Abstract
SARS-CoV-2 vaccines are effective in preventing COVID-19. Patients with cancer are at high risk for severe COVID-19 and are appropriately prioritized for vaccination. Several studies in this issue of Cancer Cell add to our knowledge of the heterogeneity of immune responses to vaccination among patients with cancer and identify important areas for future research.
SARS-CoV-2 vaccines are effective in preventing COVID-19. Patients with cancer are at high risk for severe COVID-19 and are appropriately prioritized for vaccination. Several studies in this issue of Cancer Cell add to our knowledge of the heterogeneity of immune responses to vaccination among patients with cancer and identify important areas for future research.
Main text
Patients with cancer are at increased risk for morbidity and mortality from SARS-CoV-2 infection. The increased risk of complications reflects older age and general co-morbidities that are more common in patients with cancer (e.g., co-existing lung and cardiovascular disease). Adults with hematologic malignancies are at particularly high risk for death from COVID-19, which likely reflects immune impairment from the underlying disease as well as therapies that disable innate, B cell, and T cell immunity. Following SARS-CoV-2 infection, patients with hematologic malignancies have been found to have prolonged viral shedding, impaired seroconversion, an exhausted T cell phenotype, and sustained immune-dysregulation compared to patients with solid tumors (Abdul-Jawad et al., 2021).
FDA-authorized vaccines against SARS-CoV-2 are effective in preventing COVID-19 and in reducing viral transmission, and they dramatically reduce the risk of COVID-19-related hospitalization and mortality in immunocompetent persons. Because patients with active cancer were underrepresented in these trials, an important gap in knowledge is the extent to which COVID-19 vaccines are protective in this high-risk population. Reflecting the urgent need to protect patients with cancer from COVID-19 and the safety of FDA-authorized SARS-CoV-2 vaccines in the general population, multiple professional societies and organizations (e.g., the National Comprehensive Cancer Network) strongly endorsed prioritization of such patients for SARS-CoV-2 vaccination, while noting gaps in knowledge on their efficacy.
Recently, a number of important studies have begun to elucidate the spectrum of early vaccine response among larger subsets of patients with cancer who are receiving different types of therapy. The data on COVID-19 vaccine immunogenicity in patients with cancer are preliminary and mostly limited to measurement of post-vaccine antibody titers to the viral spike protein. So far, the safety of vaccines in patients with cancer is similar to that in the general population. The vast majority of patients with solid tumors who are receiving chemotherapy generate antibody responses to two doses of BNT162b2, although titers may be lower than those for healthy controls (Massarweh et al., 2021; Monin et al., 2021). However, sub-optimal immunogenicity of COVID-19 vaccines has been observed in patients with hematologic malignancies (Herishanu et al., 2021).
Research published in this issue of Cancer Cell significantly extends our knowledge of COVID-19 vaccine immunogenicity in patients with cancer. Thakkar et al. evaluated anti-spike IgG titers in 200 patients with cancer (67% with solid tumors and 33% with hematologic tumors, predominantly lymphoid) after the patients were fully vaccinated with one of the FDA-authorized COVID-19 vaccines (Thakkar et al., 2021). Patients were from the New York City area and represented a diverse ethnic background. At the time of vaccine administration, 75% of patients had an active cancer diagnosis and 67% were receiving treatment. The overall seroconversion rate was 94%, with a significantly lower seroconversion rate in patients with hematologic malignancies (85%) versus solid tumors (98%). Substantially lower rates of seroconversion occurred in patients receiving anti-CD20 therapy (70%) or stem cell transplantation (73%). Patients receiving immune checkpoint inhibitor therapy or hormonal therapies had high seroconversion rates.
Addeo et al. assessed anti-SARS-CoV-2 spike protein antibody titers following the first and second doses of BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in 131 patients with cancer in the U.S. and Europe (Addeo et al., 2021). 81% of patients had solid tumors and 19% had hematologic cancers (the majority of these were B cell malignancy subtypes). Overall, 94% of patients seroconverted after completion of the second dose; responses were less robust after the first dose. Seroconversion rates were significantly lower in patients with hematologic malignancies (77%) than in those with solid tumors (98%). Antibody titers were highest in patients who were not receiving cancer therapy (on surveillance) or were receiving endocrine therapy compared to those receiving cytotoxic therapy. Reflecting the prolonged B cell depletion following anti-CD20 regimens, none of the vaccinated patients who received anti-CD20 antibody in the 6 months prior to vaccination developed an antibody response.
Van Oekelen et al. analyzed anti-spike IgG titers in 320 patients with multiple myeloma who received COVID-19 vaccinations (Van Oekelen et al., 2021). They observed a highly variable antibody response after the patients completed the two-dose COVID-19 vaccination regimen, with 15.8% developing no detectable anti-SARS-CoV-2 spike IgG antibodies. Patients receiving active therapy had lower antibody levels, with anti-CD38 regimens and B cell maturation antigen (BCMA)-targeted therapies correlating with lower titers. The clinical relevance of these findings is underscored by four vaccinated patients who developed severe COVID-19 that required hospitalization, one of whom died of respiratory failure. Hill et al. present a case report of a patient on B cell-depleting therapy which suggests that heterologous vaccination (mixed vaccines) against SARS-CoV-2 may enhance antibody response in this setting (Hill et al., 2021).
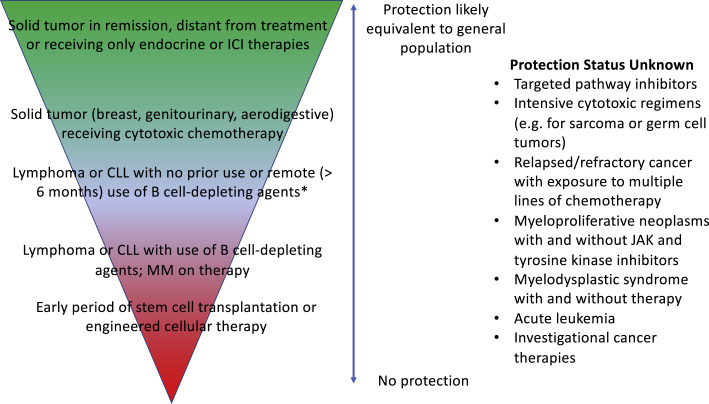
Together, these studies demonstrate the heterogeneity of patients with cancer regarding vaccine-induced immune responses (Figure 1 ). In general, antibody responses for patients with the most common solid tumor types (breast, aerodigestive, and GU malignancies), including those receiving chemotherapy, were reassuring. We do not know if those with less common solid tumors or those who receive intensive cytotoxic regimens (e.g., for sarcoma or germ cell tumors) will have impaired immune responses to vaccination. Patients with B cell malignancies who are distant from therapy or in remission appear respond to vaccination, but those on B cell-depleting agents or who have undergone cellular therapies are much less likely to be protected. Patients with myeloid malignancies and acute leukemias are not represented in these studies, and their degree of protection from vaccination is unknown.
Figure 1.
Predicted continuum of COVID-19 vaccine efficacy for patients with cancer based on cancer type and therapy
The majority of patients with cancer, including those who have solid tumors and are receiving active therapy, are expected to have protective titers following completion of COVID-19 vaccination. Specific patients with hematologic malignancies, such as cellular therapy recipients and those who are receiving B cell-depleting agents, may not mount protective responses. For a number of cancer types and regimens, vaccine-induced immune responses are unknown (right column) and warrant further research.
Abbreviations: ICI, immune checkpoint inhibitor; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; JAK, janus kinase.
∗B cell-depleting agents include anti-CD20 agents (e.g., rituximab), anti-CD38 therapy, BCMA targeted agents, and Bruton tyrosine kinase inhibitors.
Even when patients with cancer mount an immune response to COVID-19 vaccination, a number of questions remain. We do not know whether immune responses will have the same durability as in a healthy population or if post-vaccine anti-cancer therapies will impact degree and duration of protection. Although complete absence of detectable antibodies after vaccination likely equates to a lack of protection, we do not know what level of antibody titer assures protection. Moreover, although serum titers are straightforward to measure, they do not encapsulate other important features of the immune response to natural infection and vaccination, such as memory B cell and T cell responses that are likely to drive long-term immunity (Andreano et al., 2021; Kared et al., 2021). More comprehensive analysis of cellular immune responses to vaccination in patients with cancer and in other immunocompromised patients are warranted. Clinical trials of novel vaccine approaches, such as boosters and heterologous vaccination, should be prioritized in order to enhance vaccine efficacy in those who are unable to mount an adequate immune response to standard-of-care vaccination.
Finally, the Centers for Disease Control have appropriately removed restrictions (masks and social distancing) for the general public who have completed COVID-19 vaccination. Importantly, current data suggest that these liberalized guidelines should not be automatically extended to all patients with cancer; those receiving cytotoxic or targeted agents expected to impair vaccine immunogenicity should likely continue to exercise caution. This concern is especially important for patients with active hematologic cancers, stem cell transplant recipients, and those recently treated with B cell-depleting agents (e.g., anti-CD20 monoclonals, Bruton tyrosine kinase inhibitors, and BCL2 inhibitors), who are likely to have sub-optimal protection from vaccination, as well as subsets of patients in whom vaccine efficacy has not been adequately tested.
Acknowledgments
Declaration of interests
E.A.G. declares receipt of honoraria and/or consulting fees from Taiho Oncology, Takeda Pharmaceuticals, Alexion Pharmaceuticals, Abbvie, Celgene/BMS, and Novartis and research funding (to Roswell Park) from Genentech, Celgene/BMS, Celldex Therapeutics, Apellis Pharmaceuticals, Alexion Pharmaceuticals, Imago Biosciences, and Astex Pharmaceuticals. B.H.S. has no interests to declare.
References
- Abdul-Jawad S., Baù L., Alaguthurai T., Del Molino Del Barrio I., Laing A.G., Hayday T.S., Monin L., Muñoz-Ruiz M., McDonald L., Francos Quijorna I., et al. Acute Immune Signatures and Their Legacies in Severe Acute Respiratory Syndrome Coronavirus-2 Infected Cancer Patients. Cancer Cell. 2021;39:257–275.e6. doi: 10.1016/j.ccell.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addeo A., Shah P.K., Bordry N., Hudson R.D., Albracht B., Marco M.D., Kaklamani V., Dietrich P.-Y., Taylor B.S., Simand P.-F., et al. Immunogenicity of SARS-CoV-2 messenger RNA Vaccines in Patients with Cancer. Cancer Cell. 2021;39:1091–1098. doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano E., Nicastri E., Paciello I., Pileri P., Manganaro N., Piccini G., Manenti A., Pantano E., Kabanova A., Troisi M., et al. Extremely potent human monoclonal antibodies from COVID-19 convalescent patients. Cell. 2021;184:1821–1835.e16. doi: 10.1016/j.cell.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herishanu Y., Avivi I., Aharon A., Shefer G., Levi S., Bronstein Y., Morales M., Ziv T., Shorer Arbel Y., Scarfò L., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J.A., Ujjani C.S., Greninger A.L., Shadman M., Gopa A.K. Immunogenicity of a heterologous COVID-19 vaccine after failed vaccination in a lymphoma patient. Cancer Cell. 2021;39:1037–1038. doi: 10.1016/j.ccell.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kared H., Redd A.D., Bloch E.M., Bonny T.S., Sumatoh H., Kairi F., Carbajo D., Abel B., Newell E.W., Bettinotti M.P., et al. SARS-CoV-2-specific CD8+ T cell responses in convalescent COVID-19 individuals. J. Clin. Invest. 2021;131:145476. doi: 10.1172/JCI145476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarweh A., Eliakim-Raz N., Stemmer A., Levy-Barda A., Yust-Katz S., Zer A., Benouaich-Amiel A., Ben-Zvi H., Moskovits N., Brenner B., et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021;7 doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., Del Molino Del Barrio I., Alaguthurai T., Domingo-Vila C., Hayday T.S., Graham C., Seow J., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar A., Gonzalez-Lugo J.D., Goradia N., Gali R., Shapiro L.C., Pradhan K., Rahman S., Kim S.Y., Ko B., Sica R.A., et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39:1081–1090. doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oekelen O., Gleason C.R., Agte S., Srivastava K., Beach K., Aleman A., Kappes K., PVI/Seronet team, Mouhieddine T.H., Wang B., et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple. Cancer Cell. 2021;39:1028–1030. doi: 10.1016/j.ccell.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]