Abstract
Vaccine-induced thrombotic thrombocytopenia is a newly described disease process in the setting of expanding access to COVID-19 vaccination. The United States Centers for Disease Control and Prevention recommends treatment with an alternative to heparin in patients suspected of having vaccine-induced thrombotic thrombocytopenia. At this time there have been no reported outcomes from the treatment of vaccine-induced thrombotic thrombocytopenia with bivalirudin as a heparin alternative. We describe the early outcomes from the treatment of vaccine-induced thrombotic thrombocytopenia with bivalirudin as a heparin alternative. A 40-year-old Caucasian woman was found to have thrombocytopenia, cerebral venous sinus thrombosis, and pulmonary embolism following vaccination for COVID-19 with Ad26.COV2.S. She exhibited a steady rise in platelet count: 20×109/L at hospital day 0, 115×109/L at discharge on hospital day 6, and 182×109/L on outpatient follow-up on day 9. While the patient exhibited a transient drop in hemoglobin, there was no clinical evidence of bleeding. This patient did not demonstrate any clinical sequelae of thrombosis, and she reported resolution of her headache. Vaccination with Ad26.COV2.S appears to be associated with a small but significant risk for thrombotic thrombocytopenia within 13 days of receipt. The Centers for Disease Control and Prevention guidance to consider an alternative to heparin was not accompanied by specifically recommended alternatives. A single patient treated with bivalirudin for suspected vaccine-induced thrombotic thrombocytopenia subsequently experienced symptom improvement and a rise in platelet count and did not demonstrate any immediate negative outcomes. A provider may consider bivalirudin as an alternative to heparin in patients with suspected vaccine-induced thrombotic thrombocytopenia following Ad26.COV2.S vaccination, pending more definitive research.
Introduction
The Ad26.COV2.S (Johnson & Johnson/ Jansen) vaccine is a recombinant replication-incompetent adenovirus serotype 26 vector vaccine encoding the stabilized prefusion spike glycoprotein of SARS-CoV-2.1 On February 27, 2021, the United States Food and Drug Administration (FDA) granted emergency use authorization for this vaccine in patients over the age of 18 years.2 As of this writing, 6.8 million doses of Ad26.COV2.S have been administered.3
On April 13, 2021, the United States Centers for Disease Control and Prevention (CDC) and the FDA issued a joint statement recommending a pause in the administration of the Ad26.COV2.S vaccine. This recommendation was the result of 6 cases of thrombocytopenia and cerebral venous sinus thrombosis in women aged 18-48 years with symptoms occurring 6-13 days after vaccination with Ad26.COV2.S.3 The term vaccine-induced thrombotic thrombocytopenia was first used in a report of patients who received the ChAdOx1 nCoV-19 (AstraZeneca) vaccine.4 These patients presented with both thrombosis and thrombocytopenia 5-15 days post-ChAdOx1 nCoV-19 vaccination. We describe a case of a patient presenting with a headache on the same day as the CDC statement. She had both thrombocytopenia and cerebral venous sinus thrombosis, consistent with previously described cases of vaccine-induced thrombotic thrombocytopenia. Based on the CDC guidance, she was not treated with heparin, receiving bivalirudin instead. This patient’s early outcomes suggest that bivalirudin may be a safe alternative to heparin in patients demonstrating a presentation consistent with vaccine-induced thrombotic thrombocytopenia.
Case Report
A previously healthy 40-year-old woman received the Ad26.COV2.S vaccine in early April 2021 (day 0). On postvaccination day 5, she developed a headache, sinus pressure, myalgias, and sore throat with tonsillar exudate. On day 8, she presented to an urgent care clinic and was prescribed amoxicillin-clavulanate, a methylprednisolone taper, and methocarbamol. No laboratory evaluation was performed. The patient did not receive heparin at any point before her presentation. She had no history of thrombosis, clotting disorders, inflammatory bowel disease, collagen vascular disorders, or cancer. She had not been recently pregnant, was not using estrogen-containing medications or tobacco, and had no recent surgical history.
She continued to have worsening headaches, especially with movement, associated photophobia, and intermittent dizziness prompting her presentation to the emergency department (ED) on day 12. She denied fever, weakness, numbness, vision changes, speech difficulty, gait instability, and seizures. Her initial vital signs were within normal limits, with a pulse rate of 68 beats/min and SpO2 of 96% on room air. Physical examination revealed small areas of petechiae on her chest and face, with mild tonsillar exudate without cervical lymphadenopathy. A detailed neurologic examination was performed with normal findings and she had no leg swelling or pain.
She was found to have thrombocytopenia, increased D-dimer levels, normal fibrinogen levels, and mild elevation of serum transaminases (Table 1 ). Peripheral smear did not reveal schistocytes or other evidence of hemolysis. Her nasopharyngeal swab tested negative for SARS-CoV-2 RNA by reverse-transcriptase–polymerase-chain-reaction assay. An initial test for heparin-induced thrombocytopenia (HIT) or anti-platelet factor 4 (PF4)/heparin antibodies, HemosIL HIT-Ab line immunoassay, was negative. However, her confirmatory PF4 enzyme linked immunosorbent assay (ELISA) result was positive.
Table 1.
Results of laboratory evaluation.∗
| Test | Results |
|---|---|
| WBC count (4.0-11.0×109/L) | 8.1 |
| Hemoglobin (12.1-16.3 g/dl) | 15.1 |
| Hematocrit (35.7%-46.7%) | 42.4 |
| Platelet count (150-400×109/L) | 20 |
| Segmented neutrophil percentage | 73 |
| Lymphocyte percentage | 14 |
| Schistocytes | None |
| Sodium (122-145 mmol/L) | 136 |
| Potassium (3.5-5.1 mmol/L) | 4.0 |
| Chloride (98-108 mmol/L) | 101 |
| Carbon dioxide (21-31 mmol/L) | 23 |
| Blood urea nitrogen (7-25 mg/dL) | 10 |
| Creatinine (0.6-1.2 mg/dL) | 0.63 |
| Glucose (70-199 mg/dL) | 100 |
| Aspartate aminotransferase (12-39 units/L) | 65 |
| Alanine aminotransferase (7-52 units/L) | 87 |
| Total bilirubin (0.1-1.3mg/dL) | 0.9 |
| Alkaline phosphatase (39-117 U/L) | 25 |
| Total serum protein (6.4-8.9 g/dL) | 7.8 |
| Albumin (3.5-5.7 g/dL) | 4.7 |
| D-Dimer (<500 fibrinogen equivalent units) | 27,150 |
| International normalize ratio (0.9-1.1) | 1.3 |
| Prothrombin time (11.8-13.7 sec) | 16.0 |
| Partial thromboplastin time (23.8-36.2 sec) | 26.4 |
| Lactate dehydrogenase (124-271 U/L) | 238 |
| Fibrinogen (150-400 mg/dL) | 149 |
| Haptoglobin (36-195 mg/dL) | 208 |
The reference range is provided in parentheses and the abnormal values are in bold.
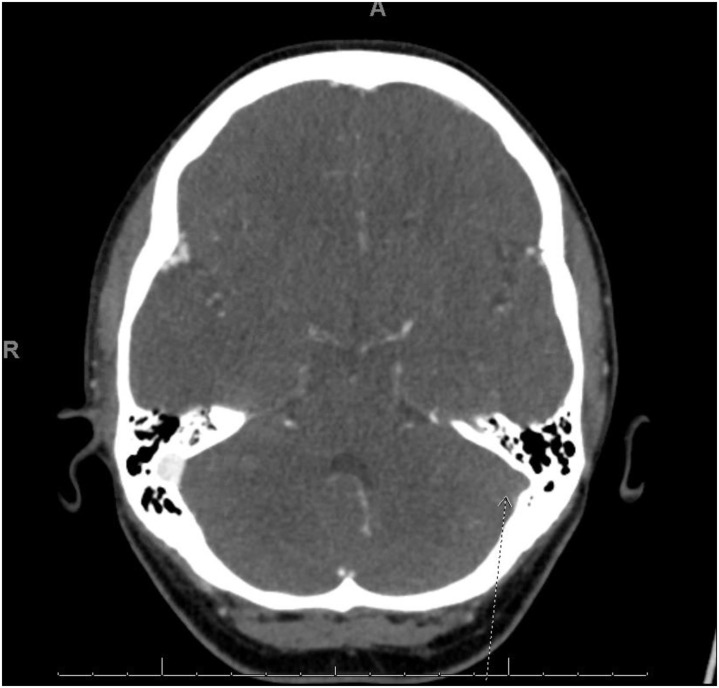
Computed tomography showed cerebral venous sinus thrombosis (Figure ) involving the left transverse and sigmoid sinuses, extending into the left internal jugular vein, with acute subsegmental pulmonary emboli. A lower extremity ultrasound revealed no deep venous thrombosis, and a right upper quadrant ultrasound confirmed no portal vein thrombosis.
Figure.
Computed tomography venogram showing cerebral venous thrombosis of the sigmoid sinus extending into the jugular vein. The arrow shows the lack of enhancement of the left sigmoid sinus.
The patient was started on bivalirudin,4, 5, 6 a direct thrombin inhibitor with a short half-life. Based on the patient’s normal renal function, bivalirudin infusion was started at 0.15 mg/kg per hour with titration parameters to target an activated partial thromboplastin time goal of 50-80 s. Additional pharmacologic interventions to aid in platelet recovery included intravenous immune globulin 1 g/kg per day for 2 days and prednisone 1 mg/kg daily, per the recommendations of Greinacher et al.4
The patient demonstrated a steady improvement in laboratory markers of thrombocytopenia (Table 2 ) until her discharge on hospital day 6. This trend continued on follow-up laboratory evaluation 3 days later. While the patient exhibited a transient drop in hemoglobin, there was no clinical evidence of bleeding. Mild transaminitis was present at the time of admission but investigation for causes such as autoimmune hepatitis did not reveal an etiology. This patient did not demonstrate any sequelae of thrombosis. At follow-up, the patient reported resolution of her headache.
Table 2.
Results of laboratory evaluation.∗
| Test | Hospital Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 9 |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin (12.1-16.3 g/dL) | 15.1 | 14.5-13.2 | 12.3 | 12.4 | 13.2 | 12.9 | 13.3 | 14.5 |
| Hematocrit (35.7%-46.7%) | 42.4 | 37.0-40.8 | 35.9 | 36 | 37.1 | 36.4 | 38.1 | 43.7 |
| Platelet count (150-400 x 109/L) | 20 | 49-52 | 64 | 68 | 83 | 115 | 115 | 182 |
| D-Dimer (<500 fibrinogen equivalent units) | 27,150-45,570 | 27,800 | 13,750 | 2,880 | 2,680 | 2,540 | ||
| Fibrinogen (150-400 mg/dL) | 149 | 195 | ||||||
| Activated partial thromboplastin time (23.8-36.2 sec) | 26.4-52.2 | 43.5-50.2 | 40.3-50.4 | 49.4-51.7 | 24.8-53.1 | 56.6 | ||
| Alkaline phosphatase (39-117 U/L) | 25 | 29 | 40 | 29 | 42 | 33 | 35 | 43 |
| Alanine aminotransferase (7-52 units/L) | 87 | 119 | 108 | 167 | 171 | 159 | 141 | 93 |
| Aspartate aminotransferase (12-39 units/L) | 65 | 78 | 66 | 101 | 83 | 64 | 49 | 28 |
| Total bilirubin (0.1-1.3 mg/dL) | 0.9 | 0.7 | 0.5 | 0.6 | 0.5 | 0.5 | 0.5 | 0.6 |
The reference range is provided in parentheses and the abnormal values are in bold.
Discussion
There have now been multiple cases of thrombotic thrombocytopenia 1-2 weeks after Ad26.COV2.S vaccination.3 , 7 Vaccine-induced thrombotic thrombocytopenia was reported in patients who received ChAdOx1 nCoV-19 vaccination and was found to be mediated by platelet-activating antibodies against PF4.4 Nine of the 11 patients described therein had cerebral venous sinus thrombosis.
The pattern of symptoms now labeled vaccine-induced thrombotic thrombocytopenia has unclear pathogenesis, though several findings have been consistent across cases. Antibodies to PF4-polyanion complexes, despite the absence of heparin, have been found by ELISA. This pattern of antibodies in the absence of prior heparin exposure has been previously described as autoimmune HIT.8 The pathophysiology of autoimmune HIT is not well understood, and this case may serve as a resource for further investigation.
Central venous sinus thrombosis is rare in ED presentations for headache9; high clinical suspicion for patients with prothrombotic risk factors must be maintained to make this diagnosis. This report and the CDC health alert3 suggest that recent Ad26.COV2.S vaccination may be considered a prothrombotic risk factor and a trigger to pursue further diagnostic testing. HIT testing that does not use ELISA technology may result in false-negative results, as in this case.4
The CDC guidance recommended treatment with heparin alternatives; however, there was no recommended alternative in the release. Bivalirudin, argatroban, fondaparinux, and rivaroxaban were described by Greinacher et al4 as potential treatment options for vaccine-induced thrombotic thrombocytopenia following the ChAdOx1 nCoV-19 vaccine. In the setting of significant thrombocytopenia and pending hepatic function tests, we selected bivalirudin for its immediate onset of action, renal elimination, short half-life (∼25 min), and ease of reversibility in the event life-threatening bleeding occurred. As both vaccine-induced thrombotic thrombocytopenia and the recommendation to avoid heparin in such cases are novel, we cannot comment on the expected outcomes or how treatment with bivalirudin would compare to heparin. However, we are pleased to report that this patient is recovering well.
In conclusion, Ad26.COV2.S vaccination may trigger vaccine-induced thrombotic thrombocytopenia up to 2 weeks after vaccination in a small subset of the population, particularly in women aged 20-50 years. Clinicians should maintain a high degree of suspicion in individuals presenting with headache or other symptoms suggestive of thrombotic events after Ad26.COV2.S vaccination. A screening complete blood count should be considered in such patients, and unexplained thrombocytopenia could direct further investigation into thrombotic pathologies. Typical findings associated with thrombosis may be absent; providers should have a low threshold to acquire advanced imaging. Due to variable sensitivity and antigen targets HIT testing may result in false negatives; in cases with high suspicion for vaccine-induced thrombotic thrombocytopenia, PF4 ELISA-based HIT testing should be pursued. Following the CDC guidance to avoid heparin products in patients with suspected vaccine-induced thrombotic thrombocytopenia is associated with a positive outcome in this case. Other providers may consider bivalirudin as a heparin alternative in similar cases. As Ad26.COV2.S and ChAdOx1 nCoV-19 vaccinations are now ubiquitous worldwide, providers everywhere must seek early recognition and treatment of this rare condition. While pursuing further investigation of the best treatment for these rare vaccination side effects, it is important that the medical community and public health officials continue to support ongoing vaccination strategies to control the COVID-19 pandemic.
Footnotes
Supervising Editor: Gregory J. Moran, MD. Specific detailed information about possible conflict of interest for individual editors is available at https://www.annemergmed.com/editors.
All authors attest to meeting the four ICMJE.org authorship criteria: (1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Fundingandsupport: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
References
- 1.Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a Phase 1-2a trial of Ad26.COV2.S COVID-19 vaccine. N Engl J Med. Published online January 13, 2021. https://doi.org/10.1056/NEJMoa2034201 [DOI] [PMC free article] [PubMed]
- 2.Oliver S.E., Gargano J.W., Scobie H., et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:329–332. doi: 10.15585/mmwr.mm7009e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marks P., Schuchat A. Joint CDC and FDA statement on Johnson & Johnson COVID-19 vaccine. https://www.fda.gov/news-events/press-announcements/joint-cdc-and-fda-statement-johnson-johnson-covid-19-vaccine
- 4.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. Published online April 9, 2021. https://doi.org/10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed]
- 5.Vo Q.A., Lin J.K., Tong L.M. Efficacy and safety of argatroban and bivalirudine in patients with suspected heparin-induced thrombocytopenia. Ann Pharmacother. 2015;49:178–184. doi: 10.1177/1060028014562949. [DOI] [PubMed] [Google Scholar]
- 6.Kiser T.H., Burch J.C., Klem P.M. Safety, efficacy, and dosing requirements of bivalirudin in patients with heparin-induced thrombocytopenia. Pharmacotherapy. 2008;28:1115–1124. doi: 10.1592/phco.28.9.1115. [DOI] [PubMed] [Google Scholar]
- 7.Muir K, Kallam A, Koepsell S, et al. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. Published online April 14, 2021. https://doi.org/10.1056/NEJMc2106075 [DOI] [PMC free article] [PubMed]
- 8.Greinacher A., Selleng K., Warkentin T.E. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15:2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 9.Fischer C., Goldstein J., Edlow J. Cerebral venous sinus thrombosis in the emergency department: retrospective analysis of 17 cases and review of the literature. J Emerg Med. 2010;38:140–147. doi: 10.1016/j.jemermed.2009.08.061. [DOI] [PubMed] [Google Scholar]