Abstract
Aims
This study aimed to determine whether anthropometric markers of thoracic skeletal muscle and abdominal visceral fat tissue correlate with outcome parameters in critically ill COVID-19 patients.
Methods
We retrospectively analysed thoracic CT-scans of 67 patients in four ICUs at a university hospital. Thoracic skeletal muscle (total cross-sectional area (CSA); pectoralis muscle area (PMA)) and abdominal visceral fat tissue (VAT) were quantified using a semi-automated method. Point-biserial-correlation-coefficient, Spearman-correlation-coefficient, Wilcoxon rank-sum test and logistic regression were used to assess the correlation and test for differences between anthropometric parameters and death, ventilator- and ICU-free days and initial inflammatory laboratory values.
Results
Deceased patients had lower CSA and PMA values, but higher VAT values (p < 0.001). Male patients with higher CSA values had more ventilator-free days (p = 0.047) and ICU-free days (p = 0.017). Higher VAT/CSA and VAT/PMA values were associated with higher mortality (p < 0.001), but were negatively correlated with ICU length of stay in female patients only (p < 0.016). There was no association between anthropometric parameters and initial inflammatory biomarker levels. Logistic regression revealed no significant independent predictor for death.
Conclusion
Our study suggests that pathologic body composition assessed by planimetric measurements using thoracic CT-scans is associated with worse outcome in critically ill COVID-19 patients.
Keywords: COVID-19, Anthropometry, Critical care, muscle(skeletal), Adipose tissue
Abbreviations
- ACE2
Angiotensin-converting enzyme 2
- BMI
Body mass index
- COVID-19
Coronavirus disease 2019
- CSA
Cross sectional area
- CRP
C-reactive protein
- CT
Computed tomography
- DICOM
Digital imaging and communications in medicine
- HU
Hounsfield unit
- ICC
Intraclass correlation coefficient
- ICU
Intensive care unit
- IL-6
Interleukin-6
- IQR
Interquartile range
- PMA
Pectoralis muscle area
- SARS-CoV2
Severe acute respiratory syndrome coronavirus 2
- VAT
Visceral adipose tissue
1. Introduction
The coronavirus disease 2019 (COVID-19) has recently become one of the most dominant health care burdens worldwide. A significant proportion of the infected patients develop critical illness with respiratory failure as leading symptom, requiring intensive care and mechanical ventilation (Docherty et al., 2020). A substantial percentage of patients treated in intensive care units (ICU) are people with overweight or obesity (Richards-Belle et al., 2020). Besides age and pre-existing illnesses, obesity has been identified as one of the most important predictors for poor outcome (Yang et al., 2020; Hussain et al., 2020). On the other hand, results of earlier studies in critically ill patients with adult respiratory distress syndrome (ARDS) reported better outcomes in patients with obesity (obesity paradox) (Ni et al., 2017). Further, several studies have already shown that sarcopenia, a state characterized by reduced muscle mass and/or function, and sarcopenic obesity were independent risk factors in both surgical and non-surgical critically ill patients (Moisey et al., 2013; Ji et al., 2018; Maeda and Akagi, 2017; Tsaousi et al., 2017).
In contrast to that, little is known about the role of skeletal muscle mass in patients with COVID-19 receiving intensive care. The vast majority of the studies evaluating nutritional status in COVID-19 cohorts categorized the patients using the body mass index (BMI). However, there is evidence that the predictive value of BMI in critically ill patients is limited, as it does not sufficiently reflect body composition (Paolini et al., 2010). Anthropometric measurements using cross-sectional thoracic computed tomography (CT) scans offer an attractive alternative to describe body composition in patients with COVID-19 for several reasons. First, suitable images are widely available in this population, as chest-CTs are frequently performed in case of suspected or diagnosed cases. Secondly, with the aid of intuitive computer programs using semi-automated methods, muscle and fat tissue compartments can easily be visualised and quantified. Thirdly, this approach provides a snapshot of the patients' current nutritional status, whereas height and weight are often estimated and inaccurate, especially in ICU patients (Bloomfield et al., 2006).
As respiratory failure and systemic inflammatory response are the key features of COVID-19, it is a reasonable assumption that body composition -reflected by thoracic skeletal muscle and visceral fat tissue with its diverse endocrine functions - might have a significant impact on patient outcome. The purpose of this study was to evaluate body composition in critically ill patients with COVID-19 and to assess if there is an association between body composition parameters and clinical outcome.
1.1. Patients and methods
1.1.1. Study population
The study complied with the edicts of the 1975 Declaration of Helsinki and was approved by the institutional review board of the university of Munich (20–0944).
This retrospective study included critically ill patients with laboratory (PCR) confirmed SARS-CoV-2 infection who were admitted due to respiratory failure to one of four COVID-19 ICUs at a tertiary care university hospital between March and July 2020.
Out of a total of 77 SARS-CoV-2 positive patients, two surgical patients were excluded since their admission was not due to respiratory failure. Another patient was excluded since - in accordance with the patient's will - the therapeutic goal was modified immediately after admission to palliative care.
1.2. Imaging technique and analysis
67 of the remaining 74 patients received a multi-slice CT scan of the thorax as part of the primary evaluation of their disease (prior to or on the day of ICU admission). The indication for CT was either suspected pneumonia or suspected pulmonary embolism. In the former case, a non-contrast examination was performed, in the latter an examination with intravenous contrast medium in the arterial phase. For further image analysis, we selected the reconstructions that provided the best image quality, typically with 2–3 mm slice thickness. Anthropometric measurements were carried out by an experienced investigator using DICOM software OsiriX™ (OsiriX Lite, Pixmeo, Geneva, Switzerland). Intraobserver reliability was tested beforehand in a random sample of 20 hospitalized patients with COVID-19. The validity of the method was verified by a second investigator, who independently reanalysed the random sample.
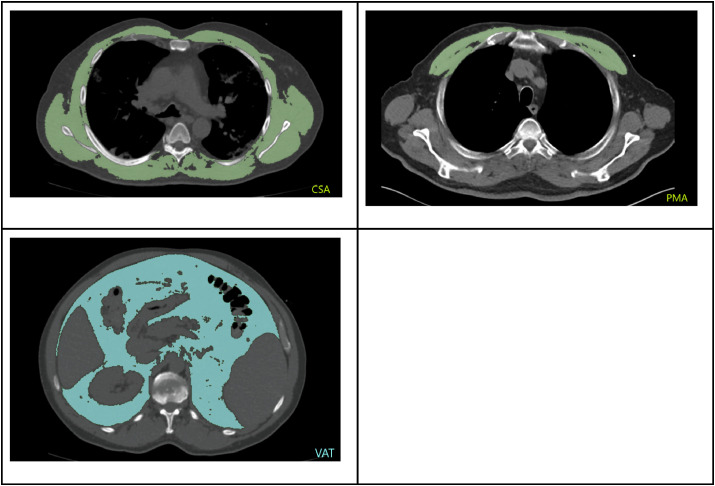
Two indices were determined to assess thoracic skeletal muscle. First, total muscle cross-sectional area (CSA) was measured at the level of the fifth thoracic vertebra, as suggested by Fintelmann et al. (2018) (Fig. 1 a.) Scans with incomplete imaging of the dorsolateral muscles - which occurred particularly (but not only) when the pulmonary embolism protocol was applied - were excluded (5 cases). Secondly, the first axial slice cranial of the aortic arch was identified and pectoralis muscle area (PMA) was quantified according to previous studies (McDonald et al., 2014; Kim et al., 2017) (Fig. 1b.). For lean muscle tissue, Hounsfield unit (HU)-thresholds were set to −29 to +150 HU.
Fig. 1.
a) Total muscle cross-sectional area (CSA), measured at the level of the 5th thoracic vertebral body, b) Pectoralis muscle area (PMA) on the first slice above the aortic arch, c) Quantification of abdominal visceral adipose tissue (VAT) at the level of the intervertebral disc space T12/L1.
Finally, by using the method described by Faron et al. abdominal visceral adipose tissue (VAT) was measured at the level of the intervertebral disc space between the 12th thoracic and the 1st lumbar vertebra (T12/L1), with a Hounsfield unit range of −190 to - 50 HU (Faron et al., 2019) (Fig. 1c.). All parameters were expressed in cm2.
1.3. Variables and outcomes
BMI was calculated based on weight and height documented in the medical record. In addition to the anthropometric parameters CSA, PMA (for muscle tissue) and VAT (for fat tissue), the ratio VAT/CSA and VAT/PMA were calculated as indicators of sarcopenic obesity.
These parameters were tested for correlations with the following outcome parameters: in-hospital death, duration of mechanical ventilation, ICU length of stay. In order to account for deceased patients, the variables “ventilator-free days” and “ICU-free days” during a 30-day period (after intubation and ICU admission, respectively) were calculated (Yehya et al., 2019).
Furthermore, we hypothesized that pathologic body constitution could have an impact on initial inflammatory activity, which was evaluated by blood levels of C-reactive protein (CRP), interleukin-6 (IL-6) and ferritine on the day of ICU admission.
1.4. Statistical analysis
Intra- and interobserver reliability were tested with the statistical software R version 3.6.0 using the package ‚irr‘ version 0.84.1. Further analyses were performed in Python 3.8.6.
The correlation between a continuous and a binary variable was tested with the point biserial correlation coefficient; the correlation between two continuous variables was tested with the Spearman's rank correlation coefficient. To test whether two sets of measurements are drawn from the same distribution, the Wilcoxon rank-sum test was used. A p-value of less than 0.05 was considered statistically significant.
We performed a logistic regression analysis to model the risk of death. Age, sex, BMI, CSA and VAT were used as independent variables.
Due to the small sample size, data were assumed to be not normally distributed. Hence, results are displayed as median and interquartile range (IQR).
2. Results
The study group consisted of 60 male (81%) and 14 female patients (19%), with a median age of 66 years (57.0; 72.75). 68 patients required mechanical ventilation, with a median ventilation time of 16 days (8; 31.25) and a median number of 7.0 ventilator-free days (0; 21.8). Median duration of ICU treatment was 20 days (10.0; 37.75) resulting in a median number of 3.5 ICU-free days (0; 18). 20 patients (27%) died during the observation period, which ended on October 7th, 2020. At this time, 1 of the 74 patients still has not been discharged from the ICU (see Table 1 ).
Table 1.
Descriptive statistics of the study group; * measurements were available for 13 female and 58 male patients; **measurements were available for 13 female and 57 male patients.
| Male (n = 60) | Female (n = 14) | All (n = 74) | |
|---|---|---|---|
| Age [years] | 64.5 (57.0; 72.3) | 71.0 (68.3; 74.3) | 66.0 (57.0; 72.8) |
| BMI [kg/m2]* | 27 (Favre et al., 2021; Park et al., 2011) | 31 (Watanabe et al., 2020; Li et al., 2020) | 27 (Favre et al., 2021; Rojas-Osornio et al., 2019) |
| Deceased | 17 | 3 | 20 |
| Hospital to ICU admission [days] | 1.0 [0; 2.25] | 1.0 [0; 1.0] | 1.0 [0; 2.0] |
| ICU length of stay [days] | 18.5 (10.0; 38.5) | 21.5 (16.0; 29.3) | 20.0 (10.0; 37.8) |
| ICU-free days [days] | 3.0 (0; 18.3) | 8.0 (0; 14.0) | 3.5 (0; 18.0) |
| Length of mechanical ventilation [days] | 14.5 (7.3; 30.0) | 21.5 (10.3; 31.3) | 16.0 (8.0; 31.3) |
| Ventilator-free days [days] | 6.0 (0; 22.0) | 8.5 (0; 19.8) | 7.0 (0; 21.8) |
| CSA [cm2] ** | 181.85 (155.02; 198.87) | 99.29 (93.68; 132.28) | 160.78 (133.79; 193.79) |
| PMA [cm2] ** | 44.66 (33.02; 53.29) | 26.72 (23.58; 32.53) | 39.95 (30.15; 49.69) |
| VAT [cm2] ** | 131.73 (94.39; 180.14) | 60.42 (52.78; 116.35) | 125.86 (67.09; 164.35) |
The median time from hospital admission to ICU admission was one day [0; 2] for all patients.
When testing for the agreement of anthropometric measurements between observers, intra-observer and inter-observer reliability were high, with overall intraclass correlation coefficients (ICC) of 0.992 (95% confidence interval [CI]: 0.987–0.995) and 0.968 (CI: 0.946–0.981), respectively.
Anthropometric characteristics of the study population are summarized in Table 2 . Elderly patients, i.e. aged 65 years and older, had reduced thoracic skeletal muscle mass compared to patients aged younger than 65 years, whereas visceral adipose tissue mass was not significantly different in the two age groups.
Table 2.
Anthropometric characteristics of the study group (Wilcoxon rank-sum test using median values).
| Male (n = 54) |
Female (n = 13) |
All (n = 67) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Age groups | <65 y (n = 29) | ≥65 y (n = 25) | p-value | <65 y (n = 3) | ≥65 y (n = 10) | p-value | <65 y (n = 32) | ≥65 y (n = 35) | p-value |
| CSA [cm2] | 189.56 | 160.78 | 0.003 | 94.39 | 110.67 | 0.612 | 187.05 | 145.89 | <0.001 |
| PMA [cm2] | 48.37 | 37.19 | <0.001 | 34.17 | 25.56 | 0.176 | 47.14 | 32.81 | <0.001 |
| VAT [cm2] | 127.04 | 144.26 | 0.391 | 60.42 | 80.64 | 0.499 | 124.92 | 131.48 | 0.801 |
A significant difference was seen in BMI measurements between deceased and non-deceased patients (p < 0.001). While deceased patients had a median BMI of 25, survivors had a median BMI of 28. Stratifying the parameter by gender, we saw that deceased male patients had lower median BMI compared to the males who survived (25 vs. 28; p < 0.001). In female patients, we observed an inverse correlation (31 vs. 32; p < 0.001).
Higher CSA and PMA values were associated with lower mortality in both genders (all p < 0.001) (see Table 3 ).
Table 3.
Differences between median anthropometric values in survivors and deceased patients (Wilcoxon rank-sum test).
| Male (n = 54) |
Female (n = 13) |
|||||
|---|---|---|---|---|---|---|
| Survived (n = 40) | Deceased (n = 14) | p-value | Survived (n = 10) | Deceased (n = 3) | p-value | |
| CSA [cm2] | 184.51 | 158.84 | <0.001 | 123.25 | 94.36 | <0.001 |
| PMA [cm2] | 45.67 | 37.19 | <0.001 | 27.06 | 25.84 | <0.001 |
| VAT [cm2] | 129.78 | 148.0 | <0.001 | 57.96 | 111.13 | <0.001 |
| VAT/CSA | 0.73 | 0.90 | <0.001 | 0.54 | 1.17 | 0.002 |
| VAT/PMA | 2.95 | 3.64 | <0.001 | 1.9 | 4.02 | <0.001 |
In male patients, CSA showed a positive correlation with ventilator-free days (Spearman's ρ = 0.27; p = 0.047) and ICU-free days (Spearman's ρ = 0.32; p = 0.017). No significant correlation was found for PMA values in male patients. In female patients, neither CSA nor PMA showed any significant correlation regarding ventilator- or ICU-free days. There was a significant difference in abdominal visceral fat tissue (VAT) in both genders: Deceased patients had higher VAT values compared to survivors (see Table 3) (female: p < 0.001; male: p < 0.001). No differences were found in male or female patients for ventilator or ICU-free days.
However, we detected a negative correlation between VAT and ICU length of stay in females (Spearman's ρ = −0.72; p = 0.005), but not in male patients (Spearman's ρ = −0.19; p = 0.164).
Further analyses revealed significant differences between the body composition parameters VAT/CSA and VAT/PMA and death, as in deceased patients these parameters were significantly higher, regardless of gender (see Table 3).
VAT/CSA and VAT/PMA values were negatively correlated to the length of ICU stay in females (Spearman's ρ of −0.65 and −0.71 respectively; both p < 0.016), but not in male patients. Positive correlations for ICU-free days were observed in female patients, but these did not reach statistical significance.
Finally, none of the anthropometric characteristics (values and ratios) showed any correlation to inflammatory biomarkers interleukin-6, CRP, or ferritin, stratified by sex. The same observation was made for BMI (all p > 0.05).
The Logit model yielded an accuracy of 0.754. Although no coefficient showed a significant p-value, we see that age, VAT and male gender increase the risk of death, whereas BMI and CSA reduced it, respectively (see Table 4 ).
Table 4.
Odds ratios and p-values of the Logit model for the risk of death.
| Variable | Odds Ratio | p-value |
|---|---|---|
| Age [years [ | 1.026 | 0.207 |
| BMI [kg/m2] | 0.945 | 0.300 |
| CSA [cm2] | 0.985 | 0.062 |
| VAT [cm2] | 1.002 | 0.620 |
| Sex (male) | 2.732 | 0.311 |
3. Discussion
The present study investigated the role of pathologic body composition in critically ill patients with COVID-19. Our aim was to identify possible associations between thoracic skeletal musculature, abdominal visceral fat tissue and key elements of the treatment course. Our results suggest that decreased thoracic muscle mass, higher values of visceral adipose tissue and increased fat/muscle tissue ratio were associated with increased mortality. Furthermore, male patients with a greater thoracic muscle area had more ventilator-free and ICU-free days. In contrast to that, in female patients with higher VAT, VAT/CSA and VAT/PMA values ICU length of stay was shorter.
The median age of 66 years and a relevant imbalance regarding gender (81% male patients) characterized the analysed population. These findings are in line with the results of previous studies in large COVID-19 cohorts (Richards-Belle et al., 2020; Grasselli et al., 2020; Gupta et al., 2020). A significant proportion of our patients met the BMI-based criterion of overweight (n = 20) or obesity (n = 23) (28 and 32%, respectively; BMI was not available in 3 patients). In a recently published prospective multicentre study in critically ill patients with COVID-19, the proportion of patients with overweight or obesity was 34,3% and 39,4% (Richards-Belle et al., 2020).
When stratified by gender, our analyses observed discordant correlations between BMI and mortality in male and female patients. These results may be explained by the fact that in the entire cohort, as well as in these gender subsets, the median values in deceased and survived patients differed only marginally. It is therefore unlikely that these results are an expression of an actual „obesity paradox” in our study cohort. But more importantly, these findings underline the importance of tissue-specific anthropometric analysis of body composition. The CT-based method used in the present study offers an attractive approach, considering its simplicity and excellent accuracy.
One of the most important results was that median thoracic skeletal muscle mass was lower in patients who died during their hospital stay. This difference was significant for muscle cross-sectional area as well as for pectoralis muscle area, both in male and female patients. In addition to that, we found that thoracic skeletal muscle was positively correlated to ventilator-free days and ICU-free days in male patients. These findings suggest a crucial impact of muscle mass in patients with COVID-19. Although reduced thoracic muscle CSA has been identified as a predictor for worse outcome in both surgical and non-surgical patients ((Fintelmann et al., 2018), (Moon et al., 2019)), to our knowledge, this parameter has not yet been utilized in assessing thoracic skeletal musculature in patients with COVID-19. In a recent investigation reduced pectoralis muscle area and index were found to be associated with adverse outcomes in adult patients with COVID-19 (Ufuk et al., 2020). In contrast to that, Moctezuma-Velázquez and colleagues could not demonstrate a negative impact of low skeletal muscle area (determined at the level of the 12th thoracic vertebra) on the outcome in 519 COVID-19 patients (Moctezuma-Velazquez et al., 2021). As accepted anthropometric cut-off values based on chest CT scans are not available, we were not able to determine the prevalence of sarcopenia in our study group. Although sarcopenia is without doubt a major problem in critical care, it has been questioned whether the reported negative effects are caused by sarcopenia itself or by conditions associated with sarcopenia (e.g.: wasting comorbidity, undernutrition) (Baggerman et al., 2020). In this context, it is important to note that 75% of all patients were admitted to the ICU within three days from their hospital admission, and CT scans were obtained before or shortly after intubation. This is an issue of utmost importance since critical illness is associated with progressive loss of muscle mass (Puthucheary et al., 2013). Therefore, measurements of skeletal muscle at a later stage of the clinical course might not represent the initial body composition accurately. Interestingly, although in our study elderly patients had reduced skeletal muscle mass, logistic regression did not show a significant correlation between age and risk of death.
Turning to the results regarding fat tissue, our study indicates that abdominal visceral fat affects mortality in critically ill patients with COVID-19. So far, CT-based assessment of visceral fat tissue has been applied only in a few COVID-19-related studies. A small study reported that hospitalized patients with COVID-19 had higher VAT values (measured on abdominal CT scans) compared to outpatients (Chandarana et al., 2021). Favre et al. reported a correlation between higher abdominal visceral fat area and poor outcome (Favre et al., 2021). Watanabe et al. applied a similar approach as used in our study and found that increased abdominal visceral fat correlated with necessity of ICU admission (Watanabe et al., 2020). The results of two other investigations using slightly different approaches reached similar conclusions (Battisti et al., 2020; Petersen et al., 2020).
It is well known that increased intra-abdominal fat can negatively influence respiration mechanics (Park et al., 2011). More importantly, visceral adipose tissue is a body compartment with diverse endocrine and immunologic functions, which has been subject to intensive research in the past (Alexopoulos et al., 2014; Rojas-Osornio et al., 2019). These aspects have presumably important consequences in COVID-19 as well (Sattar et al., 2020). For instance, the expression of the angiotensin-converting enzyme 2 (ACE2) in fat tissue represents a highly interesting aspect, since SARS-CoV2 enters the cell via the ACE2 receptor (Li et al., 2020). Beyond that, numerous other obesity-related implications has been subject to discussion, such as altered vitamin D metabolism or intracellular pH levels (Belancic et al., 2020). Contrary to expectations, we could not detect any association between parameters of abdominal fat tissue and blood levels of CRP, IL-6 or ferritine. A possible explanation might be that our study included a couple of patients who received experimental therapy (in a double-blinded setting) targeting the inflammatory cascade.
In addition, we found that higher VAT/CSA and VAT/PMA values -which indicate visceral fat mass accumulation in combination with decreased skeletal muscle mass-were associated with worse outcomes. A recent feasibility study using the ratio waist circumference per paravertebral muscle circumference also suggested that the combination of low muscle area with high fat area represents an additional prognostic information regarding patient outcome (Kottlors et al., 2020).Surprisingly, although in accordance with the results of the analysis using VAT, both quotients showed a negative correlation with length of ICU stay in female patients. However, the latter findings must be interpreted with caution, given the small number of female patients in our cohort.
There were several limitations of this study. First, although patients from four independently managed ICUs were included, therapy standards were to a large extent similar due to comprehensive centre-based guidelines, which make our results less generalizable. Second, CT-based anthropometric cut-off values of obesity and skeletal muscle tissue were originally defined utilizing abdominal scans, whereas large studies on thoracic scans are still lacking. Finally, as female patients were underrepresented in the study population, gender-dependent differences could not be investigated satisfactorily.
4. Conclusion
Reduced thoracic skeletal muscle mass and increased abdominal visceral fat tissue parameters were associated with worse outcomes in critically ill patients with COVID-19. The use of CT-based anthropometric measurements of skeletal muscle and fat tissue compartments could lead to a better understanding of their effect on the treatment course and survival in this population and promote risk stratification. Further studies are required to investigate the impact of pathologic body composition and gender-dependent differences in patients with COVID-19 treated in ICUs.
CRediT authorship contribution statement
Balázs Poros: Conceptualization, Investigation, Writing – original draft. Andrea Sabine Becker-Pennrich: Methodology, Formal analysis. Bastian Sabel: Methodology, Validation, Writing – review & editing. Hans Joachim Stemmler: Resources. Dietmar Wassilowsky: Resources. Thomas Weig: Conceptualization, Writing – review & editing. Ludwig Christian Hinske: Validation. Bernhard Zwissler: Resources, Supervision. Jens Ricke: Resources, Supervision. Dominik J. Hoechter: Conceptualization, Investigation, Writing – review & editing.
Declaration of competing interest
All authors declare to have no conflict of interest. There was no funding for this retrospective study.
References
- Alexopoulos N., Katritsis D., Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 2014;233(1):104–112. doi: 10.1016/j.atherosclerosis.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Baggerman M.R., van Dijk Dpj, Winkens B., van Gassel Rjj, Bol M.E., Schnabel R.M., et al. Muscle wasting associated co-morbidities, rather than sarcopenia are risk factors for hospital mortality in critical illness. J. Crit. Care. 2020;56:31–36. doi: 10.1016/j.jcrc.2019.11.016. [DOI] [PubMed] [Google Scholar]
- Battisti S., Pedone C., Napoli N., Russo E., Agnoletti V., Nigra S.G., et al. Computed tomography highlights increased visceral adiposity associated with critical illness in COVID-19. Diabetes Care. 2020;43(10):e129–e130. doi: 10.2337/dc20-1333. [DOI] [PubMed] [Google Scholar]
- Belancic A., Kresovic A., Racki V. Potential pathophysiological mechanisms leading to increased COVID-19 susceptibility and severity in obesity. Obes Med. 2020;19:100259. doi: 10.1016/j.obmed.2020.100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield R., Steel E., MacLennan G., Noble D.W. Accuracy of weight and height estimation in an intensive care unit: implications for clinical practice and research. Crit. Care Med. 2006;34(8):2153–2157. doi: 10.1097/01.CCM.0000229145.04482.93. [DOI] [PubMed] [Google Scholar]
- Chandarana H., Dane B., Mikheev A., Taffel M.T., Feng Y., Rusinek H. Visceral adipose tissue in patients with COVID-19: risk stratification for severity. Abdom Radiol (NY) 2021;46(2):818–825. doi: 10.1007/s00261-020-02693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faron A., Luetkens J.A., Schmeel F.C., Kuetting D.L.R., Thomas D., Sprinkart A.M. Quantification of fat and skeletal muscle tissue at abdominal computed tomography: associations between single-slice measurements and total compartment volumes. Abdom Radiol (NY) 2019;44(5):1907–1916. doi: 10.1007/s00261-019-01912-9. [DOI] [PubMed] [Google Scholar]
- Favre G., Legueult K., Pradier C., Raffaelli C., Ichai C., Iannelli A., et al. Visceral fat is associated to the severity of COVID-19. Metabolism. 2021;115:154440. doi: 10.1016/j.metabol.2020.154440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fintelmann F.J., Troschel F.M., Mario J., Chretien Y.R., Knoll S.J., Muniappan A., et al. Thoracic skeletal muscle is associated with adverse outcomes after lobectomy for lung cancer. Ann. Thorac. Surg. 2018;105(5):1507–1515. doi: 10.1016/j.athoracsur.2018.01.013. [DOI] [PubMed] [Google Scholar]
- Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Mahawar K., Xia Z., Yang W., El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. Obes. Res. Clin. Pract. 2020;14(4):295–300. doi: 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ji Y., Cheng B., Xu Z., Ye H., Lu W., Luo X., et al. Impact of sarcopenic obesity on 30-day mortality in critically ill patients with intra-abdominal sepsis. J. Crit. Care. 2018;46:50–54. doi: 10.1016/j.jcrc.2018.03.019. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Kim E.Y., Kang S.M., Ahn H.K., Kim H.S. Single cross-sectional area of pectoralis muscle by computed tomography - correlation with bioelectrical impedance based skeletal muscle mass in healthy subjects. Clin. Physiol. Funct. Imag. 2017;37(5):507–511. doi: 10.1111/cpf.12333. [DOI] [PubMed] [Google Scholar]
- Kottlors J., Zopfs D., Fervers P., Bremm J., Abdullayev N., Maintz D., et al. Body composition on low dose chest CT is a significant predictor of poor clinical outcome in COVID-19 disease - a multicenter feasibility study. Eur. J. Radiol. 2020;132:109274. doi: 10.1016/j.ejrad.2020.109274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K., Akagi J. Muscle mass loss is a potential predictor of 90-day mortality in older adults with aspiration pneumonia. J. Am. Geriatr. Soc. 2017;65(1):e18–e22. doi: 10.1111/jgs.14543. [DOI] [PubMed] [Google Scholar]
- McDonald M.L., Diaz A.A., Ross J.C., San Jose Estepar R., Zhou L., Regan E.A., et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc. 2014;11(3):326–334. doi: 10.1513/AnnalsATS.201307-229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moctezuma-Velazquez P., Miranda-Zazueta G., Ortiz-Brizuela E., Gonzalez-Lara M.F., Tamez-Torres K.M., Roman-Montes C.M., et al. Low thoracic skeletal muscle area is not associated with negative outcomes in patients with COVID-19. Am. J. Phys. Med. Rehabil. 2021;100(5):413–418. doi: 10.1097/PHM.0000000000001716. [DOI] [PubMed] [Google Scholar]
- Moisey L.L., Mourtzakis M., Cotton B.A., Premji T., Heyland D.K., Wade C.E., et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit. Care. 2013;17(5):R206. doi: 10.1186/cc12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S.W., Choi J.S., Lee S.H., Jung K.S., Jung J.Y., Kang Y.A., et al. Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir. Res. 2019;20(1):35. doi: 10.1186/s12931-019-1001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y.N., Luo J., Yu H., Wang Y.W., Hu Y.H., Liu D., et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit. Care. 2017;21(1):36. doi: 10.1186/s13054-017-1615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini J.B., Mancini J., Genestal M., Gonzalez H., McKay R.E., Samii K., et al. Predictive value of abdominal obesity vs. body mass index for determining risk of intensive care unit mortality. Crit. Care Med. 2010;38(5):1308–1314. doi: 10.1097/CCM.0b013e3181d8cd8b. [DOI] [PubMed] [Google Scholar]
- Park Y.S., Kwon H.T., Hwang S.S., Choi S.H., Cho Y.M., Lee J., et al. Impact of visceral adiposity measured by abdominal computed tomography on pulmonary function. J. Kor. Med. Sci. 2011;26(6):771–777. doi: 10.3346/jkms.2011.26.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A., Bressem K., Albrecht J., Thiess H.M., Vahldiek J., Hamm B., et al. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317. doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthucheary Z.A., Rawal J., McPhail M., Connolly B., Ratnayake G., Chan P., et al. Acute skeletal muscle wasting in critical illness. J. Am. Med. Assoc. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- Richards-Belle A., Orzechowska I., Gould D.W., Thomas K., Doidge J.C., Mouncey P.R., et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020;46(11):2035–2047. doi: 10.1007/s00134-020-06267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Osornio S.A., Cruz-Hernandez T.R., Drago-Serrano M.E., Campos-Rodriguez R. Immunity to influenza: impact of obesity. Obes. Res. Clin. Pract. 2019;13(5):419–429. doi: 10.1016/j.orcp.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Sattar N., McInnes I.B., McMurray J.J.V. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142(1):4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- Tsaousi G., Kokkota S., Papakostas P., Stavrou G., Doumaki E., Kotzampassi K. Body composition analysis for discrimination of prolonged hospital stay in colorectal cancer surgery patients. Eur. J. Canc. Care. 2017;26(6) doi: 10.1111/ecc.12491. [DOI] [PubMed] [Google Scholar]
- Ufuk F., Demirci M., Sagtas E., Akbudak I.H., Ugurlu E., Sari T. The prognostic value of pneumonia severity score and pectoralis muscle Area on chest CT in adult COVID-19 patients. Eur. J. Radiol. 2020;131:109271. doi: 10.1016/j.ejrad.2020.109271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Caruso D., Tuccinardi D., Risi R., Zerunian M., Polici M., et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319. doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehya N., Harhay M.O., Curley M.A.Q., Schoenfeld D.A., Reeder R.W. Reappraisal of ventilator-free days in critical care research. Am. J. Respir. Crit. Care Med. 2019;200(7):828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]