Abstract
Reports have emerged of myocarditis and pericarditis predominantly after the second dose of the coronavirus disease messenger ribonucleic acid vaccine. We describe 13 patients aged 12-17 years who presented with chest pain within 1 week after their second dose of the Pfizer vaccine and were found to have elevated serum troponin levels and evidence of myopericarditis.
Keywords: myocarditis, COVID-19 vaccine
Abbreviations: CMR, Cardiac magnetic resonance imaging; COVID-19, Coronavirus disease; LV, Left ventricular; LVEF, Left ventricular ejection fraction; mRNA, Messenger ribonucleic acid
See related articles p 5, p 26, and p 321
On May 10, 2021, the US Food and Drug Administration extended the Emergency Use Authorization of the Pfizer-BioNTech messenger ribonucleic acid (mRNA) coronavirus disease (COVID-19) vaccine for adolescents aged 12-15 years.1 Following this authorization, large numbers of adolescents across the country began to receive immunization. As of June 21, 2021, 98 008 adolescents aged 12-15 years and 69 489 adolescents aged 16 and 17 years in Washington state completed the 2-dose schedule of the mRNA COVID-19 vaccine.2
Reports of post-COVID-19 vaccine myocarditis and pericarditis have emerged, particularly after the second dose of the mRNA vaccine. Initial cases were noted predominantly in male adolescents and young adults in the Israeli military.3 Subsequently, US institutions have reported 7 cases in adolescents aged >16 years4 and 7 cases in young adults.5 As the age range of eligibility for the COVID-19 vaccine has broadened in Washington, we have cared for a cohort of younger patients with postvaccination myopericarditis. Here we describe clinical and cardiac magnetic resonance imaging (CMR) findings for 13 patients aged 12-17 years seen at our center.
Methods
With Institutional Review Board approval, we performed a retrospective electronic medical record review. Inclusion criteria were patients aged <18 years presenting with severe chest pain and signs of myopericarditis within 1 week of receiving the second dose of the Pfizer COVID-19 vaccine between April 1, 2021, and June 21, 2021.
Results
Clinical and laboratory findings are presented in Table I . We identified 13 patients with myopericarditis, with a median age of 15 years (range, 12-17 years). The majority of patients were male (n = 12; 92%), and non-Hispanic white (n = 10; 76.9%). The median time to presentation from the second dose of the Pfizer COVID-19 mRNA vaccine was 3 days (range, 2-4 days). According to the inclusion criteria, all patients had sudden onset of intense, persistent chest pain that was not exacerbated by movement or activity. The most common accompanying symptoms were shortness of breath (n = 6; 46.2%), tactile temperature (n = 5; 38.5%), and myalgias (n = 4; 30.7%).
Table I.
Demographic features and clinical findings in adolescents following receipt of the Pfizer mRNA COVID-19 vaccine
| Patient | Demographics |
Clinical information |
Laboratory tests |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | Sex | Race | Length of stay, d | Time from vaccination to presentation, d | Other symptoms | Peak troponin, ng/mL (normal <0.05 ng/mL) | Peak BNP, pg/mL (normal <55 pg/mL) | Peak CRP, mg/dL (normal <0.08 mg/dL) | COVID-19 immunoglobulin G nucleocapsid antibody testing | |
| 1 | 16 | M | White non-Hispanic | 1 | 2 | Fever, chills, myalgias, headache, shortness of breath | 8 | 15 | 4.3 | Negative |
| 2 | 16 | M | Asian non-Hispanic | 1 | 2 | Fever, myalgias | 11.1 | 28 | 3.5 | Not tested |
| 3 | 16 | M | White non-Hispanic | 3 | 3 | Myalgias, headache | 10.9 | <10 | 3.6 | Negative |
| 4 | 17 | M | American Indian/Alaska Native non-Hispanic | 1 | 3 | Fever, malaise | 9.18 | 14 | – | Negative |
| 5 | 15 | M | White non-Hispanic | 2 | 2 | Myalgias, shortness of breath | 4.95 | 13 | 5.5 | Negative |
| 6 | 15 | F | White non-Hispanic | 1 | 3 | Vomiting | 0.65 | 7 | 1.4 | Negative |
| 7 | 15 | M | White non-Hispanic | 3 | 3 | Fevers, shortness of breath | 9.12 | 74 | 3 | Negative |
| 8 | 15 | M | White non-Hispanic | 3 | 3 | Chills | 13.2 | 87 | 6.2 | Negative |
| 9 | 12 | M | White non-Hispanic | 2 | 3 | None | 13 | 37 | – | Negative |
| 10 | 14 | M | White non-Hispanic | 3 | 3 | Fever, headache | 18.5 | 66 | – | Negative |
| 11 | 14 | M | Asian non-Hispanic | 2 | 4 | Malaise, shortness of breath | 6.08 | 55 | 3.7 | Not tested |
| 12 | 16 | M | White non-Hispanic | 2 | 2 | Shortness of breath | 16.4 | 38 | 6.5 | Not tested |
| 13 | 15 | M | White non-Hispanic | 2 | 3 | None | 7.89 | 86 | 3.4 | Not tested |
BNP, brain natriuretic peptide; CRP, C-reactive protein; F, female; M, male.
All patients had an elevated serum troponin level (median, 9.18 ng/mL; range, 0.65-18.5 ng/mL). The median serum brain natriuretic peptide level was 37.5 pg/mL (range, 7-87 pg/mL). C-reactive protein was elevated in patients in whom it was measured (n = 10; median, 3.7 mg/dL; range, 1.4-6.5 mg/dL). COVID-19 nucleocapsid immunoglobulin G antibody was measured in 9 patients and was negative in all 9.
Cardiac testing results are presented in Table II . Nine patients had an abnormal electrocardiogram, with ST segment elevation the most common finding. All patients underwent echocardiography on admission; 11 patients had normal left ventricular (LV) systolic function, and 2 patients demonstrated mildly reduced LV systolic function as well as regional LV wall motion abnormalities. The median LV ejection fraction (LVEF) was 60% (range, 45%-69%; normal defined as >55%). No patients had significant pericardial effusion. One patient had an incidental finding of bicuspid aortic valve without regurgitation or stenosis.
Table II.
Cardiac testing results and treatment in adolescents following receipt of the Pfizer mRNA COVID-19 vaccine
| Patient | Cardiac testing |
Treatment |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ECG findings | Echocardiography |
CMR |
||||||||
| LV wall motion abnormalities | LVEF, % (normal ≥55%) | LVEF, % (normal ≥55%) | Edema | LGE | LV focal hypokinesis | IVIG | Corticosteroids | NSAIDs | ||
| 1 | Normal | No | 66 | 50.8 | Yes | Yes | No | No | No | Yes |
| 2 | ST elevation | No | 59 | 51.1 | Yes | Yes | No | No | No | Yes |
| 3 | ST elevation | No | 69 | 56.6 | Yes | Yes | No | Yes | No | Yes |
| 4 | ST elevation | No | 58 | 49.4 | Yes | Yes | No | No | No | Yes |
| 5 | Normal | No | 58 | 52 | Yes | Yes | No | No | No | Yes |
| 6 | Nonspecific T-wave changes | No | 58 | 48 | Yes | Yes | No | No | No | Yes |
| 7 | T-wave inversion | No | 61 | 61 | Yes | Yes | No | No | No | Yes |
| 8 | ST elevation | Yes | 45 | 46 | Yes | Yes | Yes | Yes | Yes | Yes |
| 9 | Normal | No | 64 | 54 | Yes | Yes | No | No | No | Yes |
| 10 | ST elevation | No | 62 | 55 | Yes | Yes | Yes | No | No | Yes |
| 11 | ST elevation | No | 60 | 58 | Yes | Yes | No | No | No | Yes |
| 12 | ST elevation | Yes | 53 | 58 | Yes | Yes | No | Yes | Yes | Yes |
| 13 | Normal | No | 61 | 53 | Yes | Yes | No | No | No | Yes |
ECG, electrocardiography; IVIG, intravenous immunoglobulin; LGE, late gadolinium enhancement; NSAID, nonsteroidal anti-inflammatory drug.
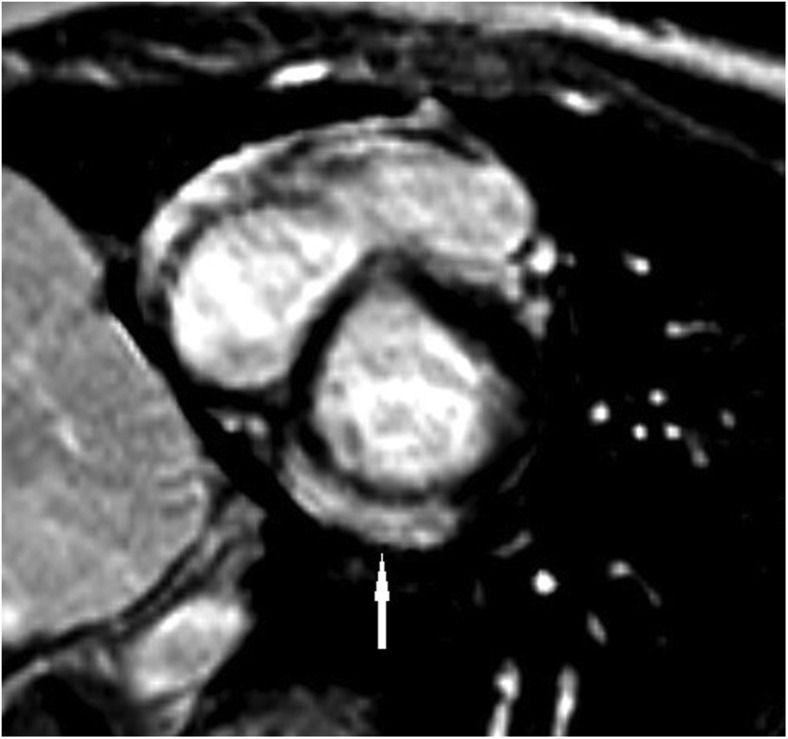
All patients underwent CMR within 1 week of presentation. All CMRs performed were abnormal, showing late gadolinium enhancement in a patchy subepicardial to transmural pattern with predilection for the inferior LV free wall (Figure ). In addition, all CMRs had evidence of edema in corresponding segments by T2-weighted CMR and met the Lake Louise criteria6 for myocarditis. LV regional wall motion abnormalities were noted in 2 patients; CMR-based LV systolic function was mildly decreased in 8 patients. The CMR-detected LVEF ranged from 46% to 61% (median, 53%). No significant pericardial effusions were seen on CMR.
Figure.
Short-axis CMR image with an arrow showing delayed enhancement in the inferior and inferolateral basal segments of the LV free wall.
All patients received scheduled doses of nonsteroidal anti-inflammatory agents (ie, ibuprofen every 8 hours, with dose dependent on patient weight). Three patients received intravenous immunoglobulin, 2 of whom were the patients with decreased LV function on echocardiography. These 2 patients also received corticosteroids according to our institutional pathway for treatment of myocarditis. One patient had isolated premature ventricular contractions on telemetry; no other patient had evidence of arrhythmia. The median hospital length of stay was 2 days (range, 1-4 days) with no intensive care unit admission, significant morbidity, or mortality. All patients had resolution of chest pain and a falling serum troponin level before discharge.
Discussion
We report 13 adolescents with myopericarditis after the second dose of the Pfizer mRNA COVID-19 vaccine. This cluster of cases was identifiable as the age of eligibility for vaccination broadened with Emergency Use Authorization by the Food and Drug Administration. Our hospital is the only freestanding children's hospital in Washington and serves as a tertiary referral institution. To our knowledge, at least 3 other cases in this age group have been cared for at other hospitals in the state. Using these numbers and Washington Department of Health data on immunization,2 we estimate a possible incidence of 0.008% in adolescents aged 16-17 years and 0.01% in those aged 12-15 years following the second dose.
All patients had evidence of myocardial inflammation and edema on CMR, similar to findings in limited case series of adults with post–COVID-19 vaccine myocarditis.7 Although the symptoms resolved rapidly in all patients, their CMR findings indicate the potential for myocardial fibrosis and unknown long-term impact. Accordingly, we are following the American Heart Association/American College of Cardiology recommendations for exercise restrictions in acute myocarditis for up to 6 months and long-term cardiac surveillance.8 In addition, follow-up CMR is planned for all patients at 3 months, which may allow us to shorten the period of exercise restriction.
We speculate that a hyperimmune response to the second dose of the vaccine is plausible. Children have demonstrated a more robust immune response to severe acute respiratory syndrome coronavirus 2 infection than adults, as observed in multisystem inflammatory syndrome in children.9 For noninferior immunogenicity, it is possible the interval between doses 1 and 2 should be longer in children than in adults or that a reduction in the content of dose 2 may be appropriate in individuals aged <18 years.
It is noteworthy that 2 of our cases had a family history of myocarditis in first-degree relatives. There is evidence that genetics may play a role in the susceptibility of patients to myopericarditis.10 This predisposition may increase the likelihood of inflammation and cardiac effects after the vaccine.
The Pfizer Phase 2/3 clinical trial included only 754 participants in the 16 to 17-year-old age group and 2260 in the 12- to 15-year-old age group. Approximately 50% were males.11 As noted earlier, we have estimated the incidence of myopericarditis in the younger group as nearly 0.01% of those receiving the second dose of vaccine. Owing to reporting issues, delays, and early inability of practitioners to associate myopericarditis with vaccine, this is likely an underestimate. Moreover, our Washington Department of Health vaccine data for these age groups are not segregated by sex. This adverse event likely would not be detected in the small population of males who received the study vaccine and highlights the need for aggressive postauthorization surveillance.
Although a causal relationship between vaccination and the development of myopericarditis cannot be concluded from a case series, the clustering in time as well as the uncommon occurrence of myopericarditis and the rapid resolution of symptoms and findings likely make this a unique vaccine-related event. Identification of myopericarditis as an adverse event should have high priority during investigations before and after authorization of COVID-19 vaccines and be considered by policy makers in the risk/benefit ratio in adolescents and children.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Hinton D.M. FDA Emergency use authorization for Pfizer-BioNTech COVID-19 vaccine [Letter] 2021. https://www.fda.gov/media/144412/download Accessed June 16, 2021.
- 2.Washington State Department of Health COVID-19 data dashboard 2021. https://www.doh.wa.gov/Emergencies/COVID19/DataDashboard Accessed June 21, 2021.
- 3.Abu Mouch S., Roguin A., Hellou E., Ishai A., Shoshan U., Mahamid L., et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790–3793. doi: 10.1016/j.vaccine.2021.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall M., Ferguson I.D., Lewis P., Jaggi P., Gagliardo C., Collins J.S., et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021 doi: 10.1542/peds.2021-052478. e2021052478. [DOI] [PubMed] [Google Scholar]
- 5.Rosner C.M., Genovese L., Tehrani B.N., Atkins M., Bakhshi H., Chaudhri S., et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021 doi: 10.1161/circulationaha.121.055891. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 7.Shaw K.E., Cavalcante J.L., Han B.K., Gössl M. Possible association between COVID-19 vaccine and myocarditis: clinical and CMR findings. JACC Cardiovasc Imaging. 2021 doi: 10.1016/j.jcmg.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canter C.E., Simpson K.E. Diagnosis and treatment of myocarditis in children in the current era. Circulation. 2014;129:115–128. doi: 10.1161/CIRCULATIONAHA.113.001372. [DOI] [PubMed] [Google Scholar]
- 9.Feldstein L.R., Tenforde M.W., Friedman K.G., Newhams M., Rose E.B., Dapul H., et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baggio C., Gagno G., Porcari A., Paldino A., Artico J., Castrichini M., et al. Myocarditis: which role for genetics? Curr Cardiol Rep. 2021;23:58. doi: 10.1007/s11886-021-01492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfizer-BioNTech announce positive topline results of pivotal COVID-19 vaccine study in adolescents. 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-biontech-announce-positive-topline-results-pivotal Accessed June 21, 2021. [Epub ahead of print]