Abstract
Recent advances in peptide research revolutionized therapeutic discoveries for various infectious diseases. In view of the ongoing threat of the COVID-19 pandemic, there is an urgent need to develop potential therapeutic options. Intense and accomplishing research is being carried out to develop broad-spectrum vaccines and treatment options for corona viruses, due to the risk of recurrent infection by the existing strains or pandemic outbreaks by new mutant strains. Developing a novel medicine is costly and time consuming, which increases the value of repurposing existing therapies. Since, SARS-CoV-2 shares significant genomic homology with SARS-CoV, we have summarized various peptides identified against SARS-CoV using in silico and molecular studies and also the peptides effective against SARS-CoV-2. Dissecting the molecular mechanisms underlying viral infection could yield fundamental insights in the discovery of new antiviral agents, targeting viral proteins or host factors. We postulate that these peptides can serve as effective components for therapeutic options against SARS-CoV-2, supporting clinical scientists globally in selectively identifying and testing the therapeutic and prophylactic agents for COVID-19 treatment. In addition, we also summarized the latest updates on peptide therapeutics against SARS-CoV-2.
Keywords: SARS-CoV-2, Spike protein, Viral entry, Peptide therapeutics, Immune system
Introduction
Human coronaviruses, causing respiratory tract infections in humans, belong to the family Coronaviridae in the order Nidovirales. They are enveloped viruses with a single stranded positive sense RNA genome. Of the seven known corona viruses causing human infection, 229E, NL63, OC43 and HKU1 are known to cause mild common cold like disease [1]. The other three, including Severe Acute Respiratory Syndrome (SARS-CoV), Middle East Respiratory Syndrome (MERS-CoV) and the novel corona virus (SARS-CoV-2) cause severe respiratory infections and deadly pneumonia outbreaks. The disease SARS, the first known epidemic caused by a coronavirus, appeared in 2002 in China [2]. In 2012, MERS-CoV, responsible for the acute human respiratory syndrome, was identified in Jeddah, Saudi Arabia [3]. SARS-CoV and SARS-CoV-2 are Beta coronaviruses [4], which show extremely high homology at the nucleotide level, upon comparative genomic analysis [150]. They also share homology at the proteomic level, in the major viral proteins such as spike protein [5], RdRp, 3CLpro etc. [6] which are all key drug targets.
The outbreak of SARS-CoV-2 and the disease COVID-19 is the major public health emergency of this century. There have been many studies published about its clinical presentation, pathophysiology and animal models [7,8]. Though we have been able to develop some successful vaccines, there is an urgent need to develop effective anti-viral regimens and strategies. SARS-CoV-2 is more infectious and transmissible than previous coronaviruses. As of June 15, 2021, over 177,057,900 cases and 3,828,400 deaths have been reported globally. COVID-19 began as an outbreak of a mysterious illness, characterized by pneumonia, came from Wuhan, in the Chinese province of Hubei. The cause of the illness was later attributed to a new coronavirus [9,10], initially called the 2019-novel coronavirus (2019-nCoV). It is currently designated as the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) by the Coronavirus Study Group of the International Committee on Taxonomy of Viruses [11], due to its close resemblance with SARS-CoV, which appeared in 2002.
Characteristic features of SARS-CoV and SARS-CoV-2 proteins and the importance in drug discovery
There are four major structural proteins in SARS-CoV-2 such as the spike (S) protein, the envelope (E) protein, the membrane (M) protein, and the nucleocapsid (N) protein [12]. In addition, there are 16 non-structural proteins (NSPs), which may also be considered for drug development [13]. S protein consists of two units namely, S1 and S2. S1 region of S protein consists of a receptor-binding domain (RBD) involved in the entry and binding to the host receptor protein ACE2 and S2 involves in viral fusion [14,15]. M and E proteins are primarily involved in virus assembly. N protein is a multivalent RNA-binding protein critical for viral replication and genome packaging. Mutational changes in the structural and non-structural proteins being highly significant in virulence and virus spread, should also be considered while designing therapeutics and vaccines [16].
SARS-CoV-2 harbors significant genetic mutations which are completely new to the innate immune system of the human population. Studies show that, this virus is capable of affecting a large section of the human populations. Ganesh et al. have compiled the findings which can be made use of while therapeutics are being designed, on comparing the epidemiology and pathobiology of SARS-CoV-2 with SARS and MERS [17]
In the fight against coronaviruses, scientists developed three strategies for developing new drugs [18]. The first strategy is to test existing broad-spectrum antivirals [19]. Though such inhibitors have certain advantages such as clarity in terms of their metabolic characteristics, dosages used, potential efficacy, and side effects, their spectrum is too broad. The second strategy is high-throughput screening of existing molecular databases to screen for molecules that may have a therapeutic effect on coronaviruses [20,21]. The third strategy is to develop new targeted drugs, directly based on the genomic information and pathological characteristics of different coronaviruses. Based on these observations, we accumulated research findings related to a specific class of therapeutic options, the peptide therapeutics and their molecular mechanisms. The compilation of publications described in this review provides an overview of the use of peptide therapeutics developed against SARS-CoV, which could be repurposed for SARS-CoV-2, as well as an update of the peptides already developed against SARS-CoV-2. However, wet lab and clinical studies are necessary to completely understand SARS-CoV-2 infection and to improve the development of these potential therapeutic options.
Literature search
We performed a literature search on the Web of Science (ISI), PubMed, Embase, Medline, and Scopus databases with keywords such as SARS-CoV, 2019-nCoV, SARS-CoV-2, peptides, vaccines, and related words, for example, peptide sequences, entry peptides, and fusion peptides. All peer reviewed research and review publications, and patent studies published in the English language from 2003 to 2021 were considered. The titles and abstracts of the publications were reviewed by two authors.
Included studies
Publications focusing on the peptides useful in terms of SARS-CoV and SARS-CoV-2 were included. Priority was given to publications related to three categories such as peptides identified in silico, evaluated with wet lab techniques, patented or in clinical trials.
Excluded studies
Publications with only an abstract available were excluded from this review. Some publications were also excluded because their focus was SARS and SARS-CoV-2, without any development of therapy or peptides studies.
Data extraction and review
Data from the publications were extracted independently by two authors and entered in a table. The title and abstract of the publications which included domains such as mechanism of action, source and sequence of peptides, proposed use were reviewed. The Mendeley library was used to import citations from all the databases. Duplicate citations were removed.
Peptide therapeutics and their importance in SARS-CoV-2 research
Existing antiviral drugs have specific limitations, including insufficient activity, the development of resistance, safety and efficacy issues and side effects [22]. Small-molecule inhibitors are often less effective at disrupting extended protein binding interfaces [23]. There is an increased interest in peptides in the pharmaceutical industry. Peptide-based drugs are a superior choice compared to small molecule drugs due to their specificity, efficiency, low molecular weight, lower toxicity and low side effects. These are synthetically accessible and can be stabilized by chemical modifications. Peptides and peptidomimetics are widely accepted because of their applications in chemical biology [24]. Peptides inhibit protein-protein interactions and mimic a protein surface to effectively compete for binding [25]. Peptide drugs have several disadvantages, including instability, susceptibility to hydrolysis and oxidation, a short half-life, and low membrane permeability [26]. There are currently more than 400 peptide based drugs in clinical development, with over 60 approved for clinical use [27]. Literature also suggests possibilities for epitope-based vaccine screening, based on the protein motif regions, making use of peptide studies [28]. However, no effective peptide-based drugs are currently available against SARS-CoV-2.
Antiviral peptides (AVPs) could represent a potential class of antiviral agents against SARS-CoV-2 [29]. AVPs are of special interest due to their higher efficacy in inhibiting viral infection by targeting various stages of the viral life cycle [30]. AVPs inhibit viral replication at the stages of adsorption, viral penetration, endosomal escape, viral uncoating, viral genome replication and release of mature virions. [31]. Studies related to natural and biological sources and computational approaches, such as high-throughput screening, are supportive in identifying a promising AVP [32]. In our previous studies, we identified seven antimicrobial peptides (AMPs) that could serve as therapeutic components for MERS [151]. The various strategies and opportunities available for peptide therapeutics have been reviewed in detail by Fosgerau et al. [33]. The feasibility of developing effective therapeutic agents against MERS-CoV by repurposing existing and clinically approved anti-viral peptide drugs has been proposed [34]. The existing antivirals and knowledge gained from the SARS and MERS outbreaks offer the easiest and fastest route to manage the current coronavirus epidemic [35,36].
The relevance of using peptides as therapeutic options for COVID-19 have been reviewed by several authors [37,38]. Virus-based and host-based drug repurposing perspectives for coronaviruses, in general and specifically for SARS-CoV-2, have been reviewed by Cherian et al. [39]. The significance of repurposing drugs for SARS-CoV-2 are highlighted by many authors [[40], [41], [42], [43]]. As it is evident that drug repurposing is a valid alternative for finding therapeutic solutions for COVID-19, we emphasize the importance of using peptides as anti-SARS-CoV-2 components. We present a comprehensive review of the peptides with therapeutic and prophylactic potential in SARS-CoV that could be repurposed for SARS-CoV-2, in addition to the peptides identified as effective against SARS-CoV-2. As we discuss in Table 1, Table 2, Table 3 , it is evident that significant progress has been made in this sector, since the discovery of SARS-CoV.
Table 1.
List of peptide drugs identified as entry/fusion inhibitors and recommended for the treatment of SARS-CoV infection.
| Peptide | Peptide sequence | Source (natural/synthetic) | Target/mechanism of action | Cell line/animal model/assay | IC50 Value/EC50 | Proposed use | Reference |
|---|---|---|---|---|---|---|---|
| P6 | EEQAKTFLDKFNHEAEDLFYQSSGLGKGDFR | Synthetic - comprised of two discontinuous segments of ACE2 (a.a. 22–44 and 351–357) artificially linked together by glycine | Interaction with S glycoprotein- entry inhibitor | VeroE6 cells or HeLa cells | ~ 0.1 μM | Therapeutic candidate that would not only inhibit SARS-CoV infection but also prevent severe lethal lung failure | [44] |
| EPL5 (DP) | KKKKYRNIRRPG | Antisense peptide based combinatorial peptide library | Prevents virus entry | ELISA assay | ∼45 μg/mL | Lead compound of potent inhibitor of SARS-CoV | [45] |
| Peptide 9626 | TLKPIFKLPLGINITNFR | SARS-CoV spike protein S residues 217–234 | Inhibit S- mediated viral entry | 293 T cells -Luciferase assay | ∼ 11 μM | For design of novel therapeutic treatments against SARS-CoV | [46] |
| HR1‐a | YENQKQIANQFNKAISQIQESLTTTSTA | Synthetic SARS-CoV spike protein | Inhibit viral entry | Vero E3-Luciferase assay | ∼ 1.16 μM | SARS-CoV entry inhibitors | [47] |
| HR2 | ISGINASVVNIQKEIDRLNEVAKNLNESLIDLQEL | Synthetic SARS-CoV spike protein | Inhibit viral entry | Vero E3-Luciferase assay | ∼ 0.34 μM | SARS-CoV entry inhibitors | [47] |
| GST‐removed HR2 | DVDLGDISGINASVVNIQKEIDRLNEVAKNLNESLIDLQELGKYEQYI | Synthetic SARS-CoV spike protein | Inhibit viral entry | Vero E3-Luciferase assay | ∼2.15 μM | SARS-CoV entry inhibitors | [47] |
| RBD-11b | YKYRYL | Synthetic-Receptor binding domain of SARS-CoV-Y438-L443 | Block SARS-CoV viral entry & also inhibits proliferation of coronavirus NL63. | Vero E6 - RT PCR | High | Can be used as lead structure to design potential entry inhibitors against SARS-CoV and related viruses | [48] |
| P8 | PSSKRFQPFQQFGRDVSDFT | Synthetic SARS-CoV spike protein S1, aa 540–559 | Inhibit virus entry- inhibited syncytia formation | HEK293T-Syncytia assay | 50% | Target for anti-SARS-CoV drug design | [49] |
| P9 | CANLLLQYGSFCTQLNRALSGIA | Synthetic SARS-CoV spike protein S2, aa 731–753 | Inhibit virus entry- inhibited syncytia formation | HEK293T-Syncytia assay | 50% | Target for anti-SARS-CoV drug design | [49] |
| HR1-1 | NGIGVTQNVLYENQKQIANQFNKAISQIQESLTTTSTA | Derived from HR1 regions in the S2 protein, aa 889–926 | Inhibit viral entry | 293T- Luciferase assay | ~ 0.14 μM | Can serve as functional probes for dissecting the fusion mechanism of SARS-CoV to find potent inhibitor peptides for SARS-CoV. | [50] |
| HR2-18 | IQKEIDRLNEVAKNLNESLIDLQELGK | Derived from HR2 regions in the S2 protein, aa 1161–1187 | Inhibit viral entry | 293T- Luciferase assay | ~ 1.19 μM | Can serve as functional probes for dissecting the fusion mechanism of SARS-CoV to find potent inhibitor peptides for SARS-CoV | [50] |
| HR2-8 | ELDSFKEELDKYFKNHTSPDVDLGDISGINASVVNIQKEIDRLNEVAKNLNESLIDLQELGKYEQYIK | Synthetic-SARS-CoV spike protein | Competitive binding to the HR1 region of the SARS-CoV spike Protein, thus blocking membrane fusion | Vero cells-Immuno fluorescence assay | 17 μM | Can be used as a lead for the further development of more effective SARS-CoV peptide inhibitors | [51] |
| EK1 | SLDQINVTFLDLEYEMKKLEEAIKKLEESYIDLKEL | Synthetic- modified OC43-HR2P peptide | Binds to HR1s of different HCoVs and form stable complexes, thereby blocking S protein mediated fusion. | Cell lines & Mouse model | 0.19 to 0.62 μM | Anti-viral agent against multiple HCoVs | [52] |
| SARSWW-I | MWKTPTLKYFGGFNFSQIL | Synthetic-S2 subunit of SARS-CoV N-terminal aa 770–788 | Fusion inhibition | Vero E6 cells -plaque assay | – | Potential therapeutics | [53] |
| SARSWW-II | ATAGWTFGAGAALQIPFAMQMAY | Synthetic- S2 subunit of SARS-CoV N-terminal, 864–886 | Fusion inhibition | Vero E6 cells plaque assay | – | Potential therapeutics | [53] |
| SARSWW-III | GYHLMSFPQAAPHGVVFLHVTW | Synthetic- S2 subunit of SARS-CoV Loop aa 1028–1049 | Fusion inhibition | Vero E6 cells plaque assay | ~ 2 μM | Potential therapeutics | [53] |
| SARSWW-IV | GVFVFNGTSWFITQRNFFS | Synthetic-S2 subunit of SARS-CoV Loop aa 1075–1093 | Fusion inhibition | Vero E6 cells plaque assay | ~ 2–4 μM | Potential therapeutics | [53] |
| SARSWW-Va | NEVAKNLNESLIDLQELGKYEQYIKWPWYVW | Synthetic-S2 subunit of SARS-CoV HR2- Aromatic aa 1169–1199 | Fusion inhibition | Vero E6 cells plaque assay | – | Potential therapeutics | [53] |
| SARSWW-Vb | AACEVAKNLNESLIDLQELGKYEQYIKW | Synthetic-S2 subunit of SARS-CoV HR2- ΔAromaticaa 1169–1194 | Fusion inhibition | Vero E6 cells plaque assay | – | Potential therapeutics | [53] |
| P6 | GINASVVNIQKEIDRLNEVAKNL | SARS-CoV spike protein, HR2 peptide | Block the fusion of SARS-CoV | HeLa cells-cell fusion inhibition assay | 2.28 ± 0.81 μM | Potential lead peptide for future drug development | [54] |
| N46 | QKQIANQFNKAISQIQESLTTTSTALGKLQDVVNQNAQALNTLVKQ | SARS-CoV spike protein, HR1 peptide | Block the fusion of SARS-CoV | HeLa cells-cell fusion inhibition assay | 3.97 ± 1.40 μM | Strategy to achieve promising inhibition by HR1 peptide for class I envelope viruses | [54] |
| N46eg | QNQSANQFQKEISQINEVLTTTNTSLGKLQDDVNQNNQSLNTLQKE | SARS-CoV spike protein- HR1 peptide (mutated version of N46) | Block the fusion of SARS-cov | HeLa cells-cell fusion inhibition assay | 5.07 μM | Strategy to achieve promising inhibition by HR1 peptide for class I envelope viruses | [54] |
Table 2.
List of peptide drugs identified as replication/maturation inhibitors and recommended for the treatment of SARS-CoV infection.
| Peptide | Peptide sequence | Source (natural/synthetic) | Target/mechanism of action | Cell line/animal model/assay | IC50 Value/EC50 | Proposed use | Reference |
|---|---|---|---|---|---|---|---|
| K12 | GGASCCLYCRCH | Synthetic-nsp10 of SARS-CoV, aa 69–80 | Replication- inhibiting 2′-O-methyltransferase activity of SARS-CoV nsp16/10 complex | E. coli-MTase Activity Assay | 169 μM | May be further developed into potential specific antivirals against coronavirus infection | [148] |
| K29 | FGGASCCLYCRCHIDHPNPKGFCDLKGKY | Synthetic-nsp10 of SARS-CoV, aa 68–96 | Replication- inhibiting 2′-O-methyltransferase activity of SARS-CoV nsp16/10 complex | E. coli -MTase Activity Assay | 160 μM | May be further developed into potential specific antivirals against coronavirus infection | [148] |
| Mucroporin-M1 | LFRLIKSLIKRLVSAFK | Synthetic-Scorpion venom | Replication- direct interaction with the virus envelope, thereby decreasing the infectivity of virus | MDCK cells-Plaque assay | 14.46 μg/mL | For developing broad-spectrum antiviral agents against RNA viruses | [149] |
| Nitrile-based peptidomimetic inhibitor | Cbz-AVLQ-CN | Synthetic-Cbz: carboxybenzyl, CN-Nitrile | Replication-inhibitory effect on 3CLpro from a broad range of coronaviruses | Protease activity assay | 4.6 ± 0.2 μM | For drug designing against various coronaviruses | [147] |
| Peptide-aldehyde | Ac-Val-Leu-NHCH(CH2CH2CON(CH3)2)-CHO | Synthetic | Virus maturation | Protease activity assay | ∼6 mM | A promising reversible SARS 3CL protease inhibitor | [145] |
| Modification: Ac: Acetyl, -CHO: Aldehyde moiety | |||||||
| Peptide-aldehyde | Ac-Ala-Val-Leu-NHCH(CH2CH2CON(CH3)2)-CHO | Synthetic | Virus maturation | Protease activity assay | 155 μM | A promising reversible SARS 3CL protease inhibitor | [145] |
| Modification: Ac: Acetyl, -CHO: Aldehyde moiety | |||||||
| Peptide-aldehyde | Ac-Ser-Ala-Val-Leu-NHCH(CH2CH2CON(CH3)2)-CHO | Synthetic | Virus maturation | -protease activity assay | 37 μM | A promising reversible SARS 3CL protease inhibitor | [145] |
| Modification: Ac: Acetyl, -CHO: Aldehyde moiety | |||||||
| Peptide-aldehyde | Ac-Thr-Ser-Ala-Val-Leu-NHCH(CH2CH2CON(CH3)2)-CHO | Synthetic | Virus maturation | -protease activity assay | 26 μM | A promising reversible SARS 3CL protease inhibitor | [145] |
| Modification: Ac: Acetyl, -CHO: Aldehyde moiety | |||||||
| Proprotein convertase inhibitor peptide | dec-RVKR-cmk | Synthetic | Virus release- blocks a PC-mediated processing step required for extensive virus spread and ensuing CPE | Vero E6- Immuno precipitation assay | – | PC-inhibitor could be used along with vaccination and/or in conjunction with interferon treatment or with the novel compounds targeting the SARS-CoV proteins | [146] |
| Modification: dec: decanoyl, cmk: chloro methyl ketone |
Table 3.
List of peptide drugs identified as immunological modulators and recommended for the treatment of SARS-CoV infection.
| Peptide | Peptide sequence | Source (natural/synthetic) | Target/mechanism of action | Cell line/animal model/assay | Proposed use | Reference |
|---|---|---|---|---|---|---|
| P15 | KLPDDFMGCV | S411–420- derived from S protein | Binding mechanism with HLA molecules. Elicited specific IFN-γ-producing CTLs from SARS-CoV S gene-based DNA-vaccinated Tg mice | Transgenic mice- ELISPOT assay and tetramer staining | SARS associated corona virus-specific CTL epitope. Potential target for characterization of virus control mechanisms and evaluation of candidate SARS vaccines | [55] |
| M1-31 | MADNGTITVEELKQLLEQWNLVIGFLFLAWI | Synthetic-derived from the N-terminal epitope | Epitope and binding mechanism of M protein- induce potent antibody responses | BALB/c mice and New Zealand White rabbits- ELISA | For developing SARS diagnostics and vaccines | [56] |
| M132-161 | LMESELVIGAVIIRGHLRMAGHPLGRCDIK | Synthetic-derived from the C-terminal epitope | Epitope and binding mechanism of M protein- induce potent antibody responses | BALB/c mice and New Zealand White rabbits- ELISA | For developing SARS diagnostics and vaccines | [56] |
| S5 | LPDPLKPTKRSFIEDLLFNKVTLADAGFMKQYG | Synthetic- residues 788-820 | Block viral invasion by eliciting an immune response specific to SARS-CoV S protein | rabbits and monkeys- ELISA | Development of synthetic peptide SARS vaccine | [57] |
| S6 | ASANLAATKMSECVLGQSKRVDFCGKGYH | Synthetic- residues 1002-1030 | Block viral invasion by eliciting an immune response specific to SARS-CoV S protein | rabbits and monkeys-ELISA | Development of synthetic peptide SARS vaccine | [57] |
| S471-503 | ALNCYWPLNDYGFYTTTGIGYQPYRVVVLSFEL | Synthetic- SARS-CoV receptor binding domain (RBD) | Block S1‑RBD: ACE‑2 binding and thus prevent entry of SARS-CoV | Vero cells-Plaque assay | Immune antigen for the development of peptide-based vaccine or for further drug evaluation against the SARS-CoV virus cell fusion | [58] |
Classification of peptide therapeutics for COVID-19 based on the mechanism of action
Potential anti-coronavirus therapies can be divided in two categories depending on the target, acting on the viral factors or on the host factors or immune system. Based on the mechanism of action, we classified potential peptide therapeutics to combat SARS-CoV-2 in three categories, including virus entry/fusion blockers, virus replication blockers and immune modulators.
Peptides affecting viral entry and fusion
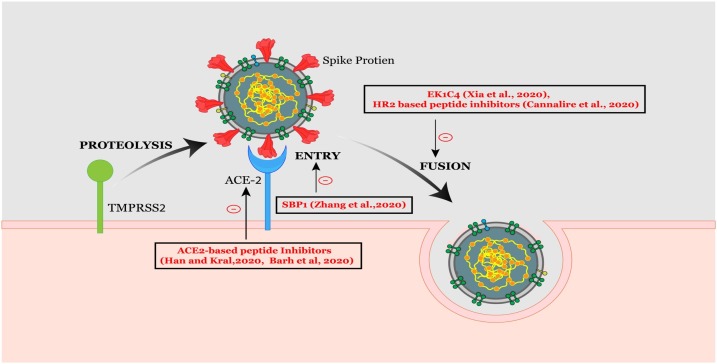
The SARS-CoV spike (S) protein, a surface glycoprotein, mediates coronavirus entry into receptor-bearing cells by binding to the human angiotensin-converting enzyme 2 (hACE2) receptor [52]. Lu et al. performed bioinformatics analysis to identify peptides containing relevant B cell epitopes within the S protein of SARS-CoV which has the potential for application in the diagnosis [49]. RBD in the S1 subunit of the spike protein is a target for the development of virus attachment inhibitors, neutralizing antibodies, and vaccines [59]. Barh et al. have identified certain potential peptides to block the SARS-CoV-2 spike RBD [60]. They designed ACE2 sequence based peptides after identifying the key residues interacting with the spike RBD using bioinformatics approaches. The α-helices extracted from the protease domain of ACE2 were used to design peptide inhibitors against SARS-CoV-2 [61]. S1 of RBD directly binds to the peptidase domain (PD) of ACE2. A 23-mer peptide fragment of the ACE2 PD α1 helix has been synthesized and named as SBP1, which is capable of blocking the SARS-CoV-2 spike protein interacting with ACE2 [62].
The coronavirus spike proteins belong to class I fusion proteins, and are characterized by the existence of two heptad repeat (HR) regions, HR1 and HR2 [63]. As soon as the S protein binds to the ACE2 receptor on the target cell, the heptad repeats interact to form a six helical bundle which is a critical and conserved mechanism for viral fusion and entry, and is shared by all coronaviruses. Hence so many attempts have been made at discovering fusion inhibitors that could act as therapeutics addressing CoV infections. A look at the sequence alignment of the HR1 and HR2 regions between SARS-CoV and SARS-CoV-2 show 92.6% and 100% sequence homology respectively, suggesting that HR2 peptides, identified against SARS-CoV, may also inhibit SARS-CoV-2 [64]. HR2 based inhibitory peptides acting on SARS-CoV and MERS-CoV were reviewed by Tang et al. [65], creating possibilities for peptide or small molecule based anti-CoV fusogenics, which can arrest membrane fusion in SARS-CoV-2. Recently, a few HR2 sequence-based fusion inhibitors have been reported to potentially inhibit cell fusion in a dual-split protein (DSP) based cell fusion assay on SARS-CoV-2 [66]. Xia et al. developed a pan-coronavirus inhibitor EK1 that targets the HR1 domain and inhibits coronavirus infection [52]. They also generated a highly potent lipopeptide EK1C4, which acts as a pan-coronavirus fusion inhibitor, targeting the spike protein [67]. EK1C4 was developed by adding cholesterol residue to the EK1 peptide to improve the inhibitory activity against SARS-CoV-2 [68]. EKIC4 was found to have remarkable inhibitory activity against membrane fusion mediated by SARS-CoV-2 spike protein and also against entry of pseudotyped human coronaviruses including SARS-CoV and MERS-CoV. EK1C4 potently inhibits SARS-CoV-2 replication, with an EC50 value of 36.5 nM, which is more potent than the EK1 peptide with an EC50 value of 2.47 μM [66]. Another HR2 sequence-based lipopeptide fusion inhibitor, termed IPB02, showed highly potent activities in inhibiting SARS-CoV-2 spike protein-mediated cell-cell fusion and pseudovirus transduction [9,10].
EK1C4 has many advantages over other peptide drugs, as it can be inhaled as an aerosol formulation, reducing the viral load in the lung, and alleviating the pulmonary inflammatory reaction [69]. The intranasal application of EK1C4 indicates that it could be used as an effective therapeutic against infection by SARS-CoV-2 [67]. The sequence of its target, the HR1 domain in the S2 subunit of the S protein, is highly conserved, which means EK1C4 cannot easily induce drug-resistant mutations.
Fig. 1 displays a graphical representation of the mechanism of entry and fusion of SARS-CoV-2, highlighting peptide therapeutics.
Fig. 1.
Graphical representation of the mechanism of entry and fusion of SARS-CoV-2. Coronavirus entry and fusion are represented along with the identified peptide compounds.
Peptides inhibiting viral replication and release
Polyprotein processing
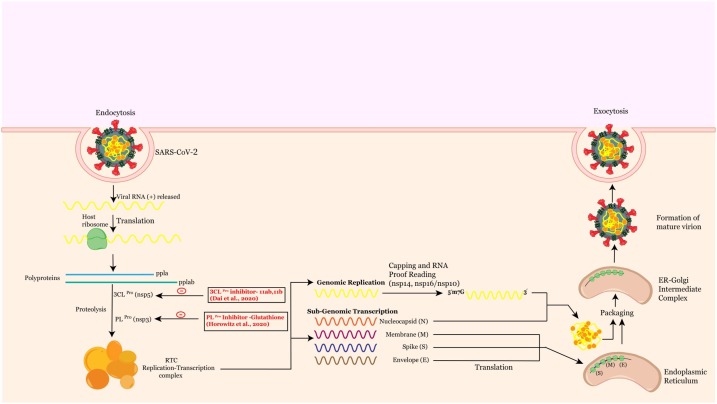
The coronavirus alters the host transcription machinery in its favor to synthesize its proteins once the virus enters the host cell. The SARS-CoV positive stranded RNA genome encodes two open reading frames (ORF), ORF1a and ORF1b, which on translation produces two polyproteins (pp), pp1a and pp1ab. These polyproteins are proteolytically cleaved to generate 16 non-structural proteins, required for viral replication [70], which include two proteases, 3-chymotrypsin-like protease (3CLpro/Nsp5) and papain-like protease (PLpro/Nsp3) that mediate the proteolytic processing of the polyprotein. The 3CLpro of SARS-CoV-2 has a 99.02% sequence identity with the SARS-CoV [71]. As the active sites of 3CLpro are highly conserved in multiple coronaviruses [72], the possibility of designing peptide inhibitors targeting the substrate binding pocket of SARS-CoV-2 3CLpro has been explored and two peptidomimetic aldehydes 11a and 11b were identified. Both exhibited excellent inhibitory activity and potent anti-SARS-CoV-2 infection activity [73] (Fig. 2 ). The another protease, PLPro (Papain like protease) involved in the proteolytic processing of the replicase polyprotein, which also plays a significant role in antagonizing the host’s innate immunity, is an attractive antiviral drug target as well [74]. Target based virtual ligand screening against the ZINC drug database identified Glutathione, currently used for the treatment of liver diseases [75], as effective against PLPro with a docking score of 20.
Fig. 2.
Shows a graphical representation of the mechanism of replication and maturation of SARS-CoV-2 highlighting peptide therapeutics.
Fig. 2 displays a graphical representation of the mechanism of replication and maturation of SARS-CoV-2, highlighting peptide therapeutics.
Viral replication and transcription
Coronavirus genome replication and transcription involve coordinated processes of both continuous and discontinuous RNA synthesis mediated by the viral replicase, a huge protein complex encoded by the 20-kb replicase gene [76]. The replicase-transcriptase proteins, together with other viral proteins and possibly cellular proteins, assemble into membrane-bound replication-transcription complexes (RTC). The central component of coronoviral replication/transcription is Nsp12 (RNA dependent RNA polymerase/RdRp) [77], which is the primary target for remdesivir, a nucleoside analog antiviral inhibitor, currently used for SARS-CoV-2 [78,79]. Remdesivir exhibits a broad spectrum of anti-viral activity against many viruses, including Ebola, Nipah and the respiratory syncytial virus (RSV), SARS-CoV and MERS-CoV [80]. This molecule is assumed to affect the viral RNA synthesis by a delayed chain termination process in the three coronaviruses (MERS-CoV, SARS-CoV and SARS-CoV-2). Remdesivir is currently being tested in the clinic in many countries and approved for emergency treatment of patients with COVID-19 [81]. A recent study indicated that remdesivir was effective in lowering the respiratory tract infection of COVID-19 adult patients in a double blind, randomized placebo controlled trial [82].
The high homology exhibited by the SARS-CoV-2 Nsp12 with SARS-CoV, indicating a conserved mechanism of action confirmed by cryo-EM structural studies [83] should be noted. The structure of Nsp12 contains a right hand RdRp domain and a nidovirus-unique N-terminal extension domain that adopts a nidovirus RdRp-associated nucleotidyl transferase architecture. The synthesis of viral RNA by Nsp12 possibly occurs with the assistance of Nsp7 and Nsp8 as cofactors [79]. Nsp8 synthesizes a primer of 6 nucleotide lengths for RdRp RNA synthesis, and the Nsp7-Nsp8 complex increases the binding of Nsp12 to RNA and enhances the RdRp enzymatic activity of Nsp12. The interactions of these non-structural proteins also offers great therapeutic possibilities, specifically investigating the Nsp12-Nsp8 interaction [84].
Nsp9, highly conserved in beta coronaviruses, is assumed to mediate viral replication, overall virulence, and viral genomic RNA reproduction. Selinexor, a synthetic peptide, an inhibitor of CRM1 (Chromosome region maintenance protein), also known as exportin 1 (XPO1) involved in nuclear export, could be effective in inhibiting the interactions between Nsp9 and nuclear pore proteins [85,86]. Low oral doses of this peptide is being tested in ongoing clinical trials against COVID-19 [87]. Ternatin 4-N-methylated cyclic hexapeptide, derived from mushrooms, inhibits adipogenesis at low concentrations (EC50 = 20 nm) [88] and becomes cytotoxic at a 10-fold higher concentration [89] and known to kill cancer cells [90], also inhibits the translation machinery. Since ternatin 4 can affect the host translation process, it is included as a preclinical compound that could be repurposed against SARS-CoV-2.
Epigenetic changes that regulate chromatin rearrangement have been implicated in the pathophysiology of viral infection [91]. Several studies have highlighted the importance of epigenetic regulators in SARS-CoV studies [92,93]. Recently, DNA methylation analysis of ACE2 expression has been shown to throw light on the epidemiology of SARS-CoV-2 infection [94]. ACE2 expression is also found to be regulated by Bromodomain and extraterminal (BET) proteins which are epigenetic readers [95]. Gordon etal has identified an interaction of BRD4 with the E protein of SARS-CoV-2, which highlights possibilities of exploring peptide based bromodomain inhibitors as SARS-CoV-2 therapeutics. In the same SARS-CoV-2 interactome study, HDAC2 was found to have a high confidence interaction with the viral protein Nsp5 [85]. Nsp5 is thought to inhibit HDAC2 transport into the nucleus, thereby enhancing inflammation and interferon response. We suggest looking for peptide inhibitors of Nsp5, which seems to be the switch to start inflammation in COVID-19. Also, HDAC inhibitors have been proposed to serve as preventive drug against COVID-19 [96]. These suggest epigenetic interventions such as epidrugs could serve as promising therapeutic or prophylactic options [97]. The approach used for the discovery of Apicidin, a natural tetrapeptide inhibitor of HDAC via a cyclic α3β-tetrapeptide scaffold [98] is interesting and can be used to identify more anti-coronaviral peptide inhibitors.
RNA processing
Besides RNA-dependent RNA polymerase, RNA helicase and protease activities, common to RNA viruses, the coronavirus replicase also employs a variety of RNA processing enzymes that are not (or extremely rarely) found in other RNA viruses [99]. Several enzymatic activities are required for the formation of a cap structure in the viral RNA, followed by Ribose 2′-O-methylation of the cap that provides a mechanism for viruses to escape host immune recognition [100]. RNA capping in CoVs involves the activities of several nonstructural proteins (nsps): Nsp13, a bifunctional RNA/NTP triphosphatase (TPase) and helicase; Nsp14, a bifunctional 3′→5′ mismatch exonuclease, and mRNA cap guanine-N7 methyltransferase, Nsp16, a cap ribose 2′-O methyltransferase, and an elusive guanylyl transferase [101]. Of the enzymes involved in capping, the N7-Methyltransferase of Nsp14 is an attractive antiviral target as this domain exhibits a non-canonical methyltransferase fold, different from cellular methyltransferases [102]. A peptide inhibitor targeting the 2′-O-methyltransferase efficiently suppresses coronavirus replication, suggesting that the 2′-O-methyltransferases of coronaviruses are ideal and potential antiviral drug targets [103].
Virus maturation
The RTC uses the genome to synthesize progeny genomes and a series of subgenomic mRNAs, using negative-stranded intermediates. The structural proteins S, M, E and N are generated by the translation of these mRNAs, which participate in the formation of mature virions. The E (envelope) protein of SARS-CoV-2 plays key roles in various phases of the viral life cycle, such as envelope formation, pathogenesis, budding and assembly. Unlike the other major structural proteins, N is the only protein that functions primarily to bind to the CoV RNA genome, constituting the nucleocapsid to make new virons [104]. The N protein of SARS-CoV-2 is highly homologous to that of SARS-CoV [105]. A recent study by Cascarina et al. reports that the N protein of SARS-CoV-2 is capable of regulating the biomolecular interaction with RNA and key host cell proteins [106]. The N protein also binds to the stress granule proteins, which induce the host’s immune response [107]. The manipulation of stress granules could be promising in inhibiting the replication of coronaviruses [108,109]. N protein is also shown to interact with the Ras-GTPase activating SH3-domain-binding-protein 1 (G3BP1) [85], which inhibits RNA virus replication [110].
Peptides that act as immune modulators
After the virus gains access to the target cell, the host immune system recognizes the whole virus or its surface epitopes, and elicits an innate or adaptive immune response. The SARS-CoV-2 virus infects through the naso-oral route, followed by infection in cells expressing the ACE2 receptor in the lung. These viruses dampen the anti-viral IFN responses by evading the innate immune cells, resulting in unrestrained virus replication. At the same time, the activation of macrophages and other immune cells, cause the production of cytokines.
Innate immune response
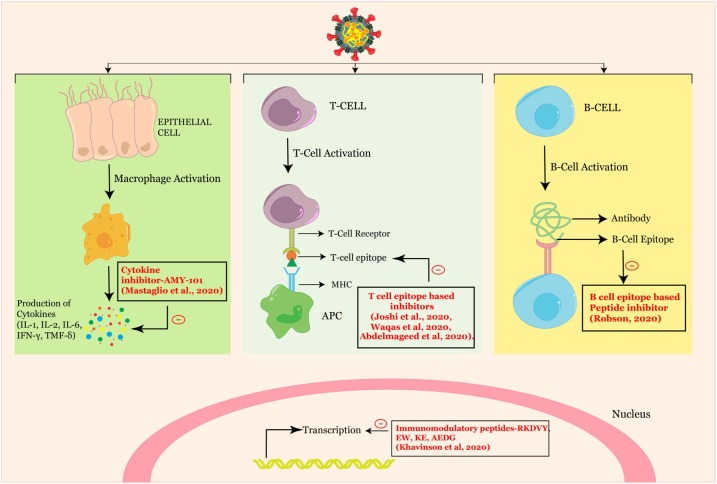
When SARS-CoV-2 infects the upper and lower respiratory tract, it can cause a mild or highly acute respiratory syndrome with consequent release of pro-inflammatory cytokines, such as the interleukins [111]. A case report shows that the compstatin-based complement C3 peptidic inhibitor AMY-101 was successful in treating a patient with severe ARDS due to COVID-19 pneumonia [112]. Anakinra, an interleukin-1 receptor antagonist, currently used for the treatment of rheumatoid arthritis [113], is beneficial in association with remdesivir in controlling the pathological immune response to SARS-CoV-2 [114].
NK cells lyse viral infected cells and hence play an important role in anti-viral immunity. A reduced number of NK cells have been found in the peripheral blood of COVID-19 patients [115]. In a murine model of SARS-CoV infection, increased production of chemokines was correlated with increased migration of NK cells into the lungs [116]. The high expression of chemokines in the lungs of COVID-19 patients also seem to facilitate NK cell migration from the peripheral blood to the lungs [117].A transcriptomic signature analysis also revealed an infiltration of the NK cells into lungs [118], possibly mediated by the CXCR3 pathway [119]. Since chemokines are vital in attracting NK cells to clear the virus, peptide inhibitors blocking such chemokine receptors are promising drugs for SARS-CoV-2. Moreover, peptide antagonism is an established mechanism for NK cell activation, and there is a major scope for more peptide therapeutics in this aspect [120].
Adaptive immune response
Adaptive immune response and immunological memory are crucial for the success of a vaccine. An effective vaccine should induce a specific immune response against the specific antigen by selectively stimulating antigen-specific B-cells or CTLs or T helper cells. In response to a SARS-CoV-2 infection, antibodies, CD4+ T cells and CD8+ T cells are synthesized in the body. During viral infection, T cells recognize the viral antigens presented by the MHC class I [MHC; Human Leukocyte Antigen (HLA) in humans], which promotes the cytokine release [121]. In other cases, MHC class II also present SARS-CoV peptides to CD4+ T cells. This should be the basis of designing effective vaccines, to stimulate immune responses against the target pathogen. Kiyotani et al. have identified 781 HLA-Class I and 418 Class II peptide epitopes for SARS-CoV-2 through the computational screening of potential epitopes present on the HLA class I molecules, frequently observed in the Japanese population [122]. Immunoinformatic tools were used to analyse the proteome of SARS-CoV-2 and an epitope with HLA allele binding affinity, ITLCFTLKR has been identified as a vaccine candidate [123] (Fig. 3 ). Similarly, peptides binding to the MHC class I and MHC class II were promising epitope based peptide vaccine candidates, when envelope protein was used as an immunogenic target [124]. A total of 10 epitopes based on the structural proteins designed by molecular docking analysis against MHC-I were identified which have the potential to be developed as peptide vaccine against SARS-CoV-2 [125]. A computational analysis identified the sequence KRSFIEDLLFNKV as particularly conserved in many coronaviruses, ranging from the common cold coronavirus to SARS-CoV-2. This sequence motif can form the basis for proposing a specific synthetic vaccine epitope and peptidomimetic agent [126].
Fig. 3.
Graphical representation of the mechanism of immune modulation of SARS-CoV-2 highlighting peptide therapeutics.
Epitopes are immunogenic components, which can also be used in vaccine discovery studies [127]. Since discovering epitopes by traditional experimental methods is often expensive and time-consuming, in silico approaches become the preferred strategy. Thus, it can be rightly said that computational immunoinformatics is the basis of epitope vaccine development [128]. Many epitope studies have been conducted with SARS-CoV proteins [129,130]. The Immune Epitope Database and Analysis Resource (IEDB) that catalogs available data related to other coronaviruses, can be used effectively to predict and identify candidate targets for immune responses to SARS-CoV-2 [131]. A study carried out by comparing the SARS-CoV-2 and the human proteomes identified certain epitopes embedded in the S protein that could serve as possible vaccine candidates [132]. In another study, two immunodominant linear B-cell epitopes on the S glycoprotein of SARS-CoV-2 that could elicit neutralizing antibodies, have been identified [133]. Nucleocapsid proteins within SARS-CoV-2 contain multiple Class I epitopes with predicted HLA restrictions, consistent with a broad population coverage, which can be used to develop a CTL peptide vaccine against COVID-19 [134]. A multiepitope vaccine construct to combat SARS-CoV-2 infection was made from spike S protein and orf1ab polyprotein against B and T lymphocytes [135]. Recently, a comprehensive reverse-vaccinology workflow that can be used to identify and shortlist potential T- and B-cell epitopes has also been proposed [136].
Possibilities of harnessing Omics approaches to combat COVID-19
Various disciplines, such as genomics and proteomics, have a major role in combating COVID-19 [137]. It is important to note that intensive research with the SARS-CoV-2 virus is ongoing to understand its pathogenesis and mechanism of virulence. Genomics is employed in identifying genomic variants of SARS-CoV-2, which is highly important, especially when the penetrance of host genetic variants is high [138]. The information related to the genetic variants of SARS-CoV-2 is a decision making tool, in terms of patient care, prioritization for therapies, clinical research, and public health containment practices. In addition, genomics studies are significant in delineating the host factors associated with the variability in susceptibility, infectivity, and also the severity of the disease [138,139]. The progress of these Omics studies can be extrapolated to applications in many fields, including immunology. This supports a deeper level of knowledge and the provision of substantial data used in computational studies, namely immunoinformatics [140].
Proteomics facilitates the deciphering of the knowledge related to structure–function relationships of protein targets. This information would provide novel insights into drug development to prevent infection and as well as to control transmission [141]. Several molecular docking, dynamics and pharmacology approaches have been investigated based on the binding affinity of drug components against COVID-19 [[142], [143], [144]]. Proteomics based directed drug repurposing might be a rapid response solution for the COVID-19 pandemic, based on the experience with SARS-CoV, MERS-CoV, or other coronaviruses. Although many countries are vaccinating their citizens, there is no effective drug to treat COVID-19. We urge the research community to exploit the omics approaches to rapidly identify new potential drug molecules.
Conclusion
The development of peptide therapeutics is considered risky, due to the poor performance in ADME (absorption, distribution, metabolism, elimination) properties. However, pharmaceutical companies are considering this strategy due to its many advantages. The data presented in this review reinforces the need for further studies and new therapeutic proposals to combat the threat of COVID-19. The knowledge achieved in the treatment of SARS-CoV may be used to enhance this process. It should be noted that the accuracy and coverage of this review will be short-lived, due to the rapid advances being made in this area; however, this review will be significant in accelerating the development of novel solutions for COVID-19.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
Acknowledgement
The authors gratefully acknowledge the contribution from Ms. Malavika Saji for creating illustrations. Special thanks to Dr. Susanna Wright for language revising and editing the manuscript.
References
- 1.Gaunt E.R., Hardie A., Claas E.C.J., Simmonds P., Templeton K.E. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol. 2010;48 doi: 10.1128/JCM.00636-10. 2940 LP-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sami A.-H. SARS: challenge of the new century. Ann Saudi Med. 2003;23:116–117. doi: 10.5144/0256-4947.2003.116. [DOI] [PubMed] [Google Scholar]
- 3.Chafekar A., Fielding B.C. MERS-CoV: understanding the latest human coronavirus threat. Viruses. 2018;10:93. doi: 10.3390/v10020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marty A.M., Jones M.K. The novel Coronavirus (SARS-CoV-2) is a one health issue. One Health (Amsterdam, Netherlands) 2020;9 doi: 10.1016/j.onehlt.2020.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neerukonda S.N., Katneni U. A review on SARS-CoV-2 virology, pathophysiology, animal models, and anti-viral interventions. Pathogens (Basel, Switzerland) 2020;9:426. doi: 10.3390/pathogens9060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med J. 2021;97 doi: 10.1136/postgradmedj-2020-138577. 312 LP–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y., Yu D., Yan H., Chong H., He Y. Design of potent membrane fusion inhibitors against SARS-CoV-2, an emerging coronavirus with high fusogenic activity. J Virol. 2020;94:e00635–20. doi: 10.1128/JVI.00635-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., et al. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Astuti I., Ysrafil Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr. 2020;14(4):407–412. doi: 10.1016/j.dsx.2020.04.020. Epub 2020 April 18. PMID: 32335367; PMCID: PMC7165108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L., Zhong L. Genomics functional analysis and drug screening of SARS-CoV-2. Genes Dis. 2020;7(4):542–550. doi: 10.1016/j.gendis.2020.04.002. Epub 2020 April 14. PMID: 32363223; PMCID: PMC7195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatmal M.M., Alshaer W., Al-Hatamleh M.A.I., Hatmal M., Smadi O., Taha M.O., et al. Comprehensive structural and molecular comparison of spike proteins of SARS-CoV-2, SARS-CoV and MERS-CoV, and their interactions with ACE2. Cells. 2020;9:2638. doi: 10.3390/cells9122638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Premkumar L., Segovia-Chumbez B., Jadi R., Martinez D.R., Raut R., Markmann A.J., et al. The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5(48):eabc8413. doi: 10.1126/sciimmunol.abc8413. PMID: 32527802; PMCID: PMC7292505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomaszewski T., DeVries R.S., Dong M., Bhatia G., Norsworthy M.D., Zheng X., et al. New pathways of mutational change in SARS-CoV-2 proteomes involve regions of intrinsic disorder important for virus replication and release. Evol Bioinform Online. 2020;16 doi: 10.1177/1176934320965149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganesh B., Rajakumar T., Malathi M., Manikandan N., Nagaraj J., Santhakumar A., et al. Epidemiology and pathobiology of SARS-CoV-2 (COVID-19) in comparison with SARS, MERS: an updated overview of current knowledge and future perspectives. Clin Epidemiol Glob Health. 2021;10 doi: 10.1016/j.cegh.2020.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.-Y. Coronaviruses — drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan J.F.W., Chan K.-H., Kao R.Y.T., To K.K.W., Zheng B.-J., Li C.P.Y., et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammadi Pour P., Fakhri S., Asgary S., Farzaei M.H., Echeverría J. The signaling pathways, and therapeutic targets of antiviral agents: focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front Pharmacol. 2019;10:1207. doi: 10.3389/fphar.2019.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith M.C., Gestwicki J.E. Features of protein-protein interactions that translate into potent inhibitors: topology, surface area and affinity. Expert Rev Mol Med. 2012;14:e16. doi: 10.1017/erm.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cretich M., Gori A., D’Annessa I., Chiari M., Colombo G. Peptides for infectious diseases: from probe design to diagnostic microarrays. Antibodies (Basel) 2019;8:23. doi: 10.3390/antib8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham A.D., Qvit N., Mochly-Rosen D. Peptides and peptidomimetics as regulators of protein-protein interactions. Curr Opin Struct Biol. 2017;44:59–66. doi: 10.1016/j.sbi.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015;17:134–143. doi: 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee A.C.-L., Harris J.L., Khanna K.K., Hong J.-H. A comprehensive review on current advances in peptide drug development and design. Int J Mol Sci. 2019;20:2383. doi: 10.3390/ijms20102383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madhavan M., Mustafa S. En route to peptide therapeutics for COVID 19: harnessing potential antigenic mimicry between viral and human proteins. Trans Indian Natl Acad Eng. 2020:1–5. doi: 10.1007/s41403-020-00132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhury S.M., Talukder S.A., Khan A.M., Afrin N., Ali M.A., Islam R., et al. Antiviral peptides as promising therapeutics against SARS-CoV-2. J Phys Chem B. 2020;124:9785–9792. doi: 10.1021/acs.jpcb.0c05621. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed A., Siman-Tov G., Hall G., Bhalla N., Narayanan A. Human antimicrobial peptides as therapeutics for viral infections. Viruses. 2019;11:704. doi: 10.3390/v11080704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilas Boas L.C.P., Campos M.L., Berlanda R.L.A., de Carvalho Neves N., Franco O.L. Antiviral peptides as promising therapeutic drugs. Cell Mol Life Sci. 2019;76:3525–3542. doi: 10.1007/s00018-019-03138-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal G., Gabrani R. Antiviral peptides: identification and validation. Int J Pept Res Ther. 2020 doi: 10.1007/s10989-020-10072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fosgerau K., Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Mustafa S., Balkhy H., Gabere M.N. Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): a review. J Infect Public Health. 2018;11:9–17. doi: 10.1016/j.jiph.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guy R.K., DiPaola R.S., Romanelli F., Dutch R.E. Rapid repurposing of drugs for COVID-19. Science (80-.) 2020;368 doi: 10.1126/science.abb9332. 829 LP–830. [DOI] [PubMed] [Google Scholar]
- 36.Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol. 2020 doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- 37.Khavinson V., Linkova N., Dyatlova A., Kuznik B., Umnov R. Peptides: prospects for use in the treatment of COVID-19. Molecules. 2020 doi: 10.3390/molecules25194389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mousavi Maleki M.S., Rostamian M., Madanchi H. Antimicrobial peptides and other peptide-like therapeutics as promising candidates to combat SARS-CoV-2. Expert Rev Anti Infect Ther. 2021:1–13. doi: 10.1080/14787210.2021.1912593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cherian S., Agrawal M., Basu A., Abraham P., Gangakhedkar R., Bhargava B. Perspectives for repurposing drugs for the coronavirus disease 2019. Indian J Med Res. 2020;151:160–171. doi: 10.4103/ijmr.IJMR_585_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y.W., Yiu C.-P.B., Wong K.-Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL (pro)) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research. 2020;9:129. doi: 10.12688/f1000research.22457.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248 doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020 doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han D.P., Penn-Nicholson A., Cho M.W. Identification of critical determinants on ACE2 for SARS-CoV entry and development of a potent entry inhibitor. Virology. 2006;350:15–25. doi: 10.1016/j.virol.2006.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Y., Zhao R., Luo J., Xiong S., Shangguan D., Zhang H., et al. Design, synthesis and screening of antisense peptide based combinatorial peptide libraries towards an aromatic region of SARS-CoV. J Mol Recognit. 2008;21:122–131. doi: 10.1002/jmr.880. [DOI] [PubMed] [Google Scholar]
- 46.Guo Y., Tisoncik J., McReynolds S., Farzan M., Prabhakar B.S., Gallagher T., et al. Identification of a new region of SARS-CoV S protein critical for viral entry. J Mol Biol. 2009;394:600–605. doi: 10.1016/j.jmb.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu L.-H.M., Chan S.-H., Tsai S.-N., Wang Y., Cheng C.H.-K., Wong K.-B., et al. Fusion core structure of the severe acute respiratory syndrome coronavirus (SARS-CoV): in search of potent SARS-CoV entry inhibitors. J Cell Biochem. 2008;104:2335–2347. doi: 10.1002/jcb.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Struck A.-W., Axmann M., Pfefferle S., Drosten C., Meyer B. A hexapeptide of the receptor-binding domain of SARS corona virus spike protein blocks viral entry into host cells via the human receptor ACE2. Antiviral Res. 2012;94:288–296. doi: 10.1016/j.antiviral.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu W., Wu X.-D., Shi M. De, Yang R.F., He Y.Y., Bian C., et al. Synthetic peptides derived from SARS coronavirus S protein with diagnostic and therapeutic potential. FEBS Lett. 2005;579:2130–2136. doi: 10.1016/j.febslet.2005.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan K., Yi L., Chen J., Qu X., Qing T., Rao X., et al. Suppression of SARS-CoV entry by peptides corresponding to heptad regions on spike glycoprotein. Biochem Biophys Res Commun. 2004;319:746–752. doi: 10.1016/j.bbrc.2004.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bosch B.J., Martina B.E.E., Van Der Zee R., Lepault J., Haijema B.J., Versluis C., et al. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc Natl Acad Sci U S A. 2004;101:8455–8460. doi: 10.1073/pnas.0400576101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia S., Yan L., Xu W., Agrawal A.S., Algaissi A., Tseng C.-T.K., et al. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci Adv. 2019;5:eaav4580. doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sainz B.J., Mossel E.C., Gallaher W.R., Wimley W.C., Peters C.J., Wilson R.B., et al. Inhibition of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) infectivity by peptides analogous to the viral spike protein. Virus Res. 2006;120:146–155. doi: 10.1016/j.virusres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu I.-J., Kao C.-L., Hsieh S.-C., Wey M.-T., Kan L.-S., Wang W.-K. Identification of a minimal peptide derived from heptad repeat (HR) 2 of spike protein of SARS-CoV and combination of HR1-derived peptides as fusion inhibitors. Antiviral Res. 2009;81:82–87. doi: 10.1016/j.antiviral.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou M., Xu D., Li X., Li H., Shan M., Tang J., et al. Screening and identification of severe acute respiratory syndrome-associated coronavirus-specific CTL epitopes. J Immunol. 2006;177:2138–2145. doi: 10.4049/jimmunol.177.4.2138. [DOI] [PubMed] [Google Scholar]
- 56.He Y., Zhou Y., Siddiqui P., Niu J., Jiang S. Identification of immunodominant epitopes on the membrane protein of the severe acute respiratory syndrome-associated coronavirus. J Clin Microbiol. 2005;43:3718–3726. doi: 10.1128/JCM.43.8.3718-3726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choy W.-Y., Lin S.-G., Chan P.K.-S., Tam J.S.-L., Lo Y.M.D., Chu I.M.-T., et al. Synthetic peptide studies on the severe acute respiratory syndrome (SARS) coronavirus spike glycoprotein: perspective for SARS vaccine development. Clin Chem. 2004;50:1036–1042. doi: 10.1373/clinchem.2003.029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu H., Li L., Kao R.Y., Kou B., Wang Z., Zhang L., et al. Screening and identification of linear B-cell epitopes and entry-blocking peptide of severe acute respiratory syndrome (SARS)-associated coronavirus using synthetic overlapping peptide library. J Comb Chem. 2005;7:648–656. doi: 10.1021/cc0500607. [DOI] [PubMed] [Google Scholar]
- 59.Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17:613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barh D., Tiwari S., Silva Andrade B., Giovanetti M., Kumavath R., Ghosh P., et al. Potential chimeric peptides to block the SARS-CoV-2 spike RBD. Virology. 2020 doi: 10.12688/f1000research.24074.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han Y., Král P. Computational design of ACE2-Based peptide inhibitors of SARS-CoV-2. ACS Nano. 2020 doi: 10.1021/acsnano.0c02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang G., Pomplun S., Loftis A.R., Loas A., Pentelute B.L. The first-in-class peptide binder to the SARS-CoV-2 spike protein. bioRxiv. 2020 doi: 10.1101/2020.03.19.999318. 2020.03.19.999318. [DOI] [Google Scholar]
- 63.Xu Y., Zhu J., Liu Yiwei, Lou Z., Yuan F., Liu Yueyong, et al. Characterization of the heptad repeat regions, HR1 and HR2, and design of a fusion core structure model of the spike protein from severe acute respiratory syndrome (SARS) coronavirus. Biochemistry. 2004;43:14064–14071. doi: 10.1021/bi049101q. [DOI] [PubMed] [Google Scholar]
- 64.Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y., et al. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cannalire R., Stefanelli I., Cerchia C., Beccari A.R., Pelliccia S., Summa V. Sars-cov-2 entry inhibitors: Small molecules and peptides targeting virus or host cells. Int J Mol Sci. 2020 doi: 10.3390/ijms21165707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahendran A.S.K., Lim Y.S., Fang C.-M., Loh H.-S., Le C.F. The potential of antiviral peptides as COVID-19 therapeutics. Front Pharmacol. 2020;11:1475. doi: 10.3389/fphar.2020.575444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X., Xia S., Wang Q., Xu W., Li W., Lu L., et al. Broad-spectrum coronavirus fusion inhibitors to combat COVID-19 and other emerging coronavirus diseases. Int J Mol Sci. 2020;21:3843. doi: 10.3390/ijms21113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Snijder E.J., Decroly E., Ziebuhr J. The nonstructural proteins directing coronavirus RNA synthesis and processing. Adv Virus Res. 2016;96:59–126. doi: 10.1016/bs.aivir.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tahir ul Qamar M., Alqahtani S.M., Alamri M.A., Chen L.-L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang H., Xie W., Xue X., Yang K., Ma J., Liang W., et al. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005;3:e324. doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dai W., Zhang B., Jiang X.-M., Su H., Li J., Zhao Y., et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science (80-.) 2020;368 doi: 10.1126/science.abb4489. 1331 LP–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lei J., Kusov Y., Hilgenfeld R. Nsp3 of coronaviruses: structures and functions of a large multi-domain protein. Antiviral Res. 2018;149:58–74. doi: 10.1016/j.antiviral.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sacco R., Eggenhoffner R., Giacomelli L. Glutathione in the treatment of liver diseases: insights from clinical practice. Minerva Gastroenterol Dietol. 2016;62:316–324. [PubMed] [Google Scholar]
- 76.Sola I., Almazán F., Zúñiga S., Enjuanes L. Continuous and discontinuous RNA synthesis in coronaviruses. Annu Rev Virol. 2015;2:265–288. doi: 10.1146/annurev-virology-100114-055218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.te Velthuis A.J.W., Arnold J.J., Cameron C.E., van den Worm S.H.E., Snijder E.J. The RNA polymerase activity of SARS-coronavirus nsp12 is primer dependent. Nucleic Acids Res. 2010;38:203–214. doi: 10.1093/nar/gkp904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shannon A., Le N.T.-T., Selisko B., Eydoux C., Alvarez K., Guillemot J.-C., et al. Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 exonuclease active-sites. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saha A., Sharma A.R., Bhattacharya M., Sharma G., Lee S.-S., Chakraborty C. Probable molecular mechanism of remdesivir for the treatment of COVID-19: need to know more. Arch Med Res. 2020;51:585–586. doi: 10.1016/j.arcmed.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hillen H.S., Kokic G., Farnung L., Dienemann C., Tegunov D., Cramer P. Structure of replicating SARS-CoV-2 polymerase. Nature. 2020;584:154–156. doi: 10.1038/s41586-020-2368-8. [DOI] [PubMed] [Google Scholar]
- 82.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kirchdoerfer R.N., Ward A.B. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat Commun. 2019;10:2342. doi: 10.1038/s41467-019-10280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mutlu O., Ugurel O.M., Sariyer E., Ata O., Inci T.G., Ugurel E., et al. Targeting SARS-CoV-2 Nsp12/Nsp8 interaction interface with approved and investigational drugs: an in silico structure-based approach. J Biomol Struct Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1819882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang A.Y., Liu H. The past, present, and future of CRM1/XPO1 inhibitors. Stem Cell Investig. 2019;6:6. doi: 10.21037/sci.2019.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uddin M.H., Zonder J.A., Azmi A.S. Exportin 1 inhibition as antiviral therapy. Drug Discov Today. 2020 doi: 10.1016/j.drudis.2020.06.014. S1359-6446(20)30239–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ito M., Ito J., Kitazawa H., Shimamura K., Fukami T., Tokita S., et al. (–)–Ternatin inhibits adipogenesis and lipid metabolism in 3T3-L1 cells. Peptides. 2009;30:1074–1081. doi: 10.1016/j.peptides.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 89.Shimokawa K., Mashima I., Asai A., Ohno T., Yamada K., Kita M., et al. Biological activity, structural features, and synthetic studies of (−)-ternatin, a potent fat-accumulation inhibitor of 3T3-L1 adipocytes. Chem Asian J. 2008;3:438–446. doi: 10.1002/asia.200700243. [DOI] [PubMed] [Google Scholar]
- 90.Carelli J.D., Sethofer S.G., Smith G.A., Miller H.R., Simard J.L., Merrick W.C., et al. Ternatin and improved synthetic variants kill cancer cells by targeting the elongation factor-1A ternary complex. Elife. 2015;4 doi: 10.7554/eLife.10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chlamydas S., Papavassiliou A.G., Piperi C. Epigenetic mechanisms regulating COVID-19 infection. Epigenetics. 2021;16:263–270. doi: 10.1080/15592294.2020.1796896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clarke N.E., Belyaev N.D., Lambert D.W., Turner A.J. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin Sci. 2014;126:507–516. doi: 10.1042/CS20130291. [DOI] [PubMed] [Google Scholar]
- 93.Schäfer A., Baric R.S. Epigenetic landscape during coronavirus infection. Pathogens (Basel, Switzerland) 2017;6:8. doi: 10.3390/pathogens6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Corley, M.J.; Ndhlovu, L.C., 2020. DNA methylation analysis of the COVID-19 host cell receptor, angiotensin I converting enzyme 2 gene (ACE2) in the respiratory system reveal age and gender differences. Prepr. 2020. 10.20944/preprints202003.0295.v1. [DOI]
- 95.Qiao Y., Wang X.-M., Mannan R., Pitchiaya S., Zhang Y., Wotring J.W., et al. Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Proc Natl Acad Sci U S A. 2020;118 doi: 10.1073/pnas.2021450118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takahashi Y., Hayakawa A., Sano R., Fukuda H., Harada M., Kubo R., et al. Histone deacetylase inhibitors suppress ACE2 and ABO simultaneously, suggesting a preventive potential against COVID-19. Sci Rep. 2021;11:3379. doi: 10.1038/s41598-021-82970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pruimboom L. Methylation pathways and SARS-CoV-2 lung infiltration and cell membrane-virus fusion are both subject to epigenetics. Front Cell Infect Microbiol. 2020;10:290. doi: 10.3389/fcimb.2020.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olsen C.A., Ghadiri M.R. Discovery of potent and selective histone deacetylase inhibitors via focused combinatorial libraries of cyclic alpha3beta-tetrapeptides. J Med Chem. 2009;52:7836–7846. doi: 10.1021/jm900850t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.03.022. S1684-1182(20)30082–30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Daffis S., Szretter K.J., Schriewer J., Li J., Youn S., Errett J., et al. 2’-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Viswanathan T., Arya S., Chan S.-H., Qi S., Dai N., Misra A., et al. Structural basis of RNA cap modification by SARS-CoV-2. Nat Commun. 2020;11:3718. doi: 10.1038/s41467-020-17496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Y., Cai H., Pan J., Xiang N., Tien P., Ahola T., et al. Functional screen reveals SARS coronavirus nonstructural protein nsp14 as a novel cap N7 methyltransferase. Proc Natl Acad Sci U S A. 2009;106 doi: 10.1073/pnas.0808790106. 3484 LP–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y., Sun Y., Wu A., Xu S., Pan R., Zeng C., et al. Coronavirus nsp10/nsp16 methyltransferase can be targeted by nsp10-Derived peptide in vitro and in vivo to reduce replication and pathogenesis. J Virol. 2015;89:8416–8427. doi: 10.1128/JVI.00948-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Haan C.A.M., Rottier P.J.M. Molecular interactions in the assembly of coronaviruses. Adv Virus Res. 2005;64:165–230. doi: 10.1016/S0065-3527(05)64006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kang S., Yang M., Hong Z., Zhang L., Huang Z., Chen X., et al. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm Sin B. 2020 doi: 10.1016/j.apsb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cascarina S.M., Ross E.D. A proposed role for the SARS-CoV-2 nucleocapsid protein in the formation and regulation of biomolecular condensates. FASEB J. 2020 doi: 10.1096/fj.202001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Onomoto K., Yoneyama M., Fung G., Kato H., Fujita T. Antiviral innate immunity and stress granule responses. Trends Immunol. 2014;35:420–428. doi: 10.1016/j.it.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miller C.L. Stress granules and virus replication. Future Virol. 2011;6:1329–1338. doi: 10.2217/fvl.11.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakagawa K., Narayanan K., Wada M., Makino S. Inhibition of stress granule formation by middle east respiratory syndrome coronavirus 4a accessory protein facilitates viral translation, leading to efficient virus replication. J Virol. 2018;92:e00902–18. doi: 10.1128/JVI.00902-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang W., Ru Y., Ren J., Bai J., Wei J., Fu S., et al. G3BP1 inhibits RNA virus replication by positively regulating RIG-I-mediated cellular antiviral response. Cell Death Dis. 2019;10:946. doi: 10.1038/s41419-019-2178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020 doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 112.Mastaglio S., Ruggeri A., Risitano A.M., Angelillo P., Yancopoulou D., Mastellos D.C., et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mertens M., Singh J.A. Anakinra for rheumatoid arthritis. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD005121.pub3. [DOI] [PubMed] [Google Scholar]
- 114.Franzetti M., Pozzetti U., Carugati M., Pandolfo A., Molteni C., Faccioli P., et al. Interleukin-1 receptor antagonist anakinra in association with remdesivir in severe Coronavirus disease 2019: a case report. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.05.050. S1201-9712(20)30357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R., et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84:1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ahmed F., Jo D.-H., Lee S.-H. Can natural killer cells Be a principal player in Anti-SARS-CoV-2 immunity? Front Immunol. 2020;11:3246. doi: 10.3389/fimmu.2020.586765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., et al. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. medRxiv. 2020 doi: 10.1101/2020.02.23.20026690. 2020.02.23.20026690. [DOI] [Google Scholar]
- 119.Zang J., Ye J., Zhang C., Sha M., Gao J. Senescent hepatocytes enhance natural killer cell activity via the CXCL-10/CXCR3 axis. Exp Ther Med. 2019;18:3845–3852. doi: 10.3892/etm.2019.8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fadda L., Borhis G., Ahmed P., Cheent K., Pageon S.V., Cazaly A., et al. Peptide antagonism as a mechanism for NK cell activation. Proc Natl Acad Sci U S A. 2010;107 doi: 10.1073/pnas.0913745107. 10160 LP–10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu J., Wu P., Gao F., Qi J., Kawana-Tachikawa A., Xie J., et al. Novel immunodominant peptide presentation strategy: a featured HLA-A*2402-restricted cytotoxic T-lymphocyte epitope stabilized by intrachain hydrogen bonds from severe acute respiratory syndrome coronavirus nucleocapsid protein. J Virol. 2010;84:11849–11857. doi: 10.1128/JVI.01464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kiyotani K., Toyoshima Y., Nemoto K., Nakamura Y. Bioinformatic prediction of potential T cell epitopes for SARS-Cov-2. J Hum Genet. 2020;65:569–575. doi: 10.1038/s10038-020-0771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Joshi A., Joshi B.C., Mannan M.A.-U., Kaushik V. Epitope based vaccine prediction for SARS-COV-2 by deploying immuno-informatics approach. Informatics Med. 2020;19 doi: 10.1016/j.imu.2020.100338. unlocked. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abdelmageed M.I., Abdelmoneim A.H., Mustafa M.I., Elfadol N.M., Murshed N.S., Shantier S.W., et al. Design of a multiepitope-based peptide vaccine against the e protein of human COVID-19: an immunoinformatics approach. Biomed Res Int. 2020;2020 doi: 10.1155/2020/2683286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Waqas M., Haider A., Sufyan M., Siraj S., Sehgal S.A. Determine the potential epitope based peptide vaccine against novel SARS-CoV-2 targeting structural proteins using immunoinformatics approaches. Front Mol Biosci. 2020;7:227. doi: 10.3389/fmolb.2020.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Robson B. Computers and viral diseases. Preliminary bioinformatics studies on the design of a synthetic vaccine and a preventative peptidomimetic antagonist against the SARS-CoV-2 (2019-nCoV, COVID-19) coronavirus. Comput Biol Med. 2020;119 doi: 10.1016/j.compbiomed.2020.103670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Palatnik-de-Sousa C.B., Soares I., da S., Rosa D.S. Editorial: epitope discovery and synthetic vaccine design. Front Immunol. 2018;9:826. doi: 10.3389/fimmu.2018.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Parvizpour S., Pourseif M.M., Razmara J., Rafi M.A., Omidi Y. Epitope-based vaccine design: a comprehensive overview of bioinformatics approaches. Drug Discov Today. 2020;25:1034–1042. doi: 10.1016/j.drudis.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 129.Lin Y., Shen X., Yang R.F., Li Y.X., Ji Y.Y., He Y.Y., et al. Identification of an epitope of SARS-coronavirus nucleocapsid protein. Cell Res. 2003;13:141–145. doi: 10.1038/sj.cr.7290158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Q., Zhang L., Kuwahara K., Li L., Liu Z., Li T., et al. Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human Primates. ACS Infect Dis. 2016;2:361–376. doi: 10.1021/acsinfecdis.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grifoni A., Sidney J., Zhang Y., Scheuermann R.H., Peters B., Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27 doi: 10.1016/j.chom.2020.03.002. 671–680.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lucchese G. Epitopes for a 2019-nCoV vaccine. Cell Mol Immunol. 2020;17:539–540. doi: 10.1038/s41423-020-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Poh C.M., Carissimo G., Wang B., Amrun S.N., Lee C.Y.-P., Chee R.S.-L., et al. Two linear epitopes on the SARS-CoV-2 spike protein that elicit neutralising antibodies in COVID-19 patients. Nat Commun. 2020;11:2806. doi: 10.1038/s41467-020-16638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Herst C.V., Burkholz S., Sidney J., Sette A., Harris P.E., Massey S., et al. An effective CTL peptide vaccine for Ebola Zaire based on Survivors’ CD8+ targeting of a particular nucleocapsid protein epitope with potential implications for COVID-19 vaccine design. Vaccine. 2020;38:4464–4475. doi: 10.1016/j.vaccine.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Almofti Y.A., Abd-elrahman K.A., Eltilib E.E.M. Vaccinomic approach for novel multi epitopes vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) BMC Immunol. 2021;22:22. doi: 10.1186/s12865-021-00412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hisham Y., Ashhab Y., Hwang S.-H., Kim D.-E. Identification of highly conserved SARS-CoV-2 antigenic epitopes with wide coverage using reverse vaccinology approach. Viruses. 2021;13 doi: 10.3390/v13050787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rana R., Rathi V., Ganguly N.K. A comprehensive overview of proteomics approach for COVID 19: new perspectives in target therapy strategies. J Proteins Proteomics. 2020;11:223–232. doi: 10.1007/s42485-020-00052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Geller G., Duggal P., Thio C.L., Mathews D., Kahn J.P., Maragakis L.L., et al. Genomics in the era of COVID-19: ethical implications for clinical practice and public health. Genome Med. 2020;12:95. doi: 10.1186/s13073-020-00792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kellam P., Weiss R.A. Infectogenomics: insights from the host genome into infectious diseases. Cell. 2006;124:695–697. doi: 10.1016/j.cell.2006.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tilocca B., Britti D., Urbani A., Roncada P. Computational immune proteomics approach to target COVID-19. J Proteome Res. 2020;19:4233–4241. doi: 10.1021/acs.jproteome.0c00553. [DOI] [PubMed] [Google Scholar]
- 141.Thakur S.S. Proteomics and its application in pandemic diseases. J Proteome Res. 2020;19:4215–4218. doi: 10.1021/acs.jproteome.0c00824. [DOI] [PubMed] [Google Scholar]
- 142.Daoud S., Alabed S.J., Dahabiyeh L.A. Identification of potential COVID-19 main protease inhibitors using structure-based pharmacophore approach, molecular docking and repurposing studies. Acta Pharm. 2021;71:163–174. doi: 10.2478/acph-2021-0016. [DOI] [PubMed] [Google Scholar]
- 143.Noureddine O., Issaoui N., Al-Dossary O. DFT and molecular docking study of chloroquine derivatives as antiviral to coronavirus COVID-19. J King Saud Univ Sci. 2021;33 doi: 10.1016/j.jksus.2020.101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vincent S., Arokiyaraj S., Saravanan M., Dhanraj M. Molecular docking studies on the anti-viral effects of compounds from Kabasura Kudineer on SARS-CoV-2 3CLpro. Front Mol Biosci. 2020;7:434. doi: 10.3389/fmolb.2020.613401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Akaji K., Konno H., Onozuka M., Makino A., Saito H., Nosaka K. Evaluation of peptide-aldehyde inhibitors using R188I mutant of SARS 3CL protease as a proteolysis-resistant mutant. Bioorg Med Chem. 2008;16:9400–9408. doi: 10.1016/j.bmc.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bergeron E., Vincent M.J., Wickham L., Hamelin J., Basak A., Nichol S.T., et al. Implication of proprotein convertases in the processing and spread of severe acute respiratory syndrome coronavirus. Biochem Biophys Res Commun. 2005;326:554–563. doi: 10.1016/j.bbrc.2004.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chuck C.-P., Chen C., Ke Z., Chi-Cheong Wan D., Chow H.-F., Wong K.-B. Design, synthesis and crystallographic analysis of nitrile-based broad-spectrum peptidomimetic inhibitors for coronavirus 3C-like proteases. Eur J Med Chem. 2013;59:1–6. doi: 10.1016/j.ejmech.2012.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ke M., Chen Y., Wu A., Sun Y., Su C., Wu H., et al. Short peptides derived from the interaction domain of SARS coronavirus nonstructural protein nsp10 can suppress the 2’-O-methyltransferase activity of nsp10/nsp16 complex. Virus Res. 2012;167:322–328. doi: 10.1016/j.virusres.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li Q., Zhao Z., Zhou D., Chen Y., Hong W., Cao L., et al. Virucidal activity of a scorpion venom peptide variant mucroporin-M1 against measles, SARS-CoV and influenza H5N1 viruses. Peptides. 2011;32:1518–1525. doi: 10.1016/j.peptides.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang Y.Z., Holmes E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181(2):223–227. doi: 10.1016/j.cell.2020.03.035. Epub 2020 March 26. PMID: 32220310; PMCID: PMC7194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mustafa S., Balkhy H., Gabere M. Peptide-protein interaction studies of antimicrobial peptides targeting middle east respiratory syndrome coronavirus spike protein: an in silico approach. Adv Bioinform. 2019 doi: 10.1155/2019/6815105. PMID: 31354813; PMCID: PMC6634063. [DOI] [PMC free article] [PubMed] [Google Scholar]