Abstract
PURPOSE
Anti-GD2 monoclonal antibody (mAb) has proven efficacy in high-risk neuroblastoma (HR-NB). A small phase I GD2/GD3 vaccine trial (n = 15) described long-term survival and a favorable safety profile among patients with a history of disease progression (PD). The kinetics of mounting antibody response to vaccine and its prognostic impact on survival are now investigated in a phase II study (ClinicalTrials.gov identifier: NCT00911560).
PATIENTS AND METHODS
One hundred two patients with HR-NB who achieved remission after salvage therapies were enrolled in this trial. They received seven subcutaneous injections of GD2/GD3 vaccine spanning 1 year plus oral β-glucan starting at week 6 after the third dose of vaccine. Serum anti-vaccine antibody titers were quantified by enzyme-linked immunosorbent assay. Single nucleotide polymorphisms (SNPs) were determined by quantitative polymerase chain reaction. Kaplan-Meier and landmark Cox Regression models were used for survival estimates.
RESULTS
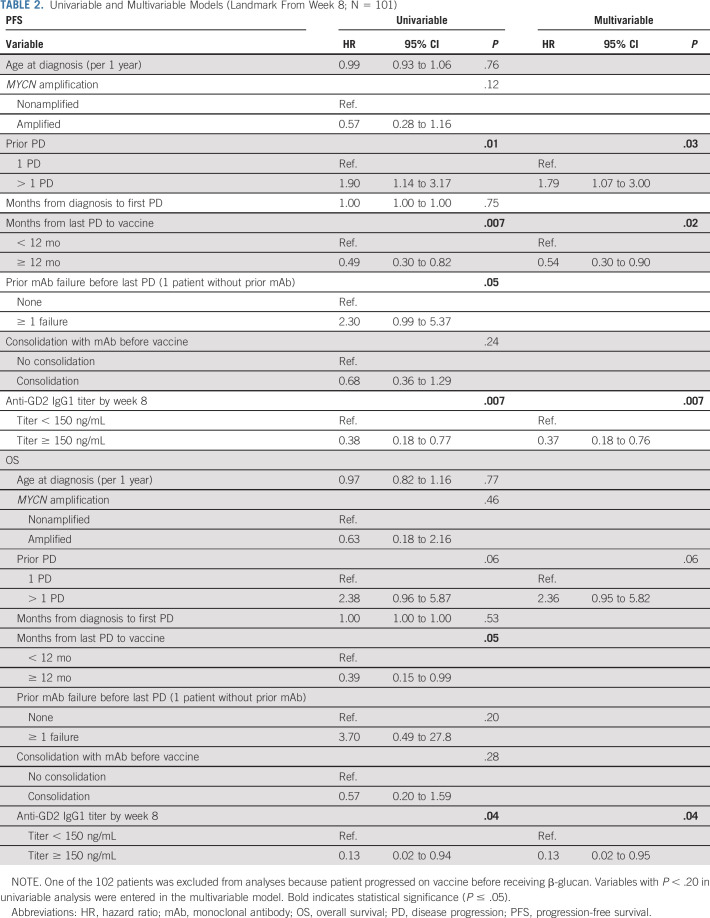
Patients had a history of one (63%), two (21%), or three to six (16%) episodes of PD. 82% of them progressed following anti-GD2 mAb (m3F8/dinutuximab/naxitamab) therapy. Vaccine-related toxicities were self-limited injection–associated local reactions and fever without any > grade 3 toxicities. The progression-free survival (PFS) was 32% ± 6%, and the overall survival (OS) was 71% ± 7% at 5 years. Serum anti-GD2 (immunoglobulin G1 [IgG1] and IgM) and anti-GD3 (IgG1) titers showed notable increases following the initiation of β-glucan at week 6. There was an association between IgG1 titer and SNP rs3901533 of dectin-1, the β-glucan receptor. Multivariable analyses showed that anti-GD2-IgG1 titer ≥ 150 ng/mL by week 8 was associated with favorable PFS and OS, while having prior episodes of PD and the time from last PD to vaccine were associated with PFS.
CONCLUSION
GD2/GD3 vaccine plus β-glucan elicited robust antibody responses in patients with HR-NB with prior PD. Higher anti-GD2-IgG1 titer was associated with improved survival.
INTRODUCTION
For children with high-risk metastatic and/or relapsed neuroblastoma, survival remains poor and long-term morbidities are common.1-3 Immunotherapy holds promise not just for the treatment of chemorefractory disease but also in improving long-term survival without additive toxicities. With the integration of anti-disialoganglioside (GD)2 monoclonal antibody (mAb) into the standard of care, 50%-60% of children with high-risk neuroblastoma (HR-NB) are long-term survivors.4,5
CONTEXT
Key Objective
Can children with high-risk neuroblastoma learn to make their own antitumor antibodies?
Knowledge Generated
Combining subcutaneous GD2/GD3 conjugate vaccine with oral β-glucan adjuvant was found to be safe and effective in inducing antibody response that correlated with dectin-1 receptor single nucleotide polymorphism rs3901533, a potential biomarker. In this phase II outpatient pediatric cancer vaccine trial among 102 patients with prior disease progression and significant prior chemotherapy, persistent anti-GD2 and anti-GD3 antibody responses could be rapidly induced, whereas high anti-GD2 immunoglobulin G1 titer was independently correlated with favorable progression-free survival and overall survival, without causing any neuropathic pain or neuropathy.
Relevance
This antineuroblastoma vaccine can potentially be a treatment alternative if anti-GD2 monoclonal antibody therapy is unsuccessful or unavailable. Since disialogangliosides GD2 and GD3 are expressed on other pediatric and adult solid tumors, this vaccine strategy deserves further investigation.
Successful vaccines could induce a protective antitumor immune response. Unlike protein antigens, GD2 and GD3, the carbohydrate antigens prevalent in NB, are poorly immunogenic, thus requiring strong and safe immune adjuvants.6 However, adjuvants for cancer vaccines currently in use or in development are all parenteral7,8 and do not fulfill all the benchmarks for efficacy: high seroconversion rates, robust and rapid immunoglobulin G (IgG) response, impact on disease outcome, and sustained immunological memory.9
To boost helper T cells, vaccines can be conjugated to highly immunogenic protein scaffolds like keyhole limpet hemocyanin (KLH)10 or the nontoxic diphtheria toxin CRM19711 and then combined with subcutaneous (sc) immune adjuvants like Quillaja saponaria (QS)-21.12,13 Despite conjugating GD2 to KLH, no anti-GD2 response was induced using adjuvant monophosphoryl lipid A.14 QS-21 plus ganglioside-KLH in patients with sarcoma induced mostly the immunoglobulin M (IgM) response15 without clinical benefit (ClinicalTrials.gov identifier: NCT01141491). Other vaccines targeting GM2 in melanoma,16 Globo H in breast cancer,17,18 and MUC1 in ovarian cancer19 have also failed to meet expectations. A common denominator was insufficient quality or quantity of antibody response.
We previously reported a phase I trial of GD2/GD3 vaccine in 15 patients with HR-NB and a history of disease progression (PD) treated in second or later remission.20 With 9.6 years of median follow-up, 14 of 15 patients are long-term survivors. The absence of any neurotoxicity was particularly reassuring given the expression of GD2 in both the central and the peripheral nervous systems and the well-documented neuropathic pain and neuropathies seen with anti-GD2 mAb therapy. Given the limitations of KLH and QS-21 alone, oral yeast β-glucan21,22 was added as an additional adjuvant. Glucans are polymers containing β-1,3-linked and β-1,4-D-glucose molecules with 1,6-linked side chains.23,24 Being a major fungal cell wall carbohydrate, glucan can activate several receptors on immune cells, including dectin-1 receptor (C-type lectin domain family 7 member A [CLEC7A]) on dendritic cells and macrophages.25 The human CLEC7A intronic single nucleotide polymorphisms (SNPs) rs3901533 and rs7309123 were associated with susceptibility to and severity of invasive fungal infections.26,27 We previously showed that oral β-glucans could enhance antibody-dependent cell-mediated cytotoxicity (ADCC) of anticancer antibodies.28-31 Good manufacturing practice (GMP) grade 1,3-1,4 (ClinicalTrials.gov identifier: NCT00492167) and 1,3-1,6 (ClinicalTrials.gov identifier: NCT00037011) purified β-glucans, administered orally, have proven to be safe in patients.32
Here, we report the results of a phase II trial of the GD2/GD3 vaccine in an expansion cohort of 102 patients with HR-NB in remission and a history of one or more episodes of PD. The kinetics of the anti-vaccine antibody response to sc KLH-conjugated GD2/GD3 plus QS21 was evaluated, with oral β-glucan being given from week 6 to the end of treatment. We also analyzed the impact of prognostic factors on survival and the possible role of dectin-1 receptor SNP as a biomarker of antibody response.
PATIENTS AND METHODS
Patient Eligibility
Patients with HR-NB staged by the International Neuroblastoma Staging System33 in clinical remission and assessed by the International Neuroblastoma Response Criteria (INRC)33 were enrolled on the Memorial Sloan Kettering Cancer Center (MSK) Protocol 05-075 (ClinicalTrials.gov identifier: NCT00911560) if they had a history of PD. Prior therapy had to be completed ≥ 21 days before enrollment. Eligibility criteria included MYCN-amplified stage 3 or 4 NB of any age and MYCN-nonamplified metastatic disease diagnosed at ≥ 18 months of age, absolute lymphocyte and neutrophil counts each ≥ 500/µL, major organ toxicity ≤ grade 3, and neurologic status ≤ grade 1. Institutional Review Board–approved informed consents were obtained from patients or their guardians. Patients who previously received GD2/GD3 vaccine were excluded from this analysis.
Protocol Study Design
GD2/GD3 vaccine treatment schedule and the source of vaccine and β-glucan were identical to those in the phase I trial.20 In brief, patients were given seven sc vaccine injections (week 1-2-3-8-20-32-52) plus oral β-glucan starting at week 6 at 40 mg/kg/d, 14 days on/14 days off, for a total planned 13 cycles. Each injection consisted of 30 μg of GD2 and 30 μg of GD3, which were stabilized as lactones, conjugated to KLH, and then mixed with OPT-821 (an analog of QS-21) at 150 μg/m2.20 The primary end point for the expansion cohort was progression-free survival (PFS) after the fourth dose of vaccine (week 8). Secondary end points were anti-GD2 and anti-GD3 antibody responses and their correlation with PFS and overall survival (OS), with PFS and OS starting from the first dose of vaccine. Toxicities were evaluated and scored using CTCAEv3.0 for 24 months after starting treatment or until PD, whichever was earlier.
Disease Evaluation
Computed tomography and/or magnetic resonance imaging plus scintigraphic studies (123I-metaiodobenzylguanidine scan) were performed at enrollment and then every 10-12 weeks through 24 months. Bone marrow (BM) aspirates and biopsies from bilateral posterior and anterior iliac crests for histology and pooled heparinized aspirates for minimal residual disease (MRD) were assessed. The response was evaluated by INRC.33
Blood Collection and Quantitation of Serum Anti-Vaccine Antibody by Enzyme-Linked Immunosorbent Assay
Patients had blood draw pretreatment, at ∼ weeks 3, 6, 9, 20, 32, 44, and 52 and at ∼3 months interval postvaccine. Sera were collected, frozen at −20°C for batch analyses, and quantified by enzyme-linked immunosorbent assay (ELISA).
Anti-GD2-IgG1.
GD2 at 20 ng/well was coated on 96-well microtiter plates, blocked with 0.5% bovine serum albumin (BSA) for 1 hour at room temperature (RT), and washed before incubation with patient serum diluted in 0.5% BSA. After 2.5 hours at 37°C and washing, peroxidase-conjugated mouse antihuman IgG1 was added. After incubation at 4°C for 1 hour and washing, chromogen o-phenylenediamine and substrate H2O2 were added for 30-minute incubation at RT. Color reaction was stopped using H2SO4, and optical density was read using an ELISA plate reader at 490 nm. Using anti-GD2 antibody Hu3F8-IgG1 as standard, the assay sensitivity was 0.6 ng/mL.
Anti-GD2-IgM.
Assay was similar to the anti-GD2-IgG1 ELISA, with the exception of using a high-IgM titer human serum to generate a standard curve, plus the addition of peroxidase-conjugated mouse antihuman IgM as the secondary antibody. IgM titer was expressed in U/mL with a detection limit of 0.3 U/mL.
Anti-GD3-IgG1.
GD3 at 20 ng/well was used to coat microtiter plates for the anti-GD3-IgG1 assay. The chimeric IgG1 anti-GD3 K6G antibody was used as the standard. Antibody titer was expressed in ng/mL with a detection limit of 8 ng/mL.
Anti-KLH-IgG1.
KLH at 250 ng/well was used to coat microtiter plates. The standard was a human serum with high levels of anti-KLH-IgG1. Antibody titer was expressed in U/mL with a detection limit of 1.25 U/mL.
MRD Detection
Quantitative reverse transcription-polymerase chain reaction to detect BM MRD was used as previously described.5
Dectin-1 (CLEC7A) Polymorphism Genotyping
40 ng of genomic DNA was used for SNP genotyping of rs16910526, rs7309123, and rs3901533 using the Applied Biosystems Sequence detection system 7300 as follows: an initial holding step of 10 minutes at 95°C, 40 cycles of 15-second denaturation at 92°C, and annealing plus extension for 1 minute at 60°C. Allelic discrimination of dectin-1 was identified using the ABI Sequence Detection System software.
Biostatistics
Survival rates using time from either the first dose of vaccine or week 8 through PD or death (PFS) or through death only (OS) were estimated using the Kaplan-Meier method and compared using the log-rank test. Patients alive without event were censored on the date of last follow-up. The potential follow-up duration was estimated using the reverse Kaplan-Meier method.34 Cox proportional hazards models were used for prognostic analyses. To be able to assess the impact of the antibody response at week 8 from starting vaccine, a landmark approach was used in the Cox models using 8 weeks as the landmark time, ie, PFS and OS were restricted to postweek 8 survivals. Prognostic impacts were expressed as hazard ratio (HR) (95% CI). Wilcoxon signed-rank tests were used to compare titer at different time points. Kruskal-Wallis tests were used for association studies between antibody titer from the initiation of β-glucan at week 6 to the end of therapy or PD and dectin-1 SNP.
RESULTS
Patient Characteristics
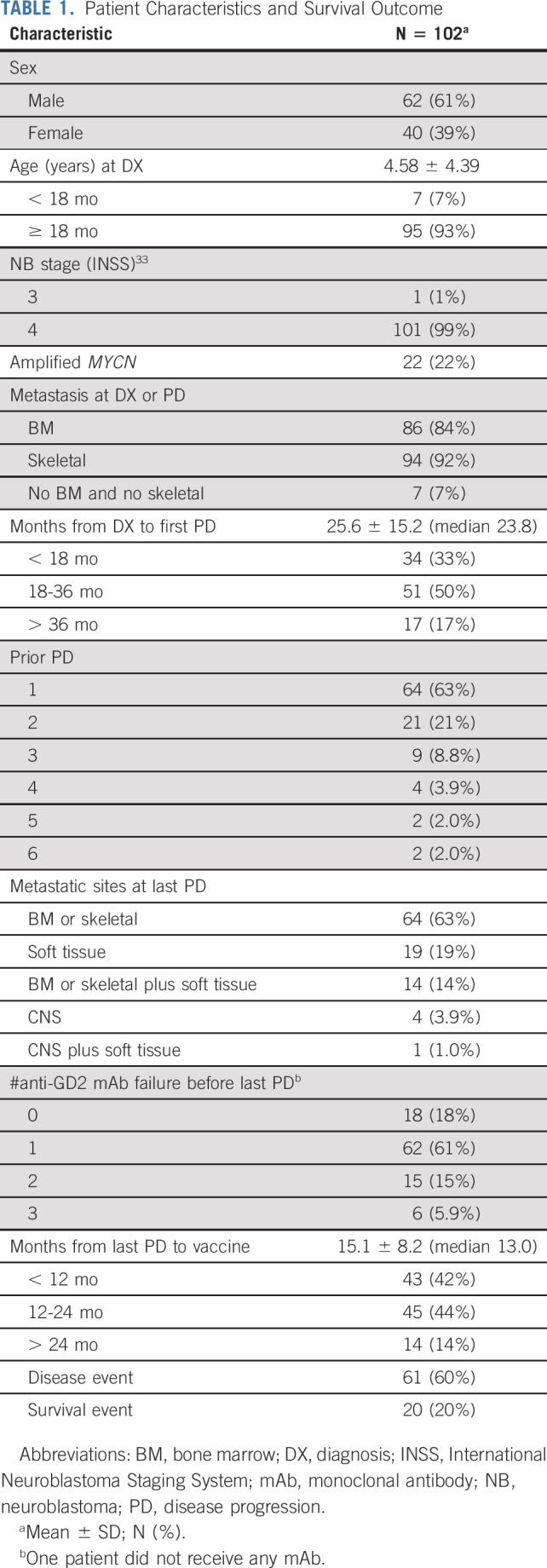
One hundred two patients with prior PD enrolled from December 2012 to September 2018 are subjects of this analysis. Prior administered therapies including induction chemotherapy, second-line therapy for refractory disease, myeloablative therapy with stem-cell transplant, anti-GD2 mAb immunotherapy, and salvage therapy for their last episode of PD before vaccine are summarized (Appendix Table A1, online only). Twenty-two percent of patients had MYCN-amplified tumors, 93% had BM and/or skeletal metastases at diagnosis and/or PD, and 5% had CNS metastasis at their last PD (Table 1). The median time from diagnosis to the first PD was 23.8 m. For 17% of the patients, the time from diagnosis to first progression was 36 months. However, PFS and OS for this cohort were not statistically different from patients who relapsed earlier than 36 months. Thirty-seven percent of patients had ≥ two episodes of PDs before enrollment, and 82% progressed despite receiving anti-GD2 mAb (m3F8, dinutuximab, or naxitamab) immunotherapy before their last PD. The median time from the last episode of PD to vaccine was 13.0 m with 58% > 12 m. Before vaccine therapy, 86 of 102 patients were consolidated with anti-GD2 mAb at a median time from mAb to vaccine of 7 m. All BM samples before vaccine enrollment were negative for MRD.
TABLE 1.
Patient Characteristics and Survival Outcome
Patient Outcome
The median follow-up was 3.4 years (range, 0.4-7.3). The PFS was 76.5% ± 4.2%, and the OS was 99% ± 1.0% at 6 months from the initiation of vaccine treatment. At 2 years, the PFS was 45.3% ± 5.0% and the OS was 88.4% ± 3.3% and 32.2% ± 6.4% and 70.7% ± 6.7% at 5 years, respectively (Fig 1A). As detailed in Table 2, patients with one episode of PD prior to study entry had better PFS and OS when compared with those with > 1 PD (Figs 1B and 1C). Favorable PFS but not OS was noted among patients who responded to mAb before their last PD, when compared with patients who progressed despite receiving mAb (Figs 1D and 1E). It was notable that the prolonged response to last salvage therapy (≥ 12 m to vaccine) was highly prognostic to survival outcomes (Figs 1F and 1G).
FIG 1.
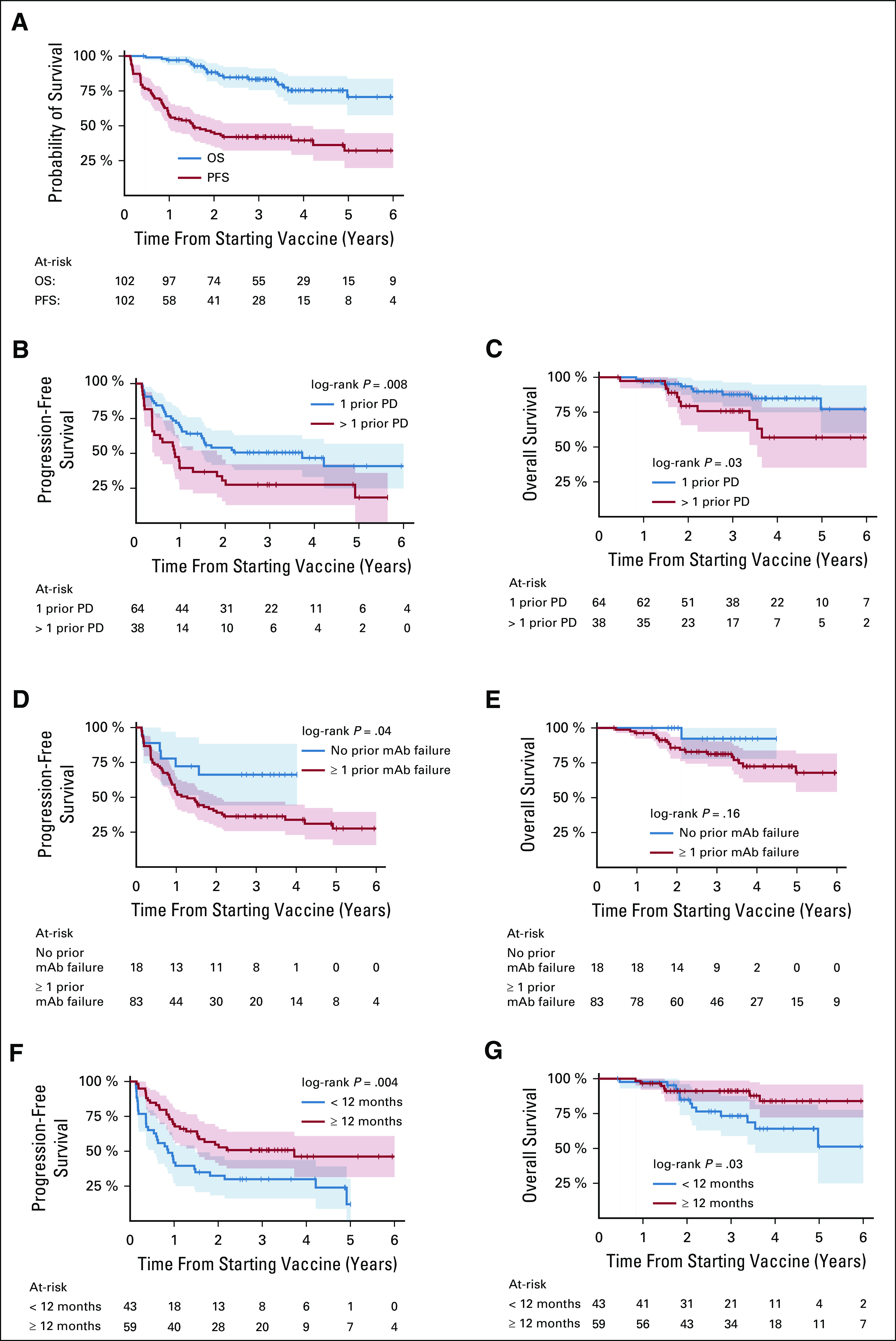
Survival outcome after vaccine therapy among 102 patients with high-risk neuroblastoma with prior disease progressions (PDs); progression-free survival (PFS) and overall survival (OS) (A); PFS (B) and OS (C) according to the number of episodes of PD prior to achieving CR and study enrollment; PFS (D) and OS (E) with respect to having successful anti-GD2 monoclonal antibody (m3F8, dinutuximab, or naxitamab) before their last PD; and PFS (F) and OS (G) according to the duration of response to last salvage therapy (≥ 12 m to vaccine). Survival time started from the first dose of vaccine. The shaded areas represent 95% CIs.
TABLE 2.
Univariable and Multivariable Models (Landmark From Week 8; N = 101)
In our cohort, 61 of 102 patients had PD after vaccine treatment, including 22 single site relapses. Forty-one remained alive with 16 achieving remission after further therapies that included anti-GD2 mAb and GD2/GD3 vaccine (Appendix Table A2).
Toxicity
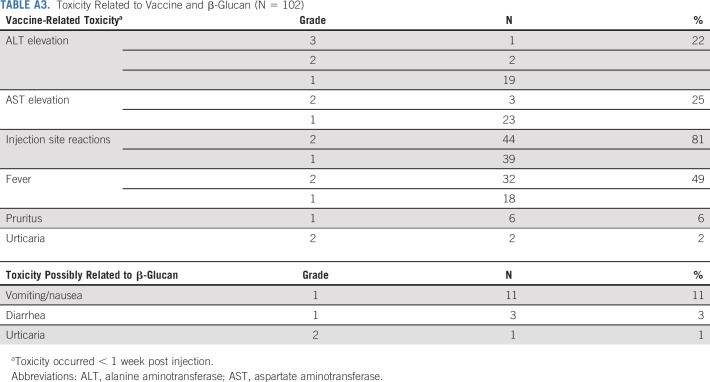
Grade 1 or 2 pain at injection sites was common. There were no related serious adverse events including > grade 3 toxicities, hospitalizations, neuropathy, neuropathic pain, ophthalmoplegia, or capillary leak syndrome. Toxicities related to vaccine and β-glucan are tabulated in Appendix Table A3. Fever was managed with antipyretics for < 48 hours, and all clinical toxicities lasted < 1 week from onset. All laboratory abnormalities were self-limited, and no long-term toxicities were reported.
Anti-Vaccine Antibody Response
The anti-GD3 IgG1 response was observed early (Fig 2C), showing statistically significant increase after the administration of oral β-glucan initiated at week 6. A similar pattern was observed for the anti-GD2 IgM response (Fig 2D). Anti-GD2 IgG1 titer (Fig 2A) rose steadily to plateau above 600 ng/mL at vaccine doses six and seven. This IgG response persisted, although diminishing over months when vaccine and β-glucan were stopped among 62 patients who completed all seven vaccine doses (Fig 2B). This kinetics contrasted with the peak anti-GD2-IgG1 titer of ∼100 ng/ml among six patients with melanoma receiving a comparable vaccine treatment that did not include oral β-glucan (Fig 2E).35
FIG 2.
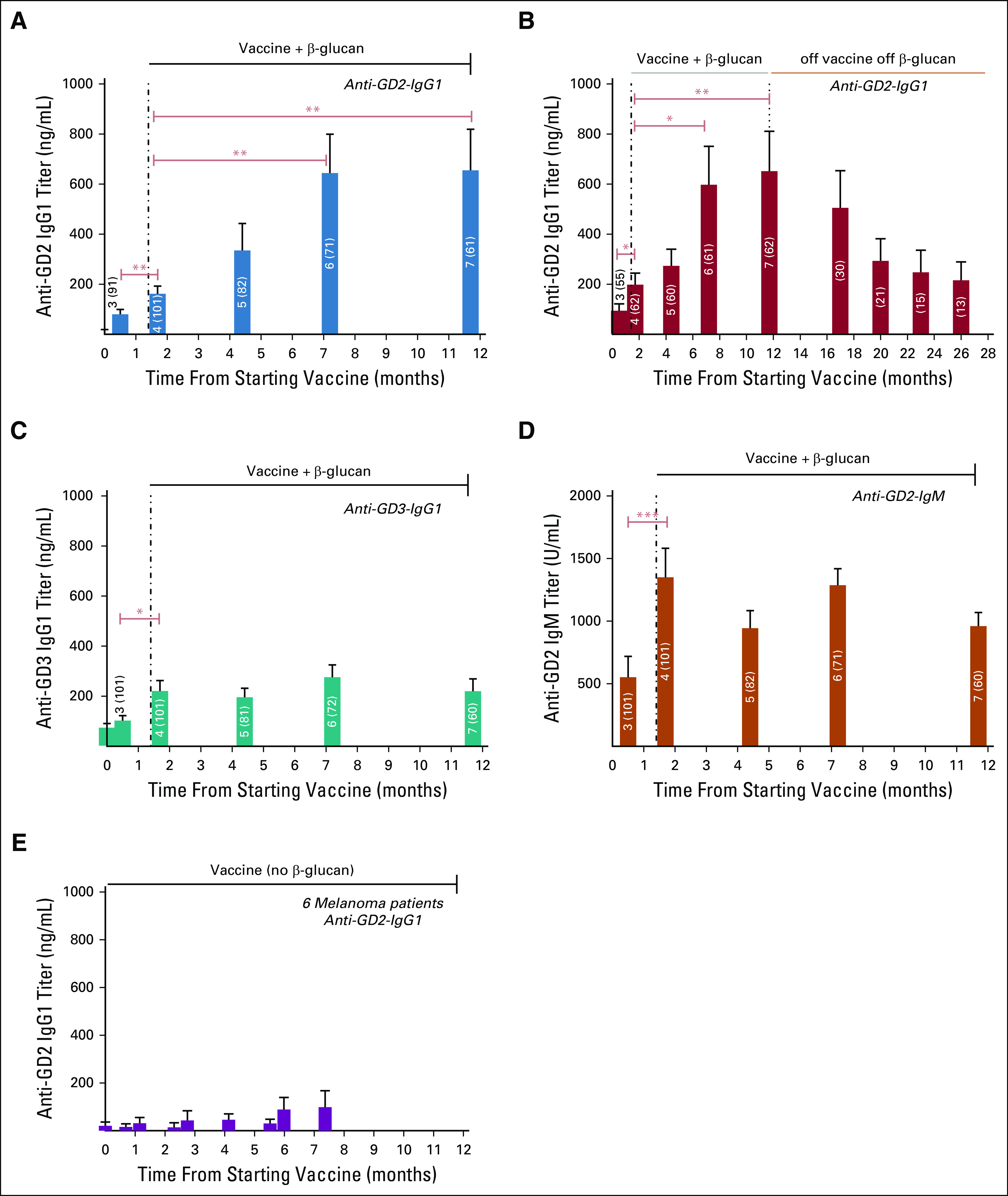
Antibody titer response during vaccine treatment among 101 patients with high-risk neuroblastoma with prior PD. One patient was excluded from this analysis because of progression before receiving β-glucan. β-glucan was initiated from week 6 to the end of vaccine (dose #7 or PD); number of each bar denotes dose #, and number in brackets denotes sample size; antibody titers were quantified by ELISA and expressed as mean + SEM; *P ≤ .05, **P ≤ .01, and ***P ≤ .001 by Wilcoxon signed-rank tests. During vaccine therapy: Anti-GD2-IgG1 titer (A), anti-GD3-IgG1 (C), and anti-GD2-IgM (D); persistence of anti-GD2-IgG1 titer among 62 patients who completed seven doses of vaccine therapy and off β-glucan (B). Anti-GD2-titer of six patients with melanoma receiving a comparable vaccine treatment that did not include oral β-glucan (E).35 PD, disease progression.
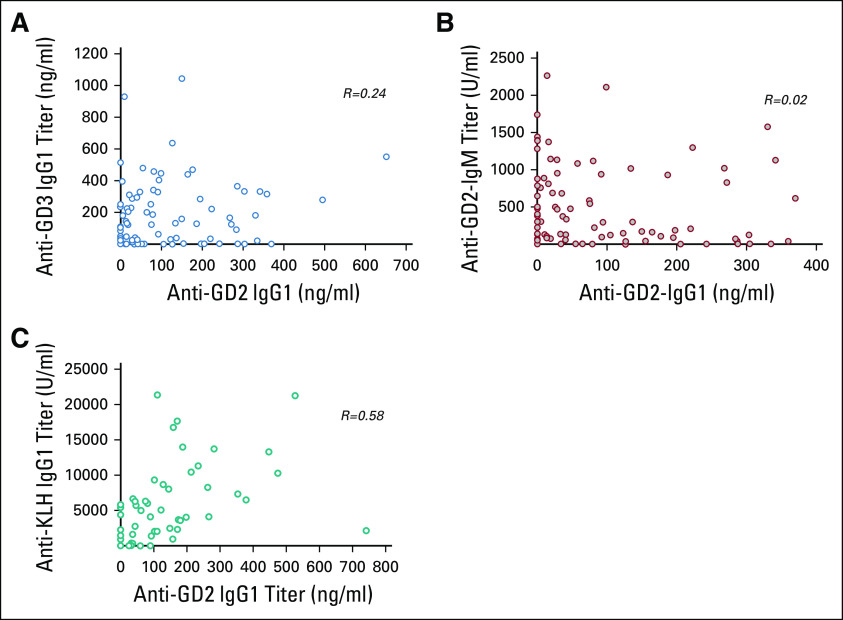
When anti-GD2-IgG1 week 8 titer (after the initiation of β-glucan) was dichotomized using the third quartile at 150 ng/mL, the antibody response was found to be prognostic for PFS and OS (Table 2; Figs 3A and 3B), respectively. This cut point for seroconversion was chosen because it was the in vitro humanized anti-GD2 antibody concentration to achieve maximal ADCC and complement-mediated cytotoxicity. In contrast, neither the induction of anti-GD3-IgG1 titer (Figs 3C and 3D) nor anti-GD2-IgM titer (Figs 3E and 3F) by week 8 was prognostic for PFS and OS, respectively. Anti-GD2-IgG1 titer did not correlate with anti-GD3-IgG, anti-GD2-IgM, or anti-KLH-IgG1, suggesting that the prognostic importance of anti-GD2 IgG1 seroconversion was independent of the global vaccine response (Appendix Fig A1).
FIG 3.
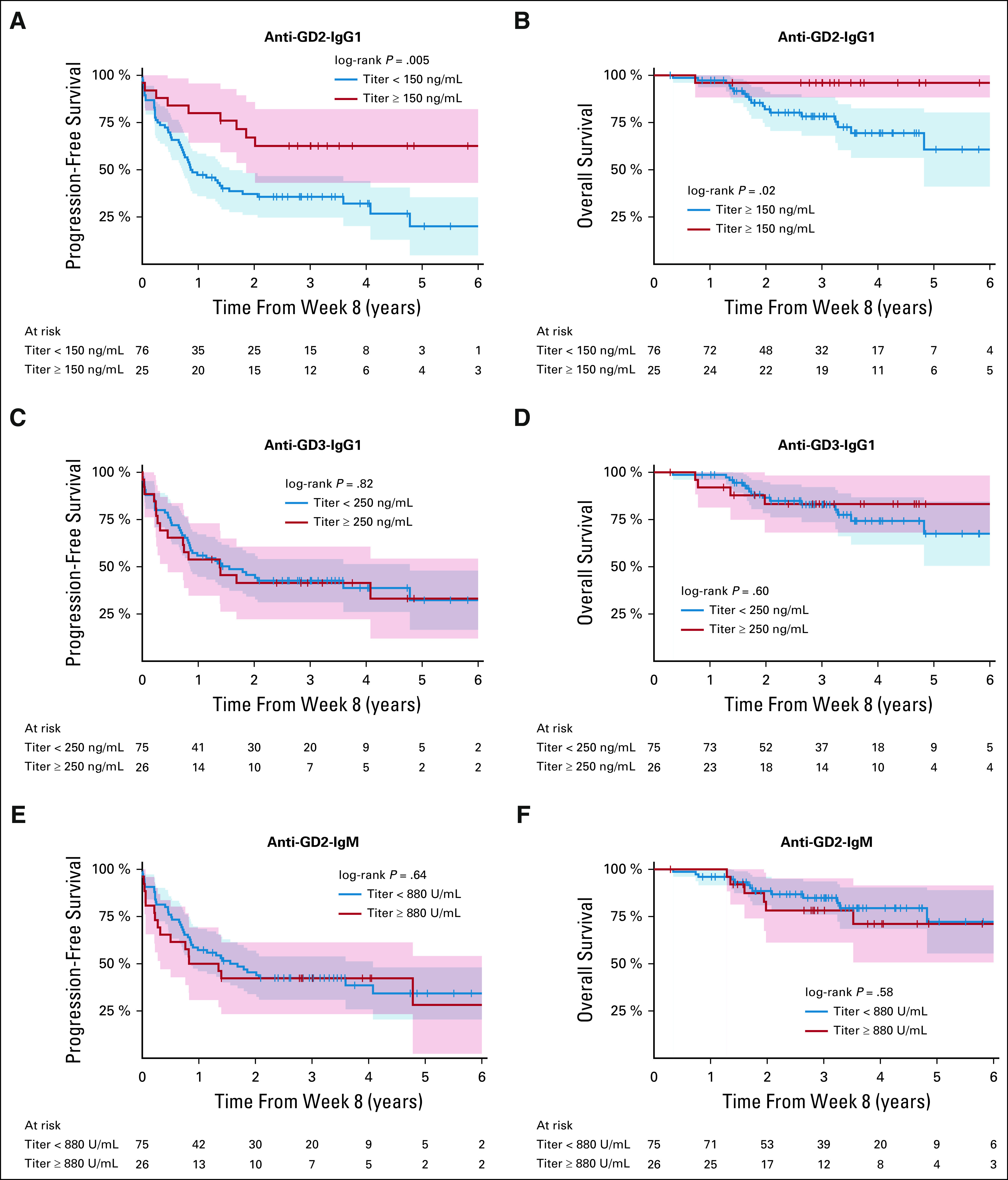
PFS (A) and OS (B) by induction of anti-GD2-IgG1 titer (≥ 150 ng/mL) by week 8, PFS (C) and OS (D) by anti-GD3-IgG1 titer by week 8, and PFS (E) and OS (F) by anti-GD2-IgM titer by week 8. Titer was dichotomized using the third quartile as high titer. One patient was excluded for this analysis because of PD before receiving β-glucan at week 6. Survival time started from week 8 of vaccine. The shaded areas represent 95% CIs. OS, overall survival; PFS, progression-free survival.
Univariable and Multivariable Prognostic Analyses
Univariable models identified the following statistically significant prognostic factors for both PFS and OS: time from last PD to vaccine and the induction of anti-GD2-IgG1 titers by week 8 (Table 2). The variables Prior PD and Prior mAb failure before last PD were associated with PFS but not OS. Consolidating patients with mAb before vaccine was not associated with improved outcome, likely because of vaccine therapy overriding mAb's influence.
By including variables with P < .20 in the univariable analysis to the multivariable model, several variables were independently associated with favorable survival outcome: Anti-GD2-IgG1 titer ≥ 150 ng/mL by week 8 for PFS and OS. Having only one prior episode of PD and ≥ 12 m from last PD to vaccine was associated with improved PFS.
Association Between Antibody Response with Dectin-1 (CLEC7A) Single Nucleotide Polymorphism
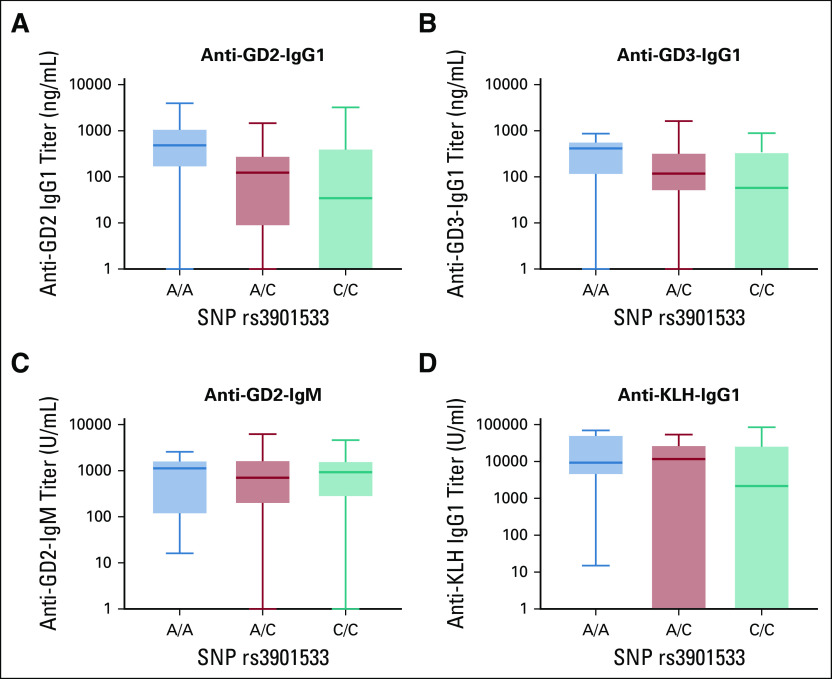
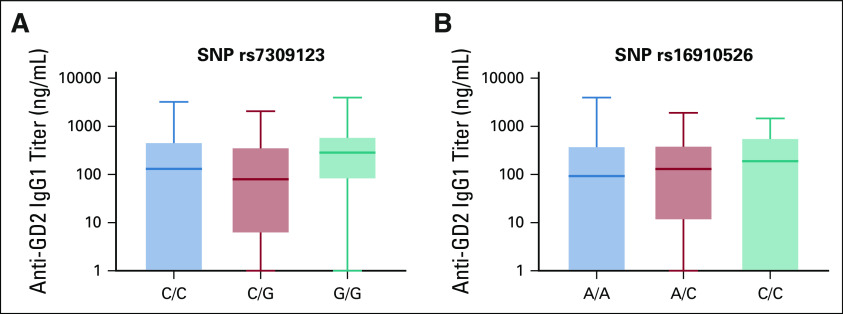
Given the adjuvant effect of oral β-glucan (Fig 2), three SNPs of dectin-1, the receptor for β-glucan, were correlated with the antibody titer from week 6 after the initiation of β-glucan until the end of treatment or PD. SNP rs3901533 had an association with anti-GD2 and anti-GD3 IgG1 titers (P = .020 and P = .042, respectively), but not anti-GD2-IgM (P = .87) nor anti-KLH-IgG1 (P = .053) (Figs 4A-D). No association with antibody titer was observed using SNP rs7309123 (P = .10) or rs16910526 (P = .82) (Appendix Fig A2).
FIG 4.
Association between antibody titer and SNP rs3901533 of dectin-1 (C-type lectin domain family 7 member A) polymorphism (N = 101). Antibody titer for individual patient was measured from the initiation of β-glucan at week 6 to the end of vaccine treatment or disease progression. Average antibody titer over time was tabulated for each patient. Statistics was performed using Kruskal-Wallis test. One patient was excluded from this analysis because of progression before receiving β-glucan. Association was tested in anti-GD2-IgG1 (A), anti-GD3-IgG1 (B), anti-GD2-IgM (C), and anti-KLH-IgG1 (D). KLH, keyhole limpet hemocyanin; SNP, single nucleotide polymorphism.
DISCUSSION
Based on the long-term survival with favorable safety profile of our earlier phase I trial of 15 patients, this phase II expansion cohort of 102 patients with HR-NB with negative marrow MRD provided additional information on survival spanning 6+ years and the kinetics of anti-vaccine antibody response. We identified the importance of eliciting anti-GD2 IgG1 antibody in improving outcome and the possible role of dectin-1 receptor SNP as a biomarker of the antibody response. The absence of pain and neurological adverse events typically seen with anti-GD2 mAb therapies was notable, suggesting an antibody concentration threshold for such sequelae and qualitative differences between the polyclonal antibody response to the vaccine and monoclonal anti-GD2 therapy.
Active immunity has theoretical advantages over mAb therapy. The induced response to vaccine is polyclonal, and autoimmune epitopes could potentially be avoided because they were either already deleted or tolerized. Polyclonal antibody responses could exploit the cooperative antibody effect not possible with mAb. More importantly, the continuous presence of antitumor antibody, in contrast to bolus injections of mAb, could improve antitumor control. This is of particular importance if GD2 expression in neuroblastoma waxed and waned.36 Success in building ganglioside-specific B cell memory should ensure a rapid recall response if tumors were to recur. These antiganglioside immune responses might prevent the emergence of other GD2(+) or GD3(+) tumors during the patient's lifetime, since soft tissue sarcoma, osteosarcoma, melanoma, and small-cell lung cancer all have abundant expression of GD2 and GD3.37,38
Conjugating the carbohydrate disialogangliosides GD2 and GD3 to the protein carrier KLH and combining with sc OPT-821 was insufficient for a robust IgG response.15 In the current study when oral β-glucan was initiated at week 6, both IgM and IgG1 titers rose, peaking within months. Compared with a historical control group of patients with melanoma receiving GD2 vaccine without β-glucan (Fig 2E),35 the anti-GD2-IgG1 titer increase was notable. This IgG response was an independent predictor of PFS and OS (Table 2), although it did diminish after vaccine and β-glucan were completed. Our study confirmed that carbohydrate epitopes like ganglioside GD2 and GD3 could be rendered immunogenic, stimulating not just IgM but also the IgG1 antibody response in children. The observation that young children, recovering from repeated damage of their immune system by high-dose chemotherapy, were able to mount an effective anticarbohydrate antibody response was reassuring.
Although the induction of anti-GD2-IgG1 was prognostic of patient outcome, neither anti-GD2-IgM, anti-GD3-IgG1, nor anti-KLH response showed any association with survival. Anti-GD2 IgM has limited antitumor properties, and KLH is not expressed on neuroblastoma and hence the lack of benefit from either. However, the absence of benefit of anti-GD3 antibody was surprising, since its presence on neuroblastoma was detected by immunohistochemistry in previous studies.38 This suggests that either GD3 was downregulated among these relapsed HR-NBs or anti-GD3 antibodies were not sufficiently high or active.39-41
Both GD2- and GD3-associated IgG1 titer correlated with SNP rs3901533 of dectin-1, suggesting the possible role of dectin-1 receptor in seroconversion via the adjuvant effect of oral β-glucan. The anti-GD2 IgM response, which was T helper independent, had no correlation with SNPs. Neither GD2 nor GD3 alone could activate helper T cells; hence, a helper effect had to be provided by KLH, the carrier protein. Unlike anti-GD2 or anti-GD3 titers, SNP association with anti-KLH IgG1 was trending toward statistical significance. This was likely a result of the effect size by dectin-1 SNP, being much more relevant for weak immunogens like GD2 or GD3 than for powerful T cell antigens like KLH. SNP rs3901533 could potentially be a biomarker for response to this carbohydrate vaccine.
In our previous report of anti-GD2 mAb m3F8 plus GM-CSF of 101 patients in clinical remission after PD, relapse was typically followed by death, such that OS and PFS converged by year 5.42 When PD was isolated to CNS43 or to single bone sites, long-term PFS was possible. In our current experience with vaccine given to patients with HR-NB who achieved clinical remission after PD, instead of OS and PFS curve convergence, there was a clear separation at least through year 5 from the start of vaccine therapy (Fig 1A).
Despite our optimism regarding the results of this trial, it must be emphasized that it was a single-arm single-institution study with potential for selection bias. Patients with different numbers of prior episodes of PD and therapies were included, even though we did find that patients with only one episode of PD had better PFS. Because patients with HR-NB have now routinely received mAb therapy as their standard of care before receiving vaccine therapy, it was not easy to tease out their potential influence on vaccine efficacy, especially when a large subset had further consolidation with mAb right before vaccine. The relative importance and interaction of these two modes of immunotherapy can only be addressed in future studies. Given the limitation of single-arm studies, a randomized trial of GD2/GD3 vaccine following uniform upfront regimen should yield a more definitive verdict on its efficacy.
ACKNOWLEDGMENT
We would like to thank our neuroblastoma clinical trial nurses and nurse practitioners for administering the vaccine and the pediatric pharmacists for dispensing the vaccine and the β-glucan. Our thanks to Biotec Pharmacon for providing MSK GMP grade β-glucan for this trial.
APPENDIX
TABLE A1.
Prior Treatment Details (N = 102)
TABLE A2.
Survival Outcome After Vaccine With Respect to the Number of Prior PD (N = 102)
TABLE A3.
Toxicity Related to Vaccine and β-Glucan (N = 102)
FIG A1.
Anti-GD2-IgG1 titer by week 8 did not correlate with the week 8 titers of anti-GD3-IgG1 (A), anti-GD2-IgM (B), and anti-KLH-IgG1 (C). KLH, keyhole limpet hemocyanin; R, correlation coefficient.
FIG A2.
No association was observed between anti-GD2-IgG1 titer with SNP rs7309123 (A) and rs16910526 of dectin-1 polymorphism (B) (N = 101). Antibody titer for individual patients was measured from the initiation of β-glucan at week 6 to the end of vaccine treatment or disease progression. Average antibody titer over time was tabulated for each patient. Statistics was performed using Kruskal-Wallis test. One patient was excluded from this analysis because of progression before receiving β-glucan. SNP, single nucleotide polymorphism.
PRIOR PRESENTATION
Presented in part at the AACR Virtual Annual Meeting, June 22, 2020.
SUPPORT
Supported in part by grants from the Band of Parents, Kids Walk for Kids with Cancer, Cycle for Survival, Arms Wide Open Childhood Cancer Foundation, Cookies for Kids' Cancer, End Kids Cancer, Press On Foundation, the National Cancer Institute grant P30 CA008748, and MSK's Clinical Grade Production Facility for vaccine production.
AUTHOR CONTRIBUTIONS
Conception and design: Irene Y. Cheung, Nai-Kong V. Cheung, Shakeel Modak, Govind Ragupathi, Brian H. Kushner
Administrative support: Nai-Kong V. Cheung
Collection and assembly of data: Irene Y. Cheung, Nai-Kong V. Cheung, Shakeel Modak, Ellen Basu, Stephen S. Roberts, Brian H. Kushner
Data analysis and interpretation: Irene Y. Cheung, Nai-Kong V. Cheung, Shakeel Modak, Audrey Mauguen, Yi Feng, Brian H. Kushner
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Survival Impact of anti-GD2 Antibody Response in a Phase II Ganglioside Vaccine Trial Among Patients With High-Risk Neuroblastoma With Prior Disease Progression
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Irene Y. Cheung
Stock and Other Ownership Interests: Y-mAbs Therapeutics (I), Eureka Therapeutics (I), Abpro (I)
Patents, Royalties, Other Intellectual Property: Methods for detecting MRD, Dectin-1 (CLEC7A) single nucleotide polymorphism as a biomarker for predicting antibody response when using beta-glucan as a vaccine adjuvant
Nai-Kong V. Cheung
Stock and Other Ownership Interests: Ymabs Therapeutics Inc, Abpro, Eureka
Consulting or Advisory Role: Abpro, Eureka Therapeutics
Research Funding: Ymabs Therapeutics Inc, Abpro
Patents, Royalties, Other Intellectual Property: scfv constructs of anti-GD2 antibodies, therapy-enhancing glucan, use of mAb 8H9, methods for preparing and using scFv, GD2 peptide mimics, methods for detecting MRD, anti-GD2 antibodies, generation and use of HLA-A2–restricted peptide-specific mAbs and CARs, high-affinity anti-GD2 antibodies, multimerization technologies, bispecific HER2 and CD3 binding molecules, affinity matured hu8H9, anti-chondroitin sulfate proteoglycan 4 antibodies and uses thereof, ROR2 antibodies, T cell receptor-like antibody agents specific for EBV latent membrane protein 2A peptide presented by human HLA, anti-CD33 antibody agents, anti-KIR3DL1 antibodies, modular self assembly disassembly (SADA) technologies, A33-C825 conjugate for pretargeted radioimmunotherapy and application as a theranostic product, anti-L1-CAM antibodies and uses thereof, anti-A33 antibodies and uses thereof, DOTA BsAb for new humanized next generation anti-GPA33 antibodies with Fc-enhanced function or bispecific properties, herceptin-C825 conjugate for pretargeted radioimmunotherapy and application as a theranostic product, anti-polysialic acid antibodies and uses thereof, methods of enhancing immunogenicity of poorly immunogenic anti-specific vaccines using oral yeast beta-glucans, small molecule hapten chelates for pretargeted radioimmunotherapy with anti-DOTA(lanthanide) bispecific antibodies (proteus), a N-acetylgalactosamino dendron-clearing agent for DOTA-pretargeted radioimmuno-therapy, 30. Heterodimeric tetravalency and specificity antibody compositions and uses thereof (HDTVS), multimerization of IL-15/IL-15R alpha complexes to enhance immunotherapy, CD22 antibodies and methods of using the same, CD33 antibodies and methods of using the same to treat cancer, CD19 antibodies and methods of using the same, anti-CD33 antibodies for treating cancer, anti-STEAP-1 antibodies and uses thereof, anti-Glypican 3 antibodies and uses thereof, multimodal fluorine-Cy3/5/7-DOTA-hapten compositions, diagnostics, fluorescence guided surgery and radioimmunotherapy, anti-CD3 antibodies and uses thereof. Anti-CD3 antibodies and uses thereof, anti-GD2 SADA conjugates and uses thereof, anti-GD2 antibodies and uses thereof, Dectin-1 (CLEC7A) single nucleotide polymorphism as a biomarker for predicting antibody response when using b-glucan as a vaccine adjuvant
Travel, Accommodations, Expenses: Partners Therapeutics
Shakeel Modak
Consulting or Advisory Role: Ymabs Therapeutics Inc, Illumina RP
Patents, Royalties, Other Intellectual Property: Two patents pending; no financial benefit
Govind Ragupathi
Stock and Other Ownership Interests: Y-mAbs Therapeutics, Inc, Adjuvance Technologies Consulting or Advisory Role: Y-mAbs Therapeutics, Inc
Research Funding: Y-mAbs Therapeutics, Inc Patents, Royalties, Other Intellectual Property: Y-mAbs Therapeutics, Inc, Adjuvance Technologies Licensed Intellectual Property Rights from MSKCC. I am a co-inventor of both patents.
No other potential conflicts of interest were reported.
REFERENCES
- 1.Basta NO, Halliday GC, Makin G, et al. Factors associated with recurrence and survival length following relapse in patients with neuroblastoma Br J Cancer 1151048–10572016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.London WB, Bagatell R, Weigel BJ, et al. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children's Oncology Group early-phase trials Cancer 1234914–49232017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreitz K, Ernst A, Schmidt R, et al. A new risk score for patients after first recurrence of stage 4 neuroblastoma aged >/=18 months at first diagnosis Cancer Med 87236–72432019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma N Engl J Med 3631324–13342010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung NK, Cheung IY, Kushner BH, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission J Clin Oncol 303264–32702012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Hagan DT, Friedland LR, Hanon E, et al. Towards an evidence based approach for the development of adjuvanted vaccines Curr Opin Immunol 4793–1022017 [DOI] [PubMed] [Google Scholar]
- 7.Saxena M, Bhardwaj N.Turbocharging vaccines: Emerging adjuvants for dendritic cell based therapeutic cancer vaccines Curr Opin Immunol 4735–432017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mbow ML, De Gregorio E, Valiante NM, et al. New adjuvants for human vaccines Curr Opin Immunol 22411–4162010 [DOI] [PubMed] [Google Scholar]
- 9.Coffman RL, Sher A, Seder RA.Vaccine adjuvants: Putting innate immunity to work Immunity 33492–5032010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston PO, Ragupathi G.Cancer vaccines targeting carbohydrate antigens Hum Vaccin 2137–1432006 [DOI] [PubMed] [Google Scholar]
- 11.Jaffe J, Wucherer K, Sperry J, et al. Effects of conformational changes in peptide–CRM197 conjugate vaccines Bioconjug Chem 3047–532019 [DOI] [PubMed] [Google Scholar]
- 12.Del Giudice G, Rappuoli R, Didierlaurent AM.Correlates of adjuvanticity: A review on adjuvants in licensed vaccines SSemin Immunol 3914–212018 [DOI] [PubMed] [Google Scholar]
- 13.Lacaille-Dubois MA. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review. Phytomedicine. 2019;60:152905. doi: 10.1016/j.phymed.2019.152905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker R, Eichler MK, Jennemann R, et al. Phase I clinical trial on adjuvant active immunotherapy of human gliomas with GD2-conjugate Br J Neurosurg 16269–2752002 [DOI] [PubMed] [Google Scholar]
- 15.Carvajal RD, Agulnik M, Ryan CW, et al. Trivalent ganglioside vaccine and immunologic adjuvant versus adjuvant alone in metastatic sarcoma patients rendered disease-free by surgery: A randomized phase 2 trial J Clin Oncol 3210520–105202014 [Google Scholar]
- 16.Eggermont AMM, Suciu S, Rutkowski P, et al. Adjuvant ganglioside GM2-KLH/QS-21 vaccination versus observation after resection of primary tumor > 1.5 mm in patients with stage II melanoma: Results of the EORTC 18961 randomized phase III trial J Clin Oncol 313831–38372013 [DOI] [PubMed] [Google Scholar]
- 17.Danishefsky SJ, Shue YK, Chang MN, et al. Development of Globo-H cancer vaccine Acc Chem Res 48643–6522015 [DOI] [PubMed] [Google Scholar]
- 18.Huang CS, Yu AL, Tseng LM, et al. Randomized phase II/III trial of active immunotherapy with OPT-822/OPT-821 in patients with metastatic breast cancer J Clin Oncol 341003–10032016 [Google Scholar]
- 19.O’Cearbhaill RE, Deng W, Chen LM, et al. A phase II randomized, double-blind trial of a polyvalent vaccine-KLH conjugate (NSC 748933 IND# 14384) + OPT-821 versus OPT-821 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer who are in second or third complete remission: An NRG Oncology/GOG study Gynecol Oncol 155393–3992019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushner BH, Cheung IY, Modak S, et al. Phase I trial of a bivalent gangliosides vaccine in combination with beta-glucan for high-risk neuroblastoma in second or later remission Clin Cancer Res 201375–13822014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han B, Baruah K, Nguyen DV, et al. Beta-glucan's varying structure characteristics modulate survival and immune-related genes expression from Vibrio harveyi-infected Artemia franciscana in gnotobiotic conditions Fish Shellfish Immunol 102307–3152020 [DOI] [PubMed] [Google Scholar]
- 22.Petrovsky N, Cooper PD.Carbohydrate-based immune adjuvants Expert Rev Vaccines 10523–5372011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohn JA, BeMiller JN.(1-3)-B-D-Glucans as biological response modifiers: A review of structure-functional activity relationships Carbohydr Polymers 283–141995 [Google Scholar]
- 24.Mueller A, Raptis J, Rice PJ, et al. The influence of glucan polymer structure and solution conformation on binding to (1-->3)-beta-D-glucan receptors in a human monocyte-like cell line Glycobiology 10339–3462000 [DOI] [PubMed] [Google Scholar]
- 25.Dambuza IM, Brown GD.C-type lectins in immunity: recent developments Curr Opin Immunol 3221–272015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sainz J, Lupiáñez CB, Segura-Catena J, et al. Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary aspergillosis infection PLoS One 7:e32273.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou P, Xie Y, Yan Z, et al. Association between dectin-1 gene single nucleotide polymorphisms and fungal infection: A systemic review and meta-analysis Biosci Rep 39:BSR20191519.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung NK, Modak S.Oral (1-->3),(1-->4)-beta-D-glucan synergizes with antiganglioside GD2 monoclonal antibody 3F8 in the therapy of neuroblastoma Clin Cancer Res 81217–121232002 [PubMed] [Google Scholar]
- 29.Cheung NK, Modak S, Vickers A, et al. Orally administered beta-glucans enhance anti-tumor effects of monoclonal antibodies Cancer Immunol Immunother 51557–5642002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong F, Yan J, Baran JT, et al. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models J Immunol 173797–8062004 [DOI] [PubMed] [Google Scholar]
- 31.Modak S, Koehne G, Vickers A, et al. Rituximab therapy of lymphoma is enhanced by orally administered (1-->3),(1-->4)-D-beta-glucan Leuk Res 29679–6832005 [DOI] [PubMed] [Google Scholar]
- 32.Modak S, Kushner BH, Kramer K, et al. Anti-GD2 antibody 3F8 and barley-derived (1-->3),(1-->4)-beta-D-glucan: A phase I study in patients with chemoresistant neuroblastoma. Oncoimmunology. 2013;2:e23402. doi: 10.4161/onci.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment J Clin Oncol 111466–14771993 [DOI] [PubMed] [Google Scholar]
- 34.Schemper M, Smith TL.A note on quantifying follow-up in studies of failure time Control Clin Trials 17343–3461996 [DOI] [PubMed] [Google Scholar]
- 35.Ragupathi G, Livingston PO, Hood C, et al. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21 Clin Cancer Res 95214–52202003 [PubMed] [Google Scholar]
- 36.Schumacher-Kuckelkorn R, Volland R, Gradehandt A, et al. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence Pediatr Blood Cancer 6446–562017 [DOI] [PubMed] [Google Scholar]
- 37.Chang HR, Cordon-Cardo C, Houghton AN, et al. Expression of disialogangliosides GD2 and GD3 on human soft tissue sarcomas Cancer 70633–6381992 [DOI] [PubMed] [Google Scholar]
- 38.Dobrenkov K, Ostrovnaya I, Gu J, et al. Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults Pediatr Blood Cancer 631780–17852016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forero A, Shah J, Carlisle R, et al. A phase I study of an anti-GD3 monoclonal antibody, KW-2871, in patients with metastatic melanoma Cancer Biother Radiopharm 21561–5682006 [DOI] [PubMed] [Google Scholar]
- 40.Tarhini AA, Moschos SJ, Lin Y, et al. Safety and efficacy of the antiganglioside GD3 antibody ecromeximab (KW2871) combined with high-dose interferon-alpha2b in patients with metastatic melanoma Melanoma Res 27342–3502017 [DOI] [PubMed] [Google Scholar]
- 41.Alpaugh RK, von Mehren M, Palazzo I, et al. Phase IB trial for malignant melanoma using R24 monoclonal antibody, interleukin-2/alpha-interferon Med Oncol 15191–1981998 [DOI] [PubMed] [Google Scholar]
- 42.Kushner BH, Ostrovnaya I, Cheung IY, et al. Prolonged progression-free survival after consolidating second or later remissions of neuroblastoma with anti-GD2 immunotherapy and isotretinoin: A prospective phase II study. Oncoimmunology. 2015;4:e1016704. doi: 10.1080/2162402X.2015.1016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramer K, Kushner BH, Modak S, et al. Compartmental intrathecal radioimmunotherapy: Results for treatment for metastatic CNS neuroblastoma J Neurooncol 97409–4182010 [DOI] [PMC free article] [PubMed] [Google Scholar]