Abstract
Background
Cardiovascular disease (CVD) can occur in COVID-19 and has impact on clinical course. Data on CVD prevalence in hospitalized COVID-19 patients and sequelae in survivors is limited. Aim of this prospective study carried out on consecutive unselected COVID-19 population, was to assess: 1) CVD occurrence among hospitalized COVID-19 patients, 2) persistence or new onset of CVD at one-month and one-year follow-up.
Methods
Over 30 days n = 152 COVID-19 patients underwent cardiovascular evaluation. Standard electrocardiogram (ECG), Troponin and echocardiography were integrated by further tests when indicated. Medical history, arterial blood gas, blood tests, chest computed tomography and treatment were recorded. CVD was defined as the occurrence of a new condition during the hospitalization for COVID-19. Survivors attended a one-month follow-up visit and a one-year telephone follow-up.
Results
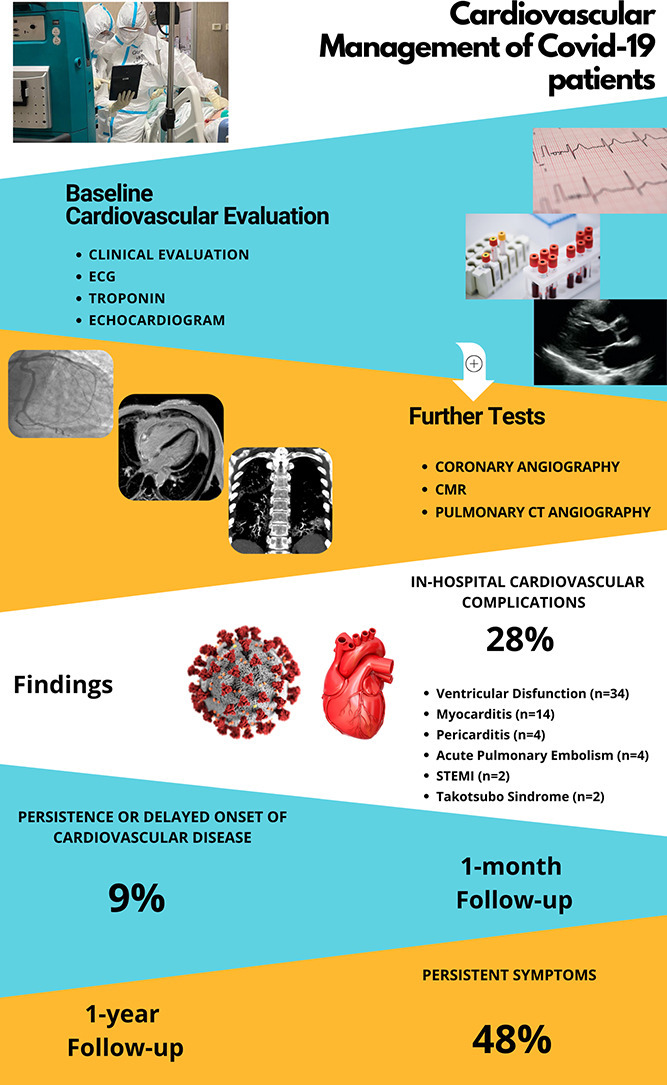
Forty-two patients (28%) experienced a wide spectrum of CVD with acute myocarditis being the most frequent. Death occurred in 32 patients (21%) and more frequently in patients who developed CVD (p = 0.032). After adjustment for confounders, CVD was independently associated with death occurrence. At one-month follow-up visit, 7 patients (9%) presented persistent or delayed CVD. At one-year telephone follow-up, 57 patients (48%) reported persistent symptoms.
Conclusion
Cardiovascular evaluation in COVID-19 patients is crucial since the occurrence of CVD in hospitalized COVID-19 patients is common (28%), requires specific treatment and increases the risk of in-hospital mortality. Persistence or delayed presentation of CVD at 1-month (9%) and persistent symptoms at 1-year follow-up (48%) suggest the need for monitoring COVID-19 survivors.
Keywords: COVID-19, Echocardiography, Cardiovascular disease, Cardiovascular magnetic resonance, Myocarditis, Follow-up
Abbreviations: ABG, arterial-blood gas; A&E, Accident & Emergency Department; CAD, coronary artery disease; CVD, Cardiovascular Diseases; CMR, Cardiac Magnetic Resonance; COPD, chronic obstructive pulmonary disease; COVID-19, COronaVIrus Disease 19; COVID-ICU, COVID intensive care unit; CPAP, continuous positive airway pressure; CT, chest computed tomography; ECG, electrocardiogram; FoCUS, Focus Cardiac UltraSound; HF, heart failure; hs-Trop, high sensitive troponin; HTN, arterial hypertension; IMV, invasive mechanical ventilation; LAD, left anterior descending artery; LV, left ventricle; MI, myocardial infarction; NIV, Non-invasive ventilation; PCI, percutaneous coronary intervention; RT-PCR, reverse-transcriptase-polymerase-chain reaction; RV, right ventricle; SARS-CoV-2, Severe Acute Respiratory Syndrome CoronaVirus 2; STEMI, ST-Elevation Myocardial Infarction; 6MWT, 6 min walking test
Graphical abstract
1. Introduction
The outbreak of COronaVIrus Disease 19 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is mainly characterized by a pulmonary involvement. However, cardiovascular complications can occur. During the first wave of the pandemic early observations reported an increased level of Troponin in a proportion of patients (10–12%) (1,2) associated with increased mortality (3). An increasing number of sporadic cases reporting cardiovascular involvement has been described afterwards, including Tako-Tsubo syndrome (4), ST-segment elevation (5) and other cases generally reported as myocarditis or myopericarditis (6,7).
Considering this growing evidence, studies based on echocardiographic evaluation were carried out which reported a high prevalence of cardiac involvement (8,9). However, these evaluations were limited to echocardiography without further cardiac examinations in case of positive findings.
The wide spectrum of cardiovascular complication can be related to the release of cytokines mediated by SARS-CoV-2, possibly causing myocardial inflammation in addition to vascular inflammation and plaque instability. Direct myocardial damage by the virus should also be considered despite the fact that undisputable localization of SARS-CoV-2 within cardiomyocytes has been not proven among the limited number of autopsies in COVID-19 ([10], [11], [12], [13], [14]). The presence of cardiovascular risk factors or cardiac disease, both pre-existing and new, is related to poor prognosis ([15], [16], [17], [18]). Despite the detection of the new CVD appears of paramount importance for in-hospital management and clinical course, data from a systematic evaluation has been not reported yet.
In addition to acute cardiovascular involvement, data from preceding Coronaviruses epidemics (severe acute respiratory syndrome coronavirus, SARS-CoV, and the Middle East respiratory syndrome - MERS) suggested the possible persistence of pulmonary and cardiovascular damage ([19], [20], [21]). Currently there are only preliminary data on short-term follow-up of COVID-19 survivors focused on cardiovascular evaluation ([22], [23], [24], [25], [26]).
The aims of this study conducted during the first wave of COVID-19 pandemic were to: 1) assess the occurrence of cardiovascular diseases (CVD) in consecutive confirmed COVID-19 patients admitted to our hospital over a 30 days period. The patients were studied by a clinical evaluation including transthoracic echocardiography integrated by further examinations when indicated, and 2) evaluate the persistence or new onset of CVD at one-month and one-year follow-up.
2. Methods
2.1. Study design and patients
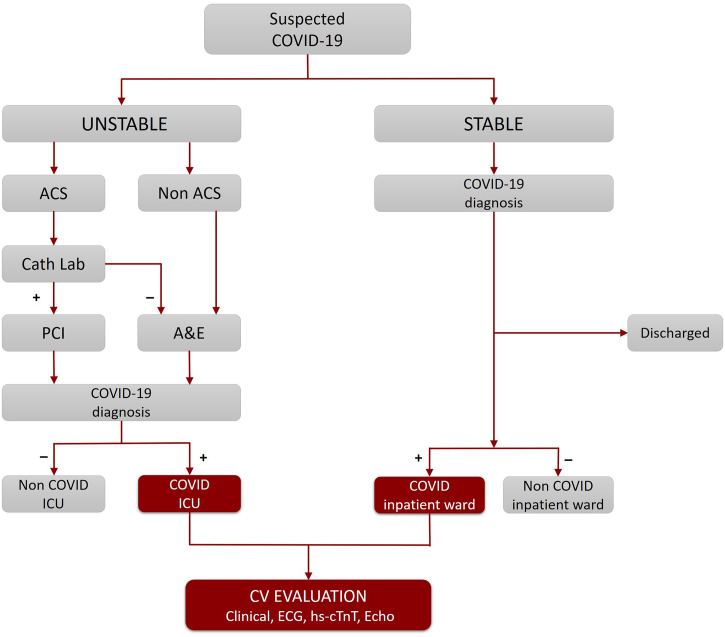
“Sapienza” University of Rome - Policlinico “Umberto I", is an academic hospital currently dedicated to COVID-19 patients in Rome (Italy). The algorithm applied for the management of patients at admission is shown in Fig. 1 . In the Accident & Emergency Department (A&E) suspected COVID-19 patients were evaluated and classified as stable or unstable. COVID-19 diagnosis was based on SARS-CoV-2 nucleic acid detection in oro/nasopharyngeal swab by reverse-transcriptase-polymerase-chain reaction (RT-PCR). COVID-19 stable patients with mild symptoms or asymptomatic were discharged recommending home isolation and care. Based on the clinical assessment, confirmed COVID-19 patients were admitted to dedicated COVID intensive care unit (COVID-ICU) or to COVID inpatient ward. All patients underwent chest computed tomography (CT).
Fig. 1.
Recruitment flow chart.
This flow chart illustrates the patient care pathway from A&E admission to hospital ward assignment.
A&E: Accident & Emergency; ACS: Acute Coronary Syndrome; CV: Cardiovascular; hs-cTnT: high-sensitivity cardiac troponin T; ICU: Intensive Care Unit; PCI: Percutaneous Coronary Intervention.
All consecutive confirmed COVID-19 patients admitted to both COVID ICU and inpatient ward underwent cardiovascular evaluation by two dedicated Cardiologists. Cardiac evaluation was performed within 48 h from admission between April 16th and May 15th 2020. The study was approved by the Ethic Committee of “Policlinico Umberto I" Hospital - “Sapienza” University of Rome (number 109/2020 approved the 07th of April 2020).
2.2. Protocol
Cardiovascular evaluation comprised clinical evaluation, 12‑lead standard electrocardiogram (ECG), high sensitive troponin (hs-Trop) and Focus Cardiac UltraSound (FoCUS) scan by portable ultrasound. At the time of cardiovascular examination, demographic and baseline characteristics, arterial-blood gas (ABG), blood tests, chest CT findings, medical history, medical and non-medical treatment including invasive/non-invasive ventilation were recorded. Cuf-off value for considering positive hs-Trop was 0.014 ng/L.
FoCUS exam was performed by hand-held Lumify (Philips) at patient bedside (27). Images were taken from three echocardiography views: parasternal, apical and subcostal. Echocardiogram quality was classified as optimal, fair, suboptimal, and poor. Only fair and optimal echocardiograms were considered (28). The presence of the following findings was evaluated according to standard guidelines and protocols: dilated left ventricle (LV) and/or right ventricle (RV), LV and/or RV systolic dysfunction, presence of pericardial effusion, significant valve insufficiency and/or stenosis (grade ≥ moderate). LV ejection fraction (LVEF) ≤ 50% was taken as cut-off for LV systolic disfunction, while a tricuspid annular plane systolic excursion (TAPSE) < 16 mm for RV systolic dysfunction. LV and RV dilatation, pericardial effusion and valve disease were classified according to cut-offs from the American and European guidelines (29,30).
According to cardiovascular findings, further examinations were requested including Cardiac Magnetic Resonance (CMR) (31), coronary angiography or CT pulmonary angiography.
CVD was defined as the occurrence of a new cardiovascular disease during the hospitalization for COVID-19. The definition of each type of CVD diagnosis was reported in the Supplements ([32], [33], [34], [35], [36], [37]).
2.3. Follow-up
After one month from hospital discharge all patients were invited to attend a follow-up evaluation at a dedicated “post-Covid-19 Outpatient Clinic”. During the evaluation all patients underwent blood sample collection, and pulmonary and cardiac assessment including ECG and transthoracic echocardiography.
After a mean of 347 ± 10 days from COVID-19 diagnosis all survivors were contacted by phone-call in order to evaluate the occurrence of re-hospitalization and if they experienced any symptoms from the discharge.
2.4. Statistical analysis
Statistical analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 21.0.V.20). Variables were presented as mean ± standard deviation (SD) if normally distributed or median (interquartile range) if not. Differences between groups were compared by Student's t-test and Mann-Whitney for continuous variables and by χ2 test (or Fisher exact test where appropriate) for categorical variables.
Univariate logistic regression for categorical variables and univariate linear regression for continuous variables were performed to explore predictors of death. A p value <0.05 was used as entry criterion.
A stepwise forward conditional logistic regression analysis was performed with death as dependent variable. The independent variables included in the analysis were selected from a bivariate analysis, with a 0.10 level as a screening criterion for the selection of candidate variables. Correlations between independent variables were checked for possible collinearity between variables (defined as rho >0.6). Adjusted odds ratios (adjOR) and 95% confidence interval (CI) were calculated from the logistic regression analyses. A two-tailed p value <0.05 was considered significant.
3. Results
3.1. Characteristics of the patients
Over an interval time of 30 days, 162 patients were recruited. Ten (6.2%) patients were excluded due to poor or suboptimal echocardiogram quality. One hhundred and fifty-two patients were finally enrolled. Characteristics of the study population are reported in Table 1 . Mean age was 70.5 years (IQR 56.2–80 and 86 patients (56.6%) were male. At least one cardiovascular risk factor was present in 108 (71.1%) of the patients. Half of the patients (n = 76) had an existing cardiac condition including coronary artery disease (CAD), heart failure (HF), valvulopathy (grade ≥ moderate), or a comorbidity including cancer, COPD and asthma. In 10 patients (6.6%) diagnosis of arterial hypertension (HTN) was made during the hospital stay.
Table 1.
Baseline characteristics of the population.
| Parameter |
All patients (n = 152) |
p-value | |
|---|---|---|---|
| CVD (n = 42) | Non CVD (n = 110) | ||
| Male sex, n (%) | 28 (66.7) | 58 (52.7) | 0.14 |
| Age, y.o * | 68 (58–82) | 71 (54–80) | 0.98 |
| Obesity n (%) | 4 (9.5) | 18 (16.4) | 0.44 |
| HTN, n (%) | 18 (42.9) | 64 (58.2) | 0.10 |
| HTN new diagnosis, n (%) | 4 (9.5) | 6 (5.5) | 0.46 |
| Diabetes, n (%) | 12 (28.6) | 12 (10.9) | 0.01 |
| Dyslipidemia, n (%) | 10 (23.8) | 24 (21.8) | 0.83 |
| At least 1 CRF-X, n (%) | 28 (66.7) | 80 (72.7) | 0.55 |
| CAD, n (%) | 10 (23.8) | 10 (9.1) | 0.02 |
| CABG, n (%) | 2 (4.8) | 2 (1.8) | 0.30 |
| PCI, n (%) | 6 (14.3) | 6 (5.5) | 0.09 |
| AF, n (%) | 6 (14.3) | 10 (9.1) | 0.38 |
| CRF, n (%) | 8 (19.04) | 12 (10.9) | 0.19 |
| HF, n (%) | 6 (14.2) | 6 (5.5) | 0.09 |
| Valvulopathy, n (%) | 8 (19.04) | 6 (5.5) | 0.02 |
| COPD, n (%) | 4 (9.5) | 10 (9.1) | 1 |
| Asthma, n (%) | 2 (4.8) | 2 (1.8) | 0.28 |
| Cancer, n (%) | 8 (19.0) | 18 (16.4) | 0.81 |
| Chemotherapy, n (%) | 6 (14.3) | 12 (10.9) | 0.58 |
| Radiotherapy, n (%) | 2 (4.8) | 4 (3.6) | 0.67 |
Unless specified, values are number of patients (%). * median (interquartile range).
AF: atrial fibrillation, CABG: coronary artery bypass grafting, CAD: coronary artery disease, CVD: cardiac disease, COPD: chronic obstructive pulmonary disease, CRF: chronic renal failure, CRF-X: classical risk factors, HF: heart failure, HTN: hypertension, PCI: percutaneous coronary intervention.
Data regarding symptoms, CT findings and blood tests are reported in Table S1 (Supplementary data) while data on clinical course and CV findings are shown in Table S2 (Supplementary data).
The admission in ICU was necessary in 44 patients (28.9%). Chest CT revealed ground glass appearance in 130 patients (85.5%) of patients, consolidation in 116 (76.3%) and pleural effusion in 26 (17.1%).
3.2. Cardiovascular disease (CVD)
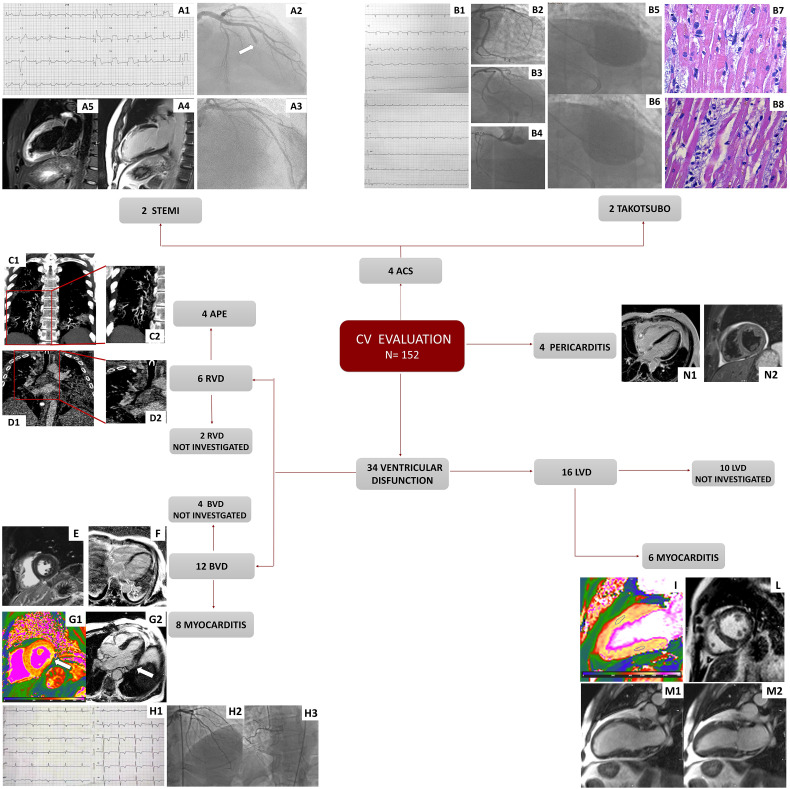
Cardiovascular disease, comprising a wide spectrum of conditions (Fig. 2 ), was observed in 27.6% of the population studied (42 patients). Specifically, two patients were admitted with anterior ST-Elevation-Myocardial Infarction (STEMI). One of these had a total occlusion of left anterior descending artery (LAD) due to thrombus and was treated by percutaneous coronary intervention (PCI) and thrombus aspiration. CMR showed an extensive anterior myocardial infarction (MI) with LV dysfunction (Fig. 2: A1-A5). Other two patients presented with Tako-Tsubo's syndrome. One had severe LV dysfunction with mid-apical ballooning at left ventricular angiography and died after a few hours from the procedure due to cardiogenic shock. Cardiac autopsy revealed diffuse oedema, multiple foci of contraction band necrosis, interstitial macrophages CD68+ and occasional presence of microthrombi in the small coronary arteries (38). Molecular analysis did not detect SARS-CoV-2 genome within the myocardium (Fig. 2: B1-B8). Four patients had an acute pulmonary embolism and showed RV dysfunction (Fig. 2: C1-C2 and D1-D2). Six patients with new onset of bi-ventricular dysfunction underwent CMR showing findings suggestive of acute myocarditis (Fig. 2: E, F, G1-G2) accordingly to the revised lake Louise consensus criteria (33). Other two patients had a diagnosis of myocarditis based on the onset of bi-ventricular dysfunction, troponin raise and diffuse ECG changes in the absence of angiographically detectable coronary artery disease. CMR in the above two cases was not performed due to claustrophobia. In other five patients with isolated LV dysfunction CMR findings were consistent with acute myocarditis (Fig. 2: I, L, M). One patient had a final diagnosis of myocarditis based on the observation of isolated LV dysfunction, troponin raise and acute chest pain with non- obstructive coronary arteries; CMR was not performed due to patient refusal. Other four patients presented acute pericarditis confirmed by CMR in two cases (Fig. 2: N1, N2).
Fig. 2.
Examples of cardiac events.
The figure shows examples of in-hospital CVD. Fig. A: patient presented with anterior STEMI (A1), with obstructed LAD (A2-A3), oedema (A4) and LGE (A5) at the mid-apical anterior segments by CMR (A4-A5). Fig. B: patient presented with diffuse ST segment elevation (B1), unobstructed coronary arteries (B2-B4) and mid-apical ballooning at the ventricular angiography (end-diastolic frame, B5, and end-systolic frame, B6). Autopsy revealed presence of contraction band in cardiomyocytes (B7) and marked interstitial oedema and mononucleate cells, in absence of myocyte necrosis (B8). Fig. C1 (magnification in C2): patient with thrombi in the segmental pulmonary arterial branches for the posterior and middle-basal segment of the right lower lobe while in D1 (magnification in D2) a patient with intraluminal thrombus in the arterial branch for the lower right lobe.
Figs. E-G: three cases of CMR findings suggestive of myocarditis: non-ischemic LGE (sub-epicardial at the inferior wall and mid-myocardial at the inferior septum) (E); sub-epicardial LGE areas at the lateral wall (breathing artefact in the image) (F); short axis high native T1 Mapping at the basal lateral wall (G1) and sub-epicardial LGE at the same level (G2) in 3 chamber view. Fig. H: patient presenting with severe biventricular dysfunction, troponin raise, diffuse ECG changes (H1) and unobstructed coronary arteries (H2−H3). Fig. I: patient with myocarditis with high native T1 Mapping (ROI in the anterior wall: 1133 ms, in the inferior wall: 1030 ms). Fig. L: non-ischemic LGE at the inferior RV insertion point. Multiple areas of increased signal in the cine (M1 end-diastolic frame and M2 end-systolic frame) sequences at the subpericardial level in the anterior and inferior wall. Patient with acute pericarditis (N1−N2).
In addition to these cases, other four patients had biventricular dysfunction, ten isolated LV dysfunction and two RV dysfunction. In such cases, further evaluations were not performed due to unstable condition or death (n = 12), acute renal failure (n = 2) or patient refusal (n = 2). Pericardial effusion was identified in 36% of patients. Significant (grade ≥ moderate) valve disease was observed in n = 14 (9%) and this was known prior the admission for COVID-19.
No differences were observed in terms of baseline characteristics, symptoms at presentation, ABG, ECG and Chest CT findings between patients who experienced CVD during hospital stay and those who did not, except for diabetes (p = 0.01) and ischemic heart disease (p = 0.02) which were more frequent in the CVD group (Table 1). Systolic blood pressure at admission was lower in CVD compared to non-CVD (p = 0.023). No differences in term of laboratory tests were observed between patients with CVD versus non-CVD except for LDH (p = 0.034) and C-reactive protein (p = 0.02) being higher and calcium (p = 0.031) and lymphocytes (p = 0.002) being lower in CVD. Furthermore, the proportion of patients with troponin raise was greater in CVD compared to non-CVD group (80.9% vs 25.5%, p < 0.001). A greater proportion of patients with CVD required ICU admission (p < 0.001) and invasive mechanical ventilation (IMV) compared to non-CVD (p < 0.001). On the contrary, a greater percentage of the non-CVD group was treated by oxygen therapy (p < 0.001). Differences in medical treatment between the two groups were related only to loop diuretics (p < 0.001) and heparin (p = 0.009) which were administered in a greater percentage to CVD group compared to non-CVD. Death occurred more often in patients presenting with CVD compared to those patients who did not experience CVD (p = 0.003). At univariate analysis, the occurrence of CVD, age, and troponin raise were associated with all-cause death (Table S4, Supplementary data). After adjustment for confounders, older age, CVD occurrence and IMV were independently associated with death (Table 2 ).
Table 2.
Results of the logistic regression analyses with death as dependent variable.
| Dependent variable | Logistic regression | Independent variables | adjOR | 95%CI | P value | Other variables (not selected by the model) |
|---|---|---|---|---|---|---|
| Death | Overall p value <0.001 | Age | 1.06 | 1.03–1.10 | 0.001 | Heparin, HTN |
| R2 = 0.272 | CVD | 3.26 | 1.30–8.13 | 0.011 | ||
| Well classified = 82.9 | IMV | 3.14 | 1.23–7.98 | 0.017 |
CVD: cardiovascular disease, HTN: arterial hypertension, IMV: Invasive mechanical ventilation.
3.3. One-month follow-up
Among the COVID-19 survivors discharged from the hospital (n = 120, 78.9%), 78 patients (65%) attended the follow-up visit. Of the remaining 42 patients: 20 (16.7%) could not be contacted, 10 (8.3%) refused, 6 (5%) moved abroad and 6 (5%) were in rehabilitation clinic.
The patients who refused and those who moved abroad (n = 16) were alive, reported to be healthy and did not have any re-hospitalization (Fig. S2). Among the 78 patients who attended the follow-up visit, sixteen had experienced a CVD during the hospital stay: 8 acute myocarditis, 2 STEMI, 4 acute pulmonary embolism and 2 acute pericarditis. No one was re-hospitalized after hospital discharged. Echocardiography results are reported in Table S3 (Supplementary data). No significant differences in terms of dimension, systolic and diastolic function were detected between patients who experienced CVD and those who did not.
At follow-up visit, the patients who experienced anterior STEMI were asymptomatic and showed an improvement in LV function. The four patients who experienced acute pulmonary embolism were in NYHA class II and presented a mixed ventilatory defect with reduced diffusing capacity of the lungs for carbon monoxide (DLCO). Two of these showed oxygen desaturation during 6MWT (6 min walking test). All had a decreased extent of the pulmonary ground glass opacity and the disappearance of the thrombo-embolic phenomena at follow-up chest CT. An improvement in the RV function and a normal systolic pulmonary arterial pressure on echocardiogram.
Among the 8 patients who experienced acute myocarditis, seven had a normal bi-ventricular EF, while one of the two patients who did not undergo CMR due to claustrophobia had a mildly impaired bi-ventricular systolic function.
The patients who experienced acute pericarditis were asymptomatic with normal value of C-reactive protein and with no pericardial effusion on echocardiography.
Of the 62 patients without CVD during hospital stay who attended the follow up visit only two patients appeared to have developed acute pericarditis and an appropriate treatment was started. The remaining patients did not report re-admission to hospital nor did they present any new onset of CVD. Only two patients had pulmonary hypertension secondary to pre-existing severe mitral stenosis.
3.4. One-year follow-up
Among COVID-19 survivors discharged from the hospital (n = 120, 78.9%), 118 patients (98.3%) were contacted by phone after a mean of 347 ± 10 days from COVID-19 diagnosis. The remaining patients (n = 2, 1.6%) were not contactable.
Fifty-seven patients (47.5%) reported symptoms, with fatigue being the most frequent (14.2%), followed by dyspnoea (10.8%), impaired memory (5.8%), palpitations (4.2%), arthomyalgia (4.2%), cutaneous manifestations (5%), chest pain (1.7%), ageusia (1.7%), gastrointestinal symptoms (1.7%) and ocular manifestations (1.7%).
With regards to the patients who experienced CVD during hospitalization, the patient with myocarditis and persistent mildly impaired bi-ventricular function at one month follow-up reported fatigue; another patient with myocarditis and the four patients with pulmonary embolism still reported being in NYHA class II; the two patients with delayed onset of pericarditis at one month follow-up reported palpitations; the remaining patients who experienced a previous CVD did not complain of symptoms.
Furthermore, 9.3% (n = 11/118) of the patients had a re-hospitalization: one of the patients reporting chest pain was hospitalized and underwent coronary angiography showing not significant coronary artery disease. The remaining rehospitalizations were due to urinary tract infections, acute renal failure or complications related to known cancer.
4. Discussion
This study was designed during the first wave of the COVID-19 pandemic given the growing number of cases reporting cardiovascular involvement. The main aim was to assess the prevalence of the new onset of CVD in consecutive unselected hospitalized COVID-19 patients based on a systematic cardiovascular evaluation. The major finding of the present study is that the occurrence of new cardiovascular conditions is common (28%) among patients admitted with severe COVID-19 disease. The conditions varied, ranging from acute myocarditis (more frequently) and pericarditis, MI, Tako-Tsubo's syndrome and acute pulmonary embolism. This study was not designed to assess the association between the occurrence of CVD and prognosis. However, we observed that CVD, older age and mechanical ventilation were independently associated with death even after adjustment for confounders. Specifically, the occurrence of CVD was associated with threefold increase in the risk of death. This systematic cardiovascular evaluation in COVID-19 patients allowed to identify patients with new onset of CVD requiring further tests and specific management.
Cardiovascular involvement including myocarditis, MI, and exacerbation of HF have been described during the previous SARS-CoV and MERS epidemic and contributed significally to mortality (39). In COVID-19 patients growing evidence on the involvement of the cardiovascular system has been accumulated with different and often coexisting possible mechanisms: myocarditis, hypercoagulability, stress cardiomyopathy and cytokine storm (40). However, there is limited evidence and the true prevalence of cardiovascular conditions is still unclear due to the lack of specific and uniform diagnostic algorithms. A large multicentric registry aimed to determine the frequency and pattern of cardiac complications reported an overall incidence of cardiac complications of 11.6% among 3011 hospitalized COVID-19 patients, with atrial fibrillation being the most frequent complication. However, the lack of central adjudication of events, missing data regarding cardiac biomarkers and echocardiography limited the results at this stage (41).
Studies based on echocardiographic evaluation reported a high prevalence of cardiac involvement (8,9,42) and results were different from ours. In two studies (8,42), a high burden of RV involvement was reported while in our cohort this was uncommon (4%) and related to acute pulmonary embolism in four cases. Our different results possibly reflect the fact that heparin was administered in 70% of our cohort, while in the previous studies heparin was a variable part of the treatment. A recent study performed in our centre showed that high dose low-molecular-weight heparin for venous thromboembolism prophylaxis reduced the incidence of thrombotic complications without an increase in bleeding events (43).
The other study (9) was a survey which included also presumed COVID-19 cases and a clinical indication was the reason of the echocardiographic evaluation. The aforementioned studies were limited to detect echocardiographic abnormalities and no further examinations were performed and a final diagnosis was then not reached.
In a multicenter retrospective cohort study of 305 patients, echocardiographic abnormalities were present in two-thirds of patents with myocardial injury. However, myocardial injury was defined as any elevation in cardiac troponin. CMR was not performed and only a small number of patients underwent cardiac catheterization (44).
In our study, patients with newly detected systolic dysfunction underwent CMR, when feasible. Tissue characterization (including Mapping technique and LGE) revealed findings consistent with acute myocarditis in thirteen cases. The patient who experienced a fatal Tako-Tsubo syndrome did not present histopathological findings of myocarditis. However, abundant macrophages CD68+ were observed in the myocardial interstitium, in absence of myocyte necrosis (38). This observation is in keeping with the finding of increased interstitial macrophages recently reported by Basso et al. as the most common feature in a series of 21 COVID-19 autopsies (13).
Patients with CVD were more often admitted in intensive care unit and in most of the cases showed increased levels of troponins. Further cardiovascular examinations were not feasible among 42% patients with abnormal echocardiogram due to different reasons (critical condition/death, refusal, acute renal failure).
In the present study we also present data on short and long term follow up of previously hospitalized COVID-19 patients. Little is known of the prevalence of cardiovascular sequelae in COVID-19 survivors, and there are no clear recommendations for follow-up of COVID-19 patients. Some reports suggest that cardiac involvement is frequent during the recovery phase or at short follow-up.
A six-weeks follow-up study on a limited number of patients (n = 33, hospitalized but not severe) COVID-19 survivors, which included echocardiography and respiratory functional assessment, reported symptoms of fatigue with no pulmonary and cardiac impairments (22).
Another follow-up study performed after 6 weeks on eighty-one patients reported residual symptoms (chest pain in 14% of patients), no severe cardiac dysfunction and no new onset of arrhythmia during 24-h ECG-monitoring (23).
A cardiopulmonary evaluation performed at 60 and 100 days after COVID-19 diagnosis reported a high rate of diastolic dysfunction, while pulmonary hypertension and pericardial effusion was observed in a smaller portion of the cohort; a reduced LVEF was described in only four patients (24).
A different study focusing on CMR findings in the sub-acute disease phase (2 weeks from diagnosis, absence of respiratory symptoms and negative swabs) revealed a frequent cardiovascular involvement characterized by acute tissue changes (25). More recently, a study reported CMR abnormalities in half of severe COVID-19 survivors with troponin elevation during hospital stay, even though these findings could be pre-existing (26).
Despite these data seems to suggest a frequent cardiovascular involvement, they are cross-sectional, heterogeneous in term of protocol applied and study patients characteristics.
Our follow-up study was performed to evaluate the possible persistence of the CVD diagnosed during the hospital stay or the delayed onset of new condition. We performed a one-month follow-up visit and a one-year follow-up by a phone-call interview to investigate the persistence of any symptoms or the occurrence of re-hospitalizations. At one month from discharge almost 9% had a persistent or a new onset cardiovascular condition. These patients reported persistence of symptoms at one year follow-up. Overall, a total of 48% of survivors complained of symptoms at one year, with fatigue being the most frequent.
These preliminary data and the experience from previous Coronavirus epidemics suggest the need for monitoring COVID-19 survivors. However, further studies on long-term follow-up including greater cohorts are needed.
The main limitations of this study are the single centre design and the limited size of the study population. FoCUS scan was preferred to standard echocardiography as recommended by international guidelines (45,46), published at the beginning of pandemic, but this precluded quantification of chamber dimension and function, as well as the detection of subtle cardiac changes. In a proportion of patients, further examinations were not performed due to patient related reasons with a consequent underestimation of cardiac condition. Endomyocardial biopsy was not performed since ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic did not recommend it in COVID-19 patients with suspected myocarditis (47). Thus, the diagnosis of myocarditis relies only on clinical, serologic and imaging findings (26).
Finally, follow-up was based on voluntary participation, possibly introducing a selection bias. Our one-year follow-up was limited to a telephone follow-up.
5. Conclusion
In conclusion, CVD occurrence in hospitalized COVID-19 patients is common (28%) covering a wide spectrum of cardiac conditions, with myocarditis being the most frequent. Acute cardiovascular event along with older age and the need for mechanical ventilation increase the risk of in-hospital mortality. Cardiovascular evaluation in COVID-19 patients is crucial since it allows to identify patients who could benefit from specific treatment with impact on prognosis.
Persistence or delayed presentation of CVD at 1-month (9%) and persistent symptoms at 1-year follow-up (48%) suggest the need for monitoring COVID-19 survivors.
Funding
None.
Authors' contributions
VM, LIB, MM: conception and design of the study, drafting the article, acquisition of data, analysis and interpretation of data. All the co-authors contributed to acquire the data and participated in finalizing the article. All the co-authors approved the final version of the article.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgements
We would like to express our gratitude to “Policlinico Umberto I COVID-19 Group” who has made substantial contributions to this study. All persons of “Policlinico Umberto I COVID-19 Group” have provided the corresponding author with permission to be named in the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2021.06.056.
Contributor Information
on behalf of Policlinico Umberto I COVID-19 Group:
Albante Alida, Araimo Morselli Fabio, Auricchio Daniela, Letizia D'Antoni, Barletta Giovanna, Bilotta Federico, Brisciani Matteo, Bruno Katia, Bucarelli Maria Clelia, Cappannoli Alessandro, Ceccarelli Giancarlo, Celli Paola, Consolo Stella, Consoli Giulia, Croce Claudia, Crocitti Beatrice, D'Antoni Letizia, De Lazzaro Francesco, De Lauri Daniela, De Rose Maria, Del Bianco Andrea, Di Bella Valerio, Di Sano Laura, Di Santo Carmela, Francavilla Santi, Giannetti Lorena, Giordano Giovanni, Ianni Stefano, Imperiale Carmela, Maestrini Ilaria, Magnanimi Eugenia, Manganelli Chiara, Maldarelli Federica, Martelli Sabina, Messina Teresa, Novelli Martina, Pasculli Patrizia, Pasqualitto Fabiola, Pattelli Elisa, Pecorari Filippo, Perrella Serena, Petroianni Angelo, Piazzolla Mario, Portieri Monica, Prosperi Silvia, Rachele Edoardo Sebastian, Ratini Fabiola, Ricci Claudia, Romano Hilde, Sabani Anna, Santopietro Pietro, Tellan Guglielmo, Titi Luca, Tordiglione Paolo, Tosi Antonella, Trigilia Fausto, Verduci Noemi, and Vaccaro Paola
Appendix A. Supplementary data
Supplementary material
References
- 1.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019. JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer P., Degrauwe S., Van Delden C., Ghadri J.R., Templin C. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur. Heart J. 2020;41:1860. doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with Covid-19 - a case series. N. Engl. J. Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szekely Y., Lichter Y., Taieb P., et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dweck M.R., Bularga A., Hahn R.T., et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging. 2020;21:949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sala S., Peretto G., Gramegna M., et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur. Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escher F., Pietsch H., Aleshcheva G., et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Failure. 2020;7:2440–2447. doi: 10.1002/ehf2.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basso C., Leone O., Rizzo S., et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur. Heart J. 2020 Oct 14;41(39):3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 17.Scudiero O., Lombardo B., Brancaccio M., et al. Exercise, immune system, nutrition, respiratory and cardiovascular diseases during COVID-19: a complex combination. Int. J. Environ. Res. Public Health. 2021 Jan 21;18(3):904. doi: 10.3390/ijerph18030904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C., Liu F., Liu W., et al. Myocardial injury and risk factors for mortality in patients with COVID-19 pneumonia. Int. J. Cardiol. 2021 Mar 1;326:230–236. doi: 10.1016/j.ijcard.2020.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui D.S., Joynt G.M., Wong K.T., et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das K.M., Lee E.Y., Singh R., et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J. Radiol. Imag. 2017;27:342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corrales-Medina V.F., Alvarez K.N., Weissfeld L.A., et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. Jama. 2015;313:264–274. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daher A., Balfanz P., Cornelissen C., et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19):pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020 Nov-Dec;174:106197. doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Graaf M.A., Antoni M.L., Ter Kuile M.M., et al. Short-term outpatient follow-up of COVID-19 patients: a multidisciplinary approach. EClinicalMedicine. 2021 Feb;32:100731. doi: 10.1016/j.eclinm.2021.100731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonnweber T., Sahanic S., Pizzini A., et al. Cardiopulmonary recovery after COVID-19 – an observational prospective multi-center trial. Eur. Respir. J. 2020 Dec;10:2003481. doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotecha T., Knight D.S., Razvi Y., et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021;42(19):1866–1878. doi: 10.1093/eurheartj/ehab075. (Feb 18:ehab075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neskovic A.N., Edvardsen T., Galderisi M., et al. Focus cardiac ultrasound: the EACVI viewpoint. Eur. Heart J. Cardiovasc. Imaging. 2014;15:956–960. doi: 10.1093/ehjci/jeu081. [DOI] [PubMed] [Google Scholar]
- 28.Galderisi M., Cosyns B., Edvardsen T., et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the EACVI. Eur. Heart J. Cardiovasc. Imaging. 2017;18:1301–1310. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]
- 29.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28(1):1–39. doi: 10.1016/j.echo.2014.10.003. Jan. (e14) [DOI] [PubMed] [Google Scholar]
- 30.Klein A.L., Abbara S., Agler D.A., et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2013;26(9):965–1012. doi: 10.1016/j.echo.2013.06.023. Sep. (e15) [DOI] [PubMed] [Google Scholar]
- 31.Galea N., Catapano F., Marchitelli L., et al. How to perform a cardio-thoracic MRI in COVID-19: comprehensive assessment of heart, pulmonary arteries and lung parenchyma. Eur. Heart J. Cardiovasc. Imaging. 2020;22(7):728–731. doi: 10.1093/ehjci/jeaa335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caforio A.L., Pankuweit S., Arbustini E., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013;34(33):2636–2648. doi: 10.1093/eurheartj/eht210. Sep. (2648a-2648d) [DOI] [PubMed] [Google Scholar]
- 33.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J. Am. Coll. Cardiol. 2018 Dec 18;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 34.Adler Y., Charron P., Imazio M., et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC)endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2015 Nov 7;36(42):2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) J. Am. Coll. Cardiol. 2018 Oct 30;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 36.Ghadri J.R., Wittstein I.S., Prasad A., et al. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur. Heart J. 2018 Jun 7;39(22):2032–2046. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konstantinides S.V., Meyer G., Becattini C., et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur. Respir. J. 2019 Oct 9;54(3):1901647. doi: 10.1183/13993003.01647-2019. [DOI] [PubMed] [Google Scholar]
- 38.Titi L., Magnanimi E., Mancone M., et al. Fatal Takotsubo Syndrome in critical COVID-19 related pneumonia. 2020. Cardiovasc. Pathol. 2021;51:107314. doi: 10.1016/j.carpath.2020.107314. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linschoten M., Peters S., van Smeden M., et al. Cardiac complications in patients hospitalised with COVID-19. Eur. Heart J. Acute Cardiovasc. Care. 2020 Dec;9(8):817–823. doi: 10.1177/2048872620974605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bleakley C., Singh S., Garfield B., et al. Right ventricular dysfunction in critically ill COVID-19 ARDS. Int. J. Cardiol. 2021 Mar 15;327:251–258. doi: 10.1016/j.ijcard.2020.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chistolini A., Ruberto F., Alessandri F., et al. Effect of low or high doses of low molecular weight heparin on thrombin generation and other hemostasis parameters in COVID-19 critical patients. British J. Haematol. 2020;190(4):e214–e218. doi: 10.1111/bjh.17003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giustino G., Croft L.B., Stefanini G.G., et al. Characterization of myocardial injury in patients with COVID-19. J. Am. Coll. Cardiol. 2020 Nov 3;76(18):2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skulstad H., Cosyns B., Popescu B.A., et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur. Heart J. Cardiovasc. Imaging. 2020;21:592–598. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirkpatrick J.N., Mitchell C., Taub C., Kort S., Hung J., Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak. JACC. 2020;75:3078–3084. doi: 10.1016/j.jacc.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material