Abstract
Objective
We aimed to analyze clinical outcomes from patients with severe COVID-19 pneumonia that received either baricitinib plus dexamethasone or dexamethasone monotherapy.
Methodology
We performed a retrospective comparative study. Data from hospitalized patients with severe COVID-19 pneumonia (saturation <93%, bilateral pulmonary infiltrates) that were treated with baricitinib plus dexamethasone or dexamethasone were collected. Our primary objective was to compare overall mortality and secondly to compare progression to mechanical ventilation and over infection rates.
Results
A total of 793 patients were assessed for inclusion criteria, 596 were excluded and 197 were analyzed for primary outcome: 123 in the baricitinib plus dexamethasone group and 74 in the dexamethasone monotherapy group. The mean age was 59.9 years (SD ± 14.5) and 62.1% (123/197) were male. 42.9% (85/197) of the cases required ICU admission and 25.8% (51/197) underwent invasive mechanical ventilation (IMV). Overall thirty-day mortality was 27.9% (55/197); Mortality was significantly lower in the baricitinib plus dexamethasone group compared to the dexamethasone monotherapy group (20.3% vs 40.5%, P = <.05). There was no difference in hospital acquired infections between both groups.
Conclusion
Thirty-day mortality was significantly lower in patients with COVID-19 pneumonia treated with baricitinib plus dexamethasone versus dexamethasone monotherapy. No difference was observed in progression to invasive mechanical ventilation and hospital acquired infections.
Keywords: COVID-19, SARS-CoV-2, Baricitinib, Dexamethasone
Introduction
The Coronavirus disease 2019 (COVID-19) pandemic is an ongoing and relapsing epidemiologic phenomenon that has resulted in over one million deaths worldwide.1
Efforts in finding an adequate universal therapy such as hydroxychloroquine,2 lopinavir/ritonavir3 and convalescent plasma4 have fallen short. The use of dexamethasone in patients with COVID-19 pneumonia who require supplementary oxygen is associated with lower 28-day mortality; the highest efficacy of dexamethasone was noticeable in patients requiring intubation and mechanical ventilation, while in patients requiring low-to high-flow oxygen the effect was less pronounced in the RECOVERY Collaborative Group.5 Remdesivir showed clinical improvement in the ACT-1 trial6 and recently tocilizumab7 have shown to improve outcomes in COVID-19 pneumonia patients.
Baricitinib is a Janus-associated tyrosine kinase (JAK) 1 and JAK 2 inhibitor. It modulates the immune response by regulating overactive signaling through the Janus kinase/signal transducers and activators of transcription (JAK-STAT) pathway.8 Furthermore, baricitinib has the advantage of providing in vitro antiviral activity at concentrations achieved with approved dosing. Therefore, it could be an attractive strategy to modulate the immunopathologic mechanisms caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.9 The effects of baricitinib on the immune system in patients with COVID-19 have been explored showing promising data.10
Two small studies have explored the use of baricitinib for COVID-19 pneumonia, both combining it with lopinavir/ritonavir or hydroxychloroquine with no sufficient power to demonstrate difference in outcome.11 , 12 The largest trial to date was the ACTT-2 study where the combination of remdesivir and baricitinib met the primary endpoint of reduction in time to recovery as compared with remdesivir monotherapy.13 In this work, we aimed to study patient's outcomes using dexamethasone and baricitinib compared to dexamethasone monotherapy for the treatment of moderate and severe COVID-19 pneumonia.
Methods
Setting
The university hospital “Dr José Eleuterio Gonzalez” in Monterrey, Mexico is a multi-building complex that includes two separate hospitals. The main building is a 600-bed hospital designated for the treatment of non-COVID-19 cases. The second building is a 76-bed hospital designated for the diagnosis and treatment of suspected or confirmed COVID-19 cases. All patients with respiratory symptoms compatible with COVID-19 are directed to the latter by a pre-arrival or pre-hospital triage located in the former unit.
Selection criteria
We collected data from patients with confirmed COVID-19 hospitalized in our unit from March 2020 through November 2020. We included patients aged >18 years, with a positive RT-PCR for SARS-CoV-2, supplemental oxygen requirement, and abnormal chest imaging that received either baricitinib plus dexamethasone or dexamethasone monotherapy. Severe COVID-19 pneumonia was defined as dyspnea, with at least one of the following: a respiratory rate of 30 or more breaths per minute, a blood oxygen saturation of 93% or less, a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) of less than 300 mm Hg, or pulmonary infiltrates in more than 50% of the lung fields.14
We excluded patients with a hospital stay less than 24 h, patients without oxygen requirement or patients that received any other therapies such as convalescent plasma, tocilizumab, remdesivir or methylprednisolone.
Medication and dosing regimens
All patients received enoxaparin 1 mg/kg once a day if d-dimer was <1000 ng/mL and every 12 h if it was higher unless otherwise contraindicated. Dexamethasone was administered at a dose of 6 mg IV every 24 h for ten days. Baricitinib was started at a dose of 4 mg/day if glomerular filtration rate (GFR) was >60 mL/min/1.73 m2, 2 mg/day if between 30 and 59 mL/min/1.73 m2, and it was withheld if GFR <30 mL/min/1.73 m2. Baricitinib was stopped after 14 days of treatment or at discharge, whichever was first. Standard of care in our center included enoxaparin, dexamethasone as described above and baricitinib could be added at the discretion of the patient's clinician. The analysis of the data was performed in a blinded matter.
Statistical analysis
Demographic and clinical characteristics were analyzed using measures of central tendency. Variables were categorized according to their normal and non-normal distribution. Student's t test was used for continuous variables with normal distribution and Mann–Whitney U test for continuous variables with non-normal distribution. For categorical variables chi-square or Fisher's exact test were used if less or more than 20% of cells have five or less expected values, respectively. A Kaplan–Meier curve was constructed and time to death at 30 days was calculated with the log-rank test (Mantel–Cox test). A P value <.05 was considered statistically significant. Proportional hazards regression (Cox regression) analysis was used to evaluate 30-day mortality with variables with statistical significance on univariate analysis. IBM SPSS Statistics 22.0 was used (IBM Corp., Armonk, NY, USA).
Results
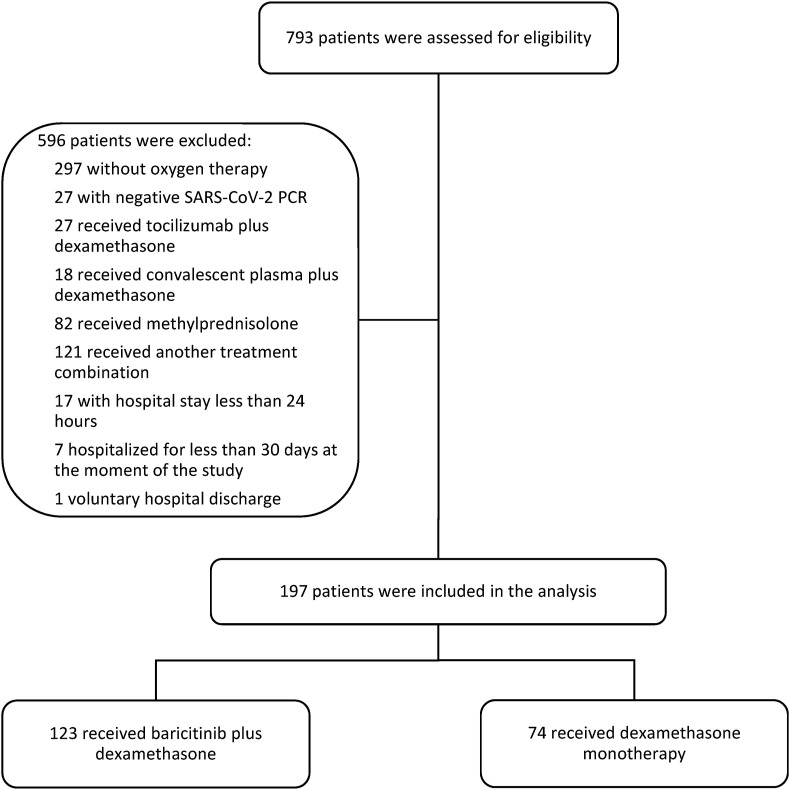
A total of 793 patients were assessed for eligibility; 596 were excluded: 297 did not require supplementary oxygen, 27 had a negative RT-PCR for SARS-CoV-2, 82 received at least one dose of methylprednisolone, 166 received other therapies, 17 had a hospital stay less than 24 h, seven were still hospitalized for less than 30 days at the time of the analysis, one was discharged against medical advice. There were 197 patients included for the primary analysis: 123 in the baricitinib plus dexamethasone group and 74 in the dexamethasone monotherapy group (Fig. 1 ).
Figure 1.
Flow chart of patient selection.
The mean age was 59.9 years (SD ± 14.5) and 123 (62.1%) patients were male. The most common comorbidities were type 2 diabetes in 81/197 (41.2%) patients, hypertension in 69/197 (35.2%) and obesity/overweight in 27/197 (13.8%). Underlying chronic kidney disease was more common in the dexamethasone monotherapy group compared to the baricitinib plus dexamethasone group (3/123 patients (20.3% vs 2.5%, P < .05)). The median of days from symptom onset until hospital admission was 7 (IQR 4–10).
Upon admission, 67.7% patients required low flow supplementary oxygen (mask or low flow nasal cannula), 13.1% HFNC and 13.1% IMV (P > .05 between groups). Median National Early Warning Score (NEWS) amongst admitted patients was 6 (IQR 4–8), which was lower in the dexamethasone monotherapy group, although not statistically significant (P = .27) (see Table 1 ). Fibrinogen, Ferritin and lactate dehydrogenase were significantly higher in the dexamethasone plus baricitinib group (see Table 2 ).
Table 1.
Characteristics of the patients at baseline, according to treatment assignment and level of respiratory support.
| Variable | Total n = 197 |
Baricitinib + Dexamethasone n = 123 |
Dexamethasone n = 74 |
P value |
|---|---|---|---|---|
| Male, n (%) | 123 (62.1) | 74 (59.7) | 49 (66.2) | .22 |
| Age yr, mean (SD) | 59.9 (14.5) | 60.7 (13.1) | 58.5 (16.5) | .30 |
| Comorbidities | ||||
| Type 2 diabetes, n (%) | 81 (41.3) | 47 (38.5) | 34 (45.9) | .19 |
| Hypertension, n (%) | 69 (35.2) | 35 (28.7) | 34 (45.9) | .01 |
| Chronic kidney disease, n (%) | 18 (9.2) | 3 (2.5) | 15 (20.3) | <.01 |
| Obesity/overweight, n (%) | 27 (13.8) | 14 (11.5) | 13 (17.6) | .16 |
| No. days of symptoms before admission, median (IQR) | 7.0 (4.0–10.0) | 7.0 (5.0–10.0) | 7.0 (3.0–8.5) | .03 |
| No. days with baricitinib, median (IQR) | 8.0 (4.0–10.0) | 8.0 (4.0–10.0) | – | – |
| No. days with dexamethasone, median (IQR) | 8.0 (5.0–10.0) | 8.0 (6.0–10.0) | 6.0 (4.0–9.5) | <.01 |
| Oxygen therapy upon admission | ||||
| Low-flow supplementary oxygen, n (%) | 134 (67.7) | 87 (70.2) | 47 (63.5) | .20 |
| High flow, n (%) | 26 (13.1) | 18 (14.5) | 8 (10.8) | .30 |
| Mechanical ventilation, n (%) | 26 (13.1) | 16 (12.9) | 10 (13.5) | .53 |
| NEWS Score, median (IQR) | 6.0 (4.0–8.0) | 6.0 (5.0–7.7) | 3.5 (2.7–7.2) | .27 |
ALT: alanine aminotransferase, AST: aspartate aminotransferase, CPK: creatinine phosphokinase, IQR: interquartile range, LDH: lactate dehydrogenase, NEWS: National Early Warning Score, SD: standard deviation.
Table 2.
Characteristics of the patients laboratory parameter at baseline, according to treatment assignment.
| Baseline laboratory test | Total n = 197 |
Baricitinib + Dexamethasone n = 123 |
Dexamethasone n = 74 |
P value |
|---|---|---|---|---|
| Hemoglobin (g/dL), median (IQR) | 13.3 (11.6–14.8) | 13.8 (12.6–15.1) | 11.9 (9.1–14.1) | <.01 |
| Leukocytes (×109/L), median (IQR) | 9.7 (7.4–13.3) | 9.670 (7.810–12.800) | 9.980 (6.430–13.700) | .79 |
| Lymphocytes (×109/L), median (IQR) | .880 (.523–1.150) | .840 (.510–1.150) | .930 (555–1.155) | .55 |
| Platelets (×109/L), median (IQR) | 233.5 (186.0–293.7) | 241,000 (187,100–295,000) | 225,000 (171,500–291,500) | .28 |
| Creatinine (mg/dL), median (IQR) | .9 (.7–1.4) | .9 (.7–1.2) | 1.1 (.7–3.1) | <.01 |
| Blood urea nitrogen (mg/dL), median (IQR) | 19.0 (12.0–33.0) | 18.0 (12.0–29.0) | 23.0 (13.0–45.5) | .11 |
| AST (IU/L), median (IQR) | 46.0 (30.5–69.5) | 49.0 (33.2–68.0) | 45.0 (27.5–75.0) | .33 |
| ALT (IU/L), median (IQR) | 37.0 (23.5–61.5) | 39.5 (26.0–60.7) | 34.0 (19.5–64.0) | .11 |
| Alkaline phosphatase (IU/L), median (IQR) | 76.0 (61.0–101.0) | 76.0 (61.0–97.0) | 75.0 (60.0–110.0) | .56 |
| Total CPK (IU/L), median (IQR) | 105.0 (60.0–200.0) | 104.5 (53.2–193.0) | 106.0 (60.7–260.0) | .49 |
| LDH (IU/L), median (IQR) | 329.0 (257.0–469.5) | 360.0 (274.0–500.0) | 283.5 (232.5–440.7) | .01 |
| C reactive protein (mg/dL), mean (SD) | 16.7 (9.6) | 16.6 (9.5) | 17.0 (9.9) | .82 |
| Ferritin (μg/L), median (IQR) | 1112.0 (547.1–2326.1) | 1282.2 (685.1–2527.2) | 866.6 (323.5–1615.2) | .05 |
| Troponin (ng/mL), median (IQR) | 14.6 (4.5–46.1) | 8.1 (4.2–29.5) | 24.9 (7.0–147.7) | .19 |
| Fibrinogen (mg/dL), mean (SD) | 876.8 (257.2) | 923.9 (256.2) | 790.2 (238.5) | <.01 |
| D dimer (ng/mL), median (IQR) | 535.0 (275.2–1832.0) | 274.0 (226.7–430.5) | 297.0 (191.5–1411.5) | .03 |
ALT: alanine aminotransferase, AST: aspartate aminotransferase, CPK: creatinine phosphokinase, IQR: interquartile range, LDH: lactate dehydrogenase, SD: standard deviation.
Progression in oxygen requirements from low-flow oxygen supplementation to High-flow nasal cannula (HFNC) was observed in 26 of 89 (29.2%) patients in the baricitinib plus dexamethasone group and in 5 out of 56 in the dexamethasone group (8.9%) (P < .01) although progression to IMV was not significantly different between both groups (15 of 107 (14.0%) in the baricitinib plus dexamethasone group vs 10 of 64 (15.6%) in the dexamethasone (P = .94)).
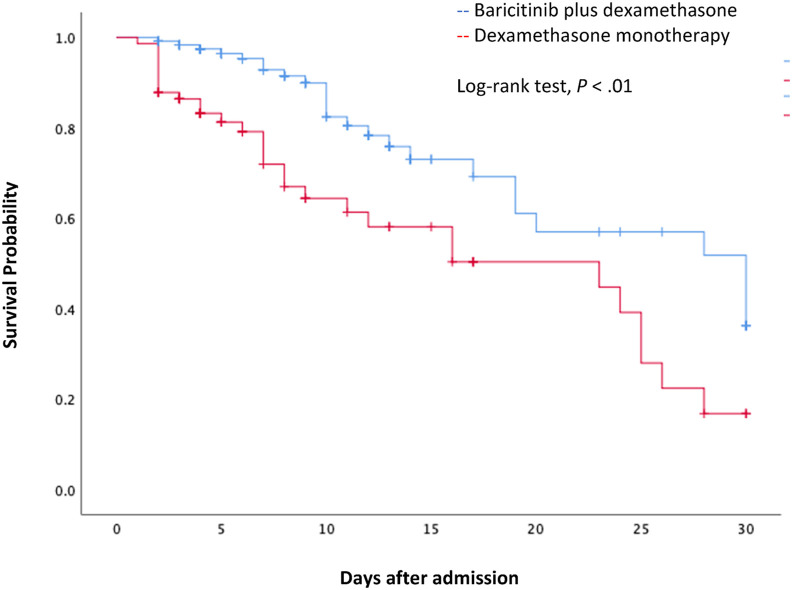
Similar proportions of patients in both groups developed acute kidney injury (AKI) (17/123 [13.7%] in the baricitinib plus dexamethasone group vs 13/74 [17.6%] in the dexamethasone monotherapy group, P = .29). Nonetheless, more patients from the latter group required kidney replacement therapy (KRT) during hospitalization (9/123 [7.3%] in the baricitinib plus dexamethasone group vs 11/74 [14.9%] in the dexamethasone group, P = .07). Thirty-day all-cause mortality was 27.9% (55/197) globally; 25 of 123 [20.3%] of patients died in the baricitinib plus dexamethasone group vs 30/74 [40.5%] in the dexamethasone monotherapy group (P < .01). Unadjusted hazard ratio for 30-day mortality was .50 (CI95% .32–.78, P < .01) with a number needed to treat of 4.94 (Fig. 2 ). Causes associated with mortality were sepsis and septic shock in 11/25 (44%) in the baricitinib plus dexamethasone group vs 15/30 (50%) in the dexamethasone group (P = .003), neurologic complications in 3 (12%) in the baricitinib plus dexamethasone group vs 4 (13.3%) in the dexamethasone group (P = .48), renal insufficiency in 1 (4%) in the baricitinib plus dexamethasone group vs 7 (23%) in the dexamethasone group (P = .004) and other causes in 10 (40%) vs 4 (13.3%) (P = .57) respectively.
Figure 2.
Kaplan–Meier survival curve for 30-day mortality between the two treatment groups.
The median length of stay was higher in the baricitinib plus dexamethasone group (8.0, IQR 5.0–13.0 days) vs the dexamethasone monotherapy group (6.0, IQR 3.0–12.2 days) (P = .01). Breakthrough infections were most frequently found in the dexamethasone monotherapy group (20/74 patients, 27.0%) compared to the baricitinib plus dexamethasone group (19/123, 15.3%) with statistical significance (P = .03). Bacterial pneumonia was more frequent in the dexamethasone group and catheter related infections were more frequent in the baricitinib–dexamethasone group, although individually the difference was not statistically significant. Conversely, thrombotic events were more common in the latter group although the difference was not significant (4/123 [3.2%] in the baricitinib plus dexamethasone group vs 1/74 [1.4%] in the dexamethasone monotherapy group, P = .39). These included stroke, pulmonary venous embolism and acute non ST elevated myocardial infraction (see Table 3 ). After multivariate analysis the difference in 30-day mortality remained significantly different (P = .001); end-stage renal disease (aHR 51.9, CI95% 5.4–496.4, P = .001) and Invasive mechanical ventilation (aHR 28.4 CI95% 4.0–193.1, P = .001) remained significant predictors of 30-day mortality.
Table 3.
Outcomes of the Patients according to the treatment assignment.
| Variable | Total n = 197 |
Baricitinib + Dexamethasone n = 123 |
Dexamethasone n = 74 |
P value |
|---|---|---|---|---|
| Length of stay, median of days (IQR) | 7.0 (4.0–13.0) | 8.0 (5.0–13.0) | 6.0 (3.0–12.25) | .01 |
| Intensive care unit admission, n (%) | 85 (42.9) | 59 (47.6) | 26 (35.1) | .05 |
| Progression to high flow, n (%) | 31 (15.7) | 26 (21.1) | 5 (6.7) | <.01 |
| Progression to invasive mechanical ventilation, n (%) | 25 (12.6) | 15 (12.1) | 10 (13.5) | .94 |
| Thrombotic events, n (%) | 5 (2.5) | 4 (3.2) | 1 (1.4) | .39 |
| Pulmonary embolism, n (%) | 4 (2.0) | 3 (2.4) | 1 (1.4) | .64 |
| Deep venous thrombosis, n (%) | 1 (.8) | 0 | 1 (.5) | .64 |
| No. patients with breakthrough infections, n (%) | 39 (19.7) | 19 (15.3) | 20 (27.0) | .03 |
| Bacterial pneumonia, n (%) | 31 (15.7) | 17 (13.7) | 14 (18.9) | .21 |
| Urinary tract infection, n (%) | 11 (5.6) | 6 (4.8) | 5 (6.8) | .39 |
| Catheter-related bloodstream infection, n (%) | 14 (7.1) | 12 (9.7) | 2 (2.7) | .05 |
| Others, n (%) | 7 (3.5) | 0 | 7 (9.5) | <.01 |
| No. days since baricitinib initiation until breakthrough infection, median (IQR) | 10.0 (4.0–10.0) | 10.0 (4.0–10.0) | – | – |
| No. days since dexamethasone initiation until breakthrough infection, median (IQR) | 10.0 (6.0–10.0) | 10.0 (7.0–10.0) | 7.0 (4.0–13.0) | .51 |
| Acute kidney injury, n (%) | 30 (15.2) | 17 (13.7) | 13 (17.6) | .29 |
| Kidney replacement therapy, n (%) | 20 (10.1) | 9 (7.3) | 11 (14.9) | .07 |
| 30-day mortality, n (%) | 55 (27.9) | 25 (20.3) | 30 (40.5) | <.01 |
Discussion
The current study showed that patients receiving baricitinib plus dexamethasone for moderate and severe COVID-19 pneumonia were associated with lower mortality compared to those receiving dexamethasone monotherapy.
Our analysis included over 100 patients treated with baricitinib plus dexamethasone as a combination therapy modality and did not include any other treatments as opposed to other studies where baricitinib was associated to medications such as hydroxychloroquine and lopinavir/ritonavir, these studies addressed safety although the open single arm designed did not permit an efficacy analysis.11 , 12
Mortality by COVID-19 pneumonia treated with dexamethasone has been reported to range between 29.3%, 32.1 and 56.3% in the 3 largest trials analyzing this endpoint (Recovery trial 2020,5 WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT), WHO Working Group 2020,15 Tomazini et al.,16 respectively). Our mortality was 40.5% in the dexamethasone group well in the range of the previously mentioned studies.
It is likely that indication bias favored the dexamethasone only arm, as evidenced by the lower NEWS score and ferritin concentrations (although the difference was not statistically significant) in that group; sicker patients would be given both drugs (or have a second drug added to the first) therefore, these findings are counter to what might be expected if the two strategies did not differ.
The baricitinib/dexamethasone arm had an average of two additional hospital days and was associated with greater need for critical care (the latter was not statistically significant). Our findings show that the addition of baricitinib to a dexamethasone standard treatment was associated with significantly less 30-day all-cause mortality when compared to dexamethasone alone, these differences could be influenced by the fact that more patients with ESRD on RRT were in the dexamethasone monotherapy group. Renal insufficiency and sepsis were associated more frequently at the time of death in the dexamethasone group.
The lack of difference in progression to mechanical ventilation between groups coupled with a lower overall mortality in the baricitinib plus dexamethasone group supports clinical benefit of this combination in our study population and promotes further clinical trials assessing this therapy including those that are currently recruiting patients (NCT04421027).
Adding baricitinib to dexamethasone does not appear to significantly increase infection risk, our findings showed that overall breakthrough infections were in fact les frequent compared to patients treated with dexamethasone. This is similar to the safety profile already known from long-term treatment in rheumatoid arthritis where the incidence rate of serious infection was 2.8/100 patient-years and stable over time.17
Limitations to our data include the absence of randomization, the retrospective nature of the study, and the fact that baricitinib was not used in patients with less than 30 mL/min GFR. We did not perform follow-up after hospital discharge for late mortality assessment, and although the primary objective was 30-day mortality information regarding infection rates and survival after hospital discharge would have added valuable information.
Similar to the study of Rodriguez-García et al. these findings could suggest a beneficial effect of combining corticosteroids and a short course of baricitinib in the treatment of SARS-CoV-2 pneumonia. This may correspond to the immunomodulation of the hosts' inflammatory reaction and the inhibition of the viral entry into the lung cells.18, 19, 20, 21, 22 However, the impact of baricitinib in combination with dexamethasone on the development of protective humoral and cell-mediated anti-viral immunity and regulating inflammation response in COVID-19 patients must be evaluated in randomized clinical trials.
We conclude that in our study thirty-day mortality was significantly lower in patients with COVID-19 pneumonia treated with baricitinib plus dexamethasone versus dexamethasone monotherapy without a difference in progression to invasive mechanical ventilation and hospital acquired infections.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all the COVID-19 patients that trusted us with their care.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard https://covid19.who.int/?gclid=CjwKCAiAwrf BRA9EiwAUWwKXp8ZBWZyESa6fOG8ox59UGNYbmEe3p1MlCdqQm5ZKYRJ-y5yOIKw7xoCLRcQAvD_BwE n.d.
- 2.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 Jul 17 doi: 10.1056/NEJMoa2021436. Epub ahead of print. PMID: 32678530; PMCID: PMC7383595. [DOI] [Google Scholar]
- 3.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021 Feb 18;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RECOVERY Collaborative Group. Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020 Nov 19;383(21):2030–2040. doi: 10.1056/NEJMoa2022926. Epub 2020 Oct 8. PMID: 33031652; PMCID: PMC7556338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., ACTT-1 Study Group Members Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020 Nov 5;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. Epub 2020 Oct 8. PMID: 32445440; PMCID: PMC7262788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horby P., Campbell M, Staplin N, Spata e, Emberson JR, Pessoa-Amorim G, RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Praveen D., Puvvada R.C., Vijey Aanandhi M. Janus kinase inhibitor baricitinib is not an ideal option for management of COVID-19. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronte V., Ugel S., Tinazzi E., Vella A., De Sanctis F., Canè S. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020 Dec 1;130(12):6409–6416. doi: 10.1172/JCI141772. PMID: 32809969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantini F., Niccoli L., Matarrese D., Nicastri E., Stobbione P., Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Titanji B.K., Farley M.M., Mehta A., Connor-Schuler R., Moanna A., Cribbs S.K. Use of baricitinib in patients with moderate to severe coronavirus disease 2019. Clin Infect Dis. 2021;72(7):1247–1250. doi: 10.1093/cid/ciaa879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V. Baricitinib plus remdesivir for the treatment of hospitalized adults with Covid-19. A randomized double-blind placebo-controlled trial. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa20319942020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N Engl J Med. 2020 Dec 17;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. Epub 2020 May 15. PMID: 32412710. [DOI] [PubMed] [Google Scholar]
- 15.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. J Am Med Assoc. 2020 Oct 6;324(13):1330–1341. doi: 10.1001/jama.2020.17023. PMID: 32876694; PMCID: PMC7489434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomazini B.M., Maia I.S., Cavalcanti A.B., Berwanger O., Rosa R.G., Veiga V.C., COALITION COVID-19 Brazil III Investigators Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. J Am Med Assoc. 2020 Oct 6;324(13):1307–1316. doi: 10.1001/jama.2020.17021. PMID: 32876695; PMCID: PMC7489411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genovese M.C., Smolen J.S., Takeuchi T., Burmester G., Brinker D., Rooney T.P. Safety profile of baricitinib for the treatment of rheumatoid arthritis over a median of 3 years of treatment: an updated integrated safety analysis. Lancet Rheumatol. 2020;2:e347–e357. doi: 10.1016/S2665-9913(20)30032-1. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Garcia L.J., Sanchez-Nievas G., Arevalo-Serrano J., Garcia-Gomez C., Maria Jimenez-Vizuete J., Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology (Oxford) 2021 Jan 5;60(1):399–407. doi: 10.1093/rheumatology/keaa587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seif F., Aazami H., Khoshmirsafa M., Kamali M., Mohsenzadegan M., Pornour M. JAK inhibition as a new treatment strategy for patients with COVID-19. Int Arch Allergy Immunol. 2020;181:467–475. doi: 10.1159/000508247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stebbing J., Krishnan V., Bono S., Ottaviani S., Casalini G., Richardson P.J. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stebbing J., Sánchez Nievas G., Falcone M., Youhanna S., Richardson P., Ottaviani S. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021 Jan 1;7(1) doi: 10.1126/sciadv.abe4724. PMID: 33187978; PMCID: PMC7775747. [DOI] [PMC free article] [PubMed] [Google Scholar]