immunosuppression compared with immunocompetent individuals but no significant difference in participants with or without lung disease was noted after the two vaccine doses. Significant endpoint differences which did not show separate dose effects included hypertension, heart disease, autoimmune disease, and diabetes, compared with healthy individuals (table 3, appendix p 7).
Neutralisation first-dose effects showed significantly lower titres for males (table 3) and older individuals aged 46 years and older. Second-dose effects measured at weeks 3–4 showed significantly lower increases in neutralisation titres in individuals with obesity with a BMI of 30 or greater compared with individuals with a BMI less than 25, and higher neutralisation titres in those with hypertension compared with healthy individuals (table 3). Overall, the endpoint analysis showed a decrease in neutralising titres of 48% in males compared with females (appendix p 7), 43% in participants aged 46·00–65·99 years compared with ages 18·00–45·99 years, and 48% in individuals with a BMI of 30 or greater, compared with those with a BMI of less than 25.
Discussion
Israel was among the first countries to roll out the Pfizer–BioNTech BNT162b2 vaccine, and health-care workers were prioritised in the vaccination programme. This unique situation allowed us to rapidly assess the vaccine-induced immune response through a longitudinal prospective study comprising a large cohort of 2607 vaccinated health-care workers across different ages, sexes, and comorbidities. Our findings show that neutralising and IgG antibodies were significantly up-regulated after vaccination and that 96·5% and 99·9% of health-care workers who were vaccinated developed neutralising and IgG antibodies against SARS-CoV-2 7 14 days after the second vaccine dose, respectively. Furthermore, we noted that IgG antibody concentrations and neutralisation titres were highly correlated. Finally, we showed that health-care workers of different ages, sexes, and comorbidities respond differently to the vaccination and that each vaccine dose elicits specific antibody responses, specifically enabling us to define non-reactive or less-reactive populations that might be more susceptible to breakthrough infections.
A national validation study9 showed that about 5% of patients with COVID-19 do not develop IgG antibodies and that most serological assays identify antibodies in 85–90% of infected individuals. The detection of neutralising and IgG antibodies in 96·5% and 99·9% of vaccinated participants in our study suggests that the vaccine is highly efficient and mounts a significantly higher and more robust antibody response compared with natural infection.11 The rapid decrease in IgM and IgA antibodies against SARS-CoV-2 receptor-binding domain after vaccination suggests that both isotypes are short lived. Additionally, IgM peak detection in 27·7% of health-care workers and the rapid and robust IgG response might suggest that the S protein, used in the BNT162b2 vaccine, is highly immunogenic and that the class switch occurs very quickly, thus preventing IgM concentrations from increasing.
The combined use of multiple regression analysis and antibody kinetics enabled us to assess the antibody response to each vaccine dose separately as well as to the complete BNT162b2 vaccine protocol. This analysis was especially important due to the shortage of COVID-19 vaccines and the suggestion in some countries to postpone the second vaccine dose. Our results show that the antibody response following the first vaccine dose is proportional to age; eg, young adults mounted a significantly higher antibody response than older individuals. The antibody response to the second dose was different; health-care workers older than 66 years and aged between 46·00 and 65·99 years had an increase in IgG concentrations by 2·66 and 1·66 times more than health-care workers younger than 45·99 years, whereas no significant differences in the increase in neutralising antibody concentrations were detected in the week after the second dose. As a result, the overall response of health-care workers younger than 45·99 years to the two doses in IgG antibody concentrations was only 1·34 to 1·57 times higher (ie, differences in the means of each subgroup); whereas neutralising antibody titres were 2·23 to 3·92 times higher in young compared with older participants. This suggests that the vaccine also elicits high concentrations of IgG antibodies in the older population; however, these antibodies are less neutralising or might take longer to become neutralising.
A similar significant difference between the IgG antibody response to the first and second vaccine doses was observed for immunosuppression and lung diseases. Given the low concentrations of neutralising antibodies at 21 days after the initial vaccination, our data suggest that the boosting second dose is perhaps important for everyone, but especially for immunosuppressed and older individuals. However, two studies reported 74% and 62% vaccine effectiveness for hospitalisation and severe disease, respectively 14–20 days after the first BNT162b2 dose12 and 80% and 85% vaccine effectiveness at preventing hospitalisation and death for individuals older than 70 years at 28 days and onward following the first vaccine dose,13 suggesting that significant protection is achieved after one vaccine dose. Overall, these data show the complexities of defining immune correlates of protection following vaccination; however, the lower immune response observed here in specific populations suggests that a decision in some countries, including the UK, to delay the second dose for 8 weeks for everyone including immunosuppressed and older individuals should be based on both efficacy and immunogenicity data.
The lower concentrations of IgG and lower detectable IgA antibodies observed in patients with diabetes (lower neutralisation concentrations in patients with diabetes were also observed but did not reach significance, most probably because of the small diabetes cohort in this subpopulation) suggest that these patients have reduced antibody response following vaccination. Indeed, a meta-analysis14 showed that patients with diabetes have an increased risk for severe COVID-19 and in-hospital mortality. Other comorbidities such as hypertension, immunosuppression, autoimmune disease, and heart disease were found in this study to be significantly correlated with a lower antibody response. It is important to note that, in general, immunosuppressed individuals belong to a very heterogeneous group and therefore the decreased serological responses seen here might not be applicable to all forms of immunosuppression. Additionally, the small numbers of allergic health-care workers, or those with liver and kidney diseases did not allow reaching powered analysis for these subgroups. Also of importance was the significant correlation of lower IgG and neutralisation antibody titres in males compared with females and in older compared with younger health-care workers. Indeed, several studies have showed that despite no significant difference in COVID-19 cases between men and women, men have been becoming severely ill at a higher rate than women with an age-dependent disease susceptibility and mortality in older individuals.15, 16, 17 Future studies should investigate the lower antibody response following vaccine administration and higher susceptibility to infection in these populations.
Our results showed that IgG antibody concentrations against the receptor-binding domain were highly correlated with neutralising antibody titres. This result, which is based on close to 1000 samples tested with both assays is particularly valuable for diagnosis purposes. Neutralisation is the gold standard assay for assessing the antibody response; however, this test is laborious and requires highly skilled personal. Therefore, high-throughput serology assays, which are correlated with neutralisation, will allow rapid and accurate detection of neutralising antibodies and in the future will hopefully allow the assessment of correlation of protection. Our study did find some discrepancies between neutralising and IgG titres, especially in specific populations such as older individuals and in obese (BMI ≥30) individuals. Future studies should assess these differential effects after more time has passed since vaccination.
This study was done in a cohort of health-care workers who self-reported any comorbidities and therefore, while all age groups were included, the proportions of older immunosuppressed and other individuals with comorbidities were smaller and do not represent the general population. Because of the small numbers in some populations with comorbidities in our study, future studies should investigate antibody response following vaccination in larger cohorts. It is also important to note that, although IgG concentrations were examined in the full health-care workers cohort, IgA and IgM data were assessed from the enriched comorbidities subgroup. Females were also over-represented in our cohort; nevertheless, this large cohort represents health-care workers, who are typically younger and healthy populations, with a higher proportion of females but are an important group in the population who are at high risk and have high exposure to COVID-19. High correlation between IgG and neutralising antibodies was observed as well as differences in antibody concentrations between distinct populations; however, it is important to keep in mind that other immune responses such as T cells6 contribute to vaccination efficacy. Moreover, since correlates of protection have not yet been determined, we are unable to predict antibody and neutralizing titres needed for protection. We also cannot entirely exclude the possibility that pre-vaccination infections could have been missed due to an absence of PCR testing or waning antibodies, although this is highly unlikely as we did not observe a significant and rapid antibody response following the first vaccine dose in our cohort as one study recently showed.18 Further studies are needed to define correlates of protection and assess the clinical significance of this occurrence and clinical determinants of antibody titres in other distinct comorbidity groups, as well as other innate and adaptive immune responses.
In this study we found that almost all study participants developed IgG and neutralising antibodies rapidly after two doses, 3 weeks apart, of BNT162b2 COVID-19 vaccine. Furthermore, we showed a significantly high correlation between IgG and neutralising antibodies and show age related, sex-related, and comorbidity-related reactivity of IgG. Finally, our study showed that individuals with certain comorbidities, especially older age and immunosuppression mount a significantly lower antibody response following the first dose compared with younger and healthy adults, suggesting that the longer gap between vaccine doses, currently in effect in some countries, should be re-evaluated, especially to these more susceptible populations. Overall, our study is the first to assess the early response to vaccination among a large cohort composed of several demographic populations and comorbidities. Follow-up studies on these individuals are needed to evaluate the extended response to vaccination and breakthrough infections, which can shed light on the correlation of protection of this promiscuous virus.
Data sharing
De-identified clinical data for the patients in this study might be made available to other investigators after approval by the institutional review board. Requests should be directed to the corresponding author (YL).
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the internal sources of Sheba Medical Center (Tel-Hashomer, Israel) and the Central Virology Laboratory of Israel's Ministry of Health (Tel-Hashomer, Israel). YL is supported by the Nehemia Rubin Excellence in Biomedical Research—The TELEM Program of Chaim, Sheba Medical Center. We thank Ravit Koren, Shiri Katz-Likvornik, Osnat Halpern, Tal Levin, and Yara Kanaaneh (Central Virology Laboratory of Israel's Ministry of Health, Tel-Hashomer, Israel) for their technical assistance.
Contributors
YL and ES contributed to study concept and design, data collection, analysis and interpretation of data, and drafting of the manuscript. GR-Y contributed to study concept and design, analysis and interpretation of data, and drafting of the manuscript. CC contributed to data collection; administrative, technical, or material support; and critical revision of the manuscript for important intellectual content. RF and LO contributed to statistical analysis, and critical revision of the manuscript for important intellectual content. VI, MM, SA, and EM contributed to critical revision of the manuscript for important intellectual content. RD contributed to data collection, and critical revision of the manuscript for important intellectual content. AZ, AH, CR, and LF contributed to statistical analysis, and critical revision of the manuscript for important intellectual content. YK contributed to study concept and design, critical revision of the manuscript for important intellectual content, and supervision. All authors reviewed and approved the final manuscript. YL, ES, and GR-Y accessed and verified the data and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices' interim recommendation for use of Pfizer–BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration Pfizer–BioNTech COVID-19 vaccine. Vaccines and Related Biological Products Advisory Committee briefing document, 2020. Sponsor: Pfizer and BioNTech. Dec 10, 2020. https://www.fda.gov/media/144245/download
- 5.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 7.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 8.Israel Ministry of Health Israel COVID-19 data tracker. Cases and deaths dashboard. https://datadashboard.health.gov.il/COVID-19/general?utm_source=go.gov.il&utm_medium=referral
- 9.Oved K, Olmer L, Shemer-Avni Y, et al. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Indenbaum V, Koren R, Katz-Likvornik S, et al. Testing IgG antibodies against the RBD of SARS-CoV-2 is sufficient and necessary for COVID-19 diagnosis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch KL, Whitman JD, Lacanienta NP, et al. Magnitude and kinetics of anti-SARS-CoV-2 antibody responses and their relationship to disease severity. Clin Infect Dis. 2021;72:301–308. doi: 10.1093/cid/ciaa979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernal JL, Andrews N, Gower C, et al. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. medRxiv. 2021 doi: 10.1101/2021.03.01.21252652. published online March 2. (preprint). [DOI] [Google Scholar]
- 14.Mantovani A, Byrne CD, Zheng MH, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30:1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandi ML, Giustina A. Sexual dimorphism of coronavirus 19 morbidity and lethality. Trends Endocrinol Metab. 2020;31:918–927. doi: 10.1016/j.tem.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified clinical data for the patients in this study might be made available to other investigators after approval by the institutional review board. Requests should be directed to the corresponding author (YL).