Abstract
Lactic acid bacteria (LAB) are used as a probiotic alternative to antibiotics in livestock production. Microencapsulation technology is widely used for probiotic preservation. A variety of microencapsulation protocols have been proposed and compared based on chemicals and mechanical procedures. This study aimed to develop a double-encapsulated coating from alginate (1.5%) and chitosan (0.5%) by extrusion, emulsion, and spray drying methods using the LAB strains Lactobacillus plantarum strains 31F, 25F, 22F, Pediococcus pentosaceus 77F, and P. acidilactici 72N, and to monitor the basic probiotic properties of the encapsulated prototypes. The final products from each microencapsulation protocol were analysed for their appearance, probiotic properties and viable cell count. Using the spray drying method, particles smaller than 15 μm in diameter with a regular spherical shape were obtained, whereas the other methods produced larger (1.4–52 mm) and irregularly shaped microcapsules. After storage for 6 months at room temperature, the LAB viability of the spray-dried particles was the highest among the three methods. In all the LAB strains examined, the encapsulated LAB retained their probiotic properties in relation to acid-bile tolerance and antibacterial activity. This study highlights the efficacy of double-coating microencapsulation for preserving LAB properties and survival rate, and demonstrates its potential for probiotic application in livestock farms.
Subject terms: Biotechnology, Microbiology
Introduction
Probiotic bacteria are well-defined live micro-organisms which, when consumed in sufficient numbers, confer a beneficial health effect to the host’s gastrointestinal tract (GIT)1. In order to provide these benefits, probiotic bacteria must be presented at a minimum level of 106 to 107 colony forming units (CFU) per g or mL when they reach the gut1–4. Several stress factors during manufacturing, storage, and GIT transit are detrimental to the viability of probiotic bacteria5–7.
Microencapsulation coating of core material into capsules in the size range of micrometers up to millimeters is the most prominent technique to protect probiotic bacteria under adverse conditions and improve their survival at the final destination8–11. Alginate has often been used in microencapsulation procedures because it is simple to manipulate, compatible with almost all encapsulation methods, low-cost, and is a non-toxic material12,13. However, alginate microcapsules readily disintegrate in highly acidic environments, as occurs in the stomach14–16. To decrease these drawbacks, good biofilm-forming materials, such as chitosan, have been chosen for coating alginate beads to enhance their protection15,17,18. Various methods have been widely used for probiotic cell encapsulation, including extrusion, emulsion, and spray drying12,19. Production of stable microparticles of a low diameter and homogeneous size distribution is an advantage of the spray drying method compared to the extrusion or emulsion methods. Nonetheless, this method can reduce the cell vitality due to dehydration and thermal inactivation of the probiotic cells during the process12,20–22. There has been relatively little comparison of the efficiency and viability of these different encapsulation methods for probiotic cells. Moreover, there is no evidence to confirm the persistence of probiotic properties post-encapsulation.
This study aimed to evaluate the efficacies of three microencapsulation methods (extrusion, emulsion, and spray drying) on the viability, thermotolerance, encapsulation yield (EY) percentage, and probiotic properties (acid-bile tolerance and antimicrobial activity) of LAB strains double-coated with alginate-chitosan.
Results
Size and morphology of microcapsules
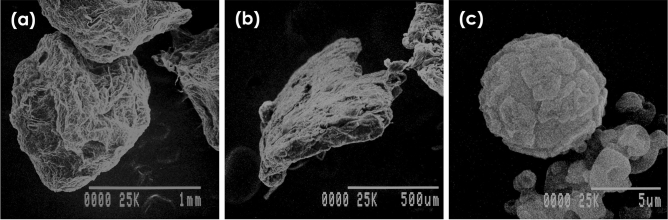
From the SEM analysis, the size of the encapsulated products among the three methods were significantly (P < 0.05) different, and their shapes varied, but within each method they were uniform (Table 1 and Fig. 1). The microcapsules obtained by the extrusion method were droplet-like particles with a mean diameter of approximately 1.5 mm. Those formed by emulsion were tiny flake shaped (0.5 mm in diameter), whereas those from the spray drying method were spherical powdery particles with a mean diameter of less than 15 μm. For each method, there was no difference in the size (Table 1) or morphology (not shown) of the particles between the five LAB strains.
Table 1.
Size and EY of the microcapsules. The different lowercase letters within each column indicate significant differences between methods (P < 0.05).
| Method | Size of microcapsule (μm) | EY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L22F | L25F | L31F | P77F | P72N | L22F | L25F | L31F | P77F | P72N | |
| Extrusion | 1516 ± 304.86c | 1469 ± 273.51c | 1429 ± 412.11c | 1504 ± 282.42c | 1443 ± 370.89c | 93.52 ± 0.11b | 93.49 ± 0.34b | 93.22 ± 0.17b | 93.11 ± 0.08b | 93.39 ± 0.32b |
| Emulsion | 482.11 ± 58.59b | 497.11 ± 67.51b | 527.75 ± 57.69b | 495.75 ± 70.04b | 524.38 ± 67.56b | 94.10 ± 0.10b | 93.55 ± 0.21b | 93.27 ± 0.63b | 92.66 ± 0.05b | 93.83 ± 0.21b |
| Spray dry | 12.74 ± 1.710a | 13.10 ± 1.83a | 12.90 ± 2.02a | 12.58 ± 1.74a | 13.10 ± 3.35a | 74.56 ± 0.11a | 74.50 ± 0.02a | 74.67 ± 0.27a | 73.64 ± 0.09a | 75.03 ± 0.05a |
| P-value | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
Figure 1.
Representative SEM images of alginate-chitosan microcapsules of L22F (as a representative example of the five LAB strains) prepared by the (a) extrusion, (b) emulsion, and (c) spray drying methods.
Efficacy of microencapsulation
Encapsulation yield (EY)
The EY rates were found to range from 73.64 to 94.10% (Table 1). The EY was higher in the extrusion and emulsion methods (at 93% approximately) than in the spray drying method (P < 0.05), but there was no significant difference between different LAB strains.
Viability test
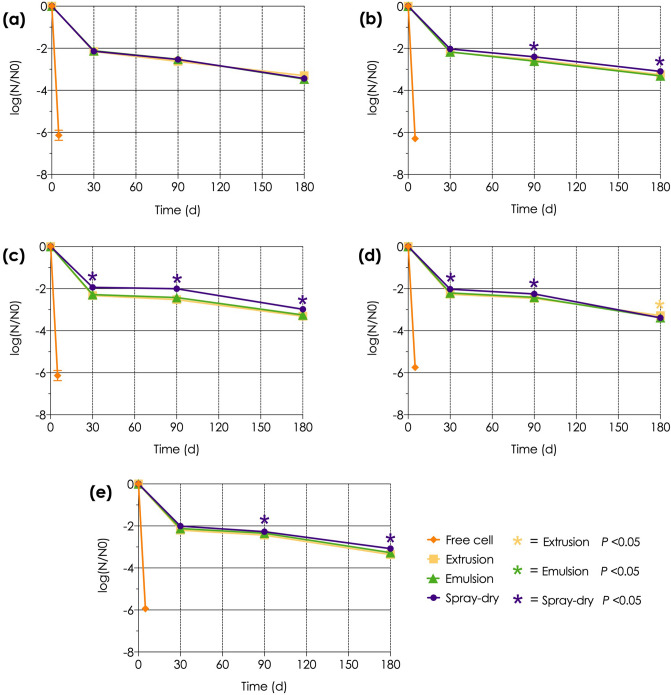
The viability of the microencapsulated LAB obtained from all three methods were significantly (P < 0.05) different from the free cells (Fig. 2). The alginate-chitosan coated LAB showed a greater viable cell count during the 6-months storage at room temperature (25–28 °C) than the others. For all three microencapsulated methods, the number of viable cells was reduced by about 2.0 log CFU/g after 1-month storage but by less than 3.5 log CFU/g after 6 months. Meanwhile, the free LAB cells could not be grown after storage for only 3 days.
Figure 2.
Comparison of the survival level between the non-capsulated and differently encapsulated LAB (a) L22F, (b) L25F (c) L31F, (d) P77F and (e) P72N strains over 6 months storage at room temperature. The asterisks represent statistically significant differences (P < 0.05). The yellow, green, and purple asterisks represent the significance of differences for the extrusion, emulsion, and spray-drying methods, respectively.
Thermotolerance test
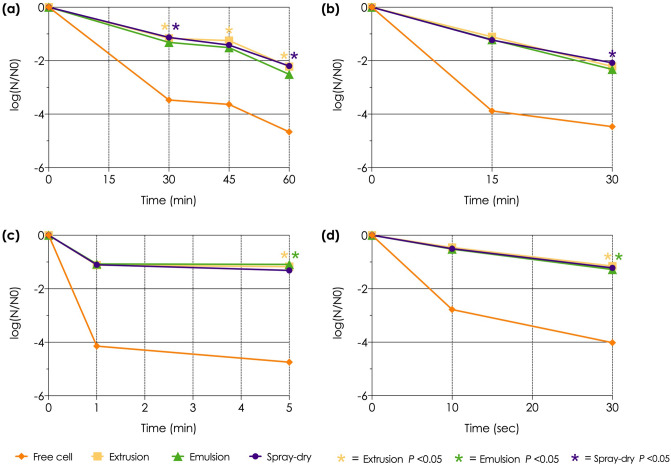
LAB microencapsulated by each of the three methods tolerated high-temperature treatment significantly (P < 0.05) better than did the free cells (Fig. 3). For all three microencapsulation methods, the number of viable cells was reduced by approximately 2.5 log CFU/g after 60 °C for 60 min and 70 °C for 30 min, compared to about 1.5 log CFU/g after 80 °C for 5 min and 100 °C for 30 s.
Figure 3.
Comparison of survival between the non-capsulated and differently encapsulated L22F strains after heat treatment at (a) 60 °C, (b) 70 °C (c) 80 °C, and (d) 100 °C. The spray drying procedures were performed under an inlet temperature of 130 °C. The asterisks represent statistically significant differences (P < 0.05). The yellow, green, and purple asterisks represent the significance of the extrusion, emulsion, and spray-drying methods, respectively.
Confirmation of probiotic properties after encapsulation
Acid and bile tolerance test
After 6 months storage, all five tested strains of LAB encapsulated by the three different methods still showed a similar tolerance (viability) to acid and bile adjusted MRS broth compared to the fresh cultures of LAB strains (Table 2).
Table 2.
Viable colony count of live-cultures and 6-month stored encapsulated LAB after acid-bile incubation. Colony counts ≥ 104 CFU/mL indicate tolerance to acid and bile.
| Method | Colony count | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCl adjusted MRS | Oxgall adjusted MRS | |||||||||
| L22F | L25F | L31F | P77F | P72N | L22F | L25F | L31F | P77F | P72N | |
| Live culture | 1.10 × 105 ± 1.41 | 0.43 × 105 ± 1.26 | 0.98 × 105 ± 1.71 | 0.17 × 105 ± 0.42 | 2.08 × 105 ± 1.26 | 1.33 × 104 ± 4.11 | 1.25 × 104 ± 1.30 | 1.48 × 104 ± 3.60 | 1.08 × 104 ± 2.50 | 2.28 × 104 ± 3.95 |
| Extrusion | 1.10 × 105 ± 0.81 | 0.48 × 105 ± 2.36 | 0.80 × 105 ± 2.58 | 0.17 × 105 ± 0.38 | 1.88 × 105 ± 1.71 | 1.23 × 104 ± 3.70 | 1.10 × 104 ± 3.56 | 1.50 × 104 ± 5.66 | 1.09 × 104 ± 3.11 | 2.13 × 104 ± 1.71 |
| Emulsion | 1.13 × 105 ± 1.70 | 0.77 × 105 ± 0.50 | 0.88 × 105 ± 1.71 | 0.17 × 105 ± 0.10 | 2.08 × 105 ± 1.26 | 1.40 × 104 ± 4.16 | 1.23 × 104 ± 3.40 | 1.60 × 104 ± 2.16 | 1.03 × 104 ± 2.22 | 2.25 × 104 ± 1.30 |
| Spray dry | 1.03 × 105 ± 1.50 | 0.23 × 105 ± 2.31 | 0.95 × 105 ± 1.73 | 0.15 × 105 ± 0.13 | 1.93 × 105 ± 2.1 | 1.00 × 104 ± 0.82 | 1.10 × 104 ± 1.83 | 1.70 × 104 ± 1.83 | 1.05 × 104 ± 4.04 | 2.43 × 104 ± 2.62 |
Antibacterial effect
After 6 months of storage, the encapsulated LAB still retained an antibacterial ability against pathogenic bacteria that ranged from an intermediate to a strong inhibition level relative to the live culture form (Table 3). However, LAB strains L31F and L22F showed a weaker inhibition to ETEC and EHEC after 180 days of storage when the microcapsules were formed by the emulsion or extrusion methods.
Table 3.
Antibacterial activity of the cell free supernatant (CFS)* of live-cultured and 6-month stored encapsulated LAB. *Non-neutralized CFS; The diameters of the inhibition zones were measured and interpreted as (+) weak inhibition (6–9 mm), (++) intermediate inhibition (10–13 mm), (+++) strong inhibition (14–16 mm) and (++++) very strong inhibition (> 17 mm).
| Method | ETEC | EHEC | Salmonella choleraesuis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L22F | L25F | L31F | P77F | P72N | L22F | L25F | L31F | P77F | P72N | L22F | L25F | L31F | P77F | P72N | |
| Live culture | +++ | ++ | +++ | ++ | ++ | +++ | ++ | +++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Extrusion | +++ | ++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Emulsion | ++ | ++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ | ++ | +++ | +++ | +++ | +++ | +++ |
| Spray dry | +++ | ++ | +++ | ++ | ++ | +++ | ++ | +++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
Discussion
In this study, the double-coated bead size formed by extrusion and spray drying resembled those reported previously14,23, whereas those formed by the emulsion method were larger than previously reported24,25. In previous studies, extrusion beads over 100 μm in size did not enhance nutrient availability or reduce the palatability of the consolidated food product26,27. From size alone, the alginate-chitosan powder obtained from the spray drying method would be the most suitable to apply for delivery to hosts via feed or water. Thus, encapsulation by spray drying could transform free LAB cells into a usable powdery form that could be applicable for combining in a livestock meal.
The lower EY rate obtained by spray drying could be of concern in a scaled-up process, but is still in the acceptable range and consistent with that previously reported28. The high EY rates of 97.4–99.9% that were obtained with the extrusion or emulsion methods were concordant with previous studies14,24,25. The higher EY obtained by the extrusion and emulsion methods were due to the use of a lower temperature and less osmotic stress in the process compared to spray drying6,20,29. To improve the EY in the process, it has been suggested that increasing the number of live probiotic cells, supplementing them with certain protective agents (such as maltodextrin, inulin, reconstituted skimmed milk, or low melting point fat), or reducing the inlet temperature during the drying step could be used20,30.
Although the results of this study were comparable to findings with emulsion-encapsulation of Bifidobacterium animalis BB-12 during storage for 90 days31, they contrasted with those of another study where the viability of microencapsulated L. lactis formed by extrusion showed a reduction in viability of up to 5 log CFU/g after only 30 days in storage32. Single alginate coated probiotic bacteria are fragile and tend to lose viability more than double-coated ones17. Therefore, the double coating of alginate beads with chitosan, as used in this study, could be recommended to protect the viability of LAB for at least 6 months at room temperature. In contrast, the viability of L. acidophilus La-5 and L. rhamnosus B442 have been reported to decrease by only 0.43–0.62 log CFU/g after storage for 32–90 days when prepared by the spray dry method26,28. However, those studies maintained the moisture content of the spray-dried products at 1.8–4.79% during storage, whereas in the current study the products were not subjected to moisture control: this might have impacted on the viable cell count since the survival of encapsulated probiotics after spray drying is related to the water content33,34. To promote LAB viability, the water content should be reduced, and the storage temperature kept at 4 °C35–37. Nevertheless, after six months of storage, the LAB double-coated by the spray drying method in the current study exhibited a better (lower) logarithmic reduction in the viable cell count than those formed by the extrusion or emulsion methods for all five tested LAB strains. In contrast, in another study the viability of L. casei encapsulated with alginate-whey protein by the emulsion method was reported to be higher by 0.8 log CFU/g than with the spray dry method coating with alginate-chitosan after three months of storage at 4 °C22. These differences may be due to the different properties of the encapsulating materials applied. In contrast to the stability of the viable cell count at room temperature over 180 days of storage found in the current study, the loss in cell viability during the initial spray dry method was clear, and in accord with previous studies that found that spray-dried products can be stored for prolonged periods20,34,38.
The reductions in the microencapsulated LAB count after heat treatment (60 °C for 60 min, 70 °C for 30 min, 80 °C for 5 min, and 100 °C for 30 s) were less than 2.5 log CFU/g. In contrast, it has been previously reported that the viability of L. rhamnosus GG double-coated with alginate-chitosan by the external gelation method was reduced by 5.9, 7.4, 6.5 and 3.3 log CFU/mL after incubating at 60 °C for 30 min, 70 °C for 30 min, 80 °C for 1 min, or 100 °C briefly, respectively39. Therefore, it seems probable that the extrusion, emulsion, and spray drying methods used in the current study gave a better thermos-resistance performance (higher LAB cell viability) than would have been achieved with the alternative external gelation method. Furthermore, at 60 and 70 °C, the L22F strain that was double-coated by the emulsion or spray drying methods showed a higher viability than was achieved with the extrusion method. In contrast, the extrusion and emulsion double-coated L22F cells exhibited a higher viability after the 80 and 100 °C treatments than did the spray-dried L22F. Interestingly, previous studies showed a rather dissimilar trend. L. casei NCDC-298 encapsulated with alginate by the emulsion method at 60 °C for 20 min was reported to have a reduction in cell viability of 2–3 log CFU/g40, which was lower than with chitosan-coated alginate-starch LAB formed by the extrusion method that had a 1.42 log CFU/mL reduction after heat treatment at 60 °C for 30 min41. The results of the current study suggest that the methods used might be useful for industrial processing, such as feed pellet production that requires at least 80 °C for 17 s to form pellets. In addition, the encapsulation could prolong the viability of LAB by protecting from temperature fluctuations during storage in livestock farms.
Under long-term storage, LAB-containing microcapsules might be exposed to various stresses, such as starvation, heat, osmosis, or oxidation. Such stresses cause LAB to shift their physiological state by reducing the synthesis of DNA and proteins, and finally they may lose some functionality42,43. In this study, all five LAB strains still maintained their acid and bile tolerance, although the degree of such was specific to each LAB strain43.
Previously, the antibacterial properties of P. acidilactici UL5, L. reuteri, and L. salivarius were found to decline after encapsulation and prolonged storage44. This may be explained by metabolic changes following carbohydrate starvation that reduced synthesis of metabolic end products42,43. Moreover, active antimicrobial substances (bacteriocins or antimicrobial peptides) secreted by LAB strains may remain trapped inside microcapsules, which could reduce the amount of these active substances in cell free supernatants (CFS) and lower the apparent antibacterial effect. The results of this study indicated that the emulsion and extrusion methods slightly reduced the antibacterial ability of LAB, although this depended on the LAB strain and the target pathogenic bacteria. Nevertheless, all three encapsulation methods still showed a satisfactory outcome in preserving antibacterial activity, which might be applicable for industrial processes and livestock farm usage.
Conclusion
For alginate-chitosan double-coated LAB, this study revealed that three methods of encapsulation (extrusion, emulsion, and spray drying) could provide a high final bacterial concentration. The spray dry method gave smaller diameter sized particles as an easily handled powder for mixing with feed product. The alginate-chitosan beads from all three methods significantly improved the survival of LAB over 6-month storage at room temperature and after high-temperature treatment. Moreover, the six-month stored encapsulated LAB still retained their probiotic properties of acid-bile tolerance and antibacterial ability. Therefore, the use of microencapsulation by the spray drying method is strongly recommended for probiotic preparation for livestock farms.
Materials and methods
Bacterial strains and culture condition
The five strains of LAB used in this study included Lactobacillus plantarum 31F (L31F), L. plantarum 25F (L25F), L. plantarum 22F (L22F), Pediococcus pentosaceus 77F (P77F), and Pediococcus acidilactici 72N (P72N), and were selected since they have been reported to have a high potential as swine probiotic strains45–47. The strains were maintained in de Man Rogosa Sharpe (MRS) broth (Becton, Dickinson and Company, Maryland, USA) containing 20% (v/v) glycerol at − 80 °C. Frozen stocks were cultured in MRS broth and incubated at 37 °C for 18–20 h. Each LAB strain was harvested by centrifugation at 3000g for 10 min at 4 °C and then washed twice in 0.85% (w/v) saline solution. The cell pellets were resuspended in normal saline and prepared at a final concentration of 109 CFU/mL48. The cell suspension of each LAB strain was divided into four groups; free cells (control) and three methods of microencapsulation.
Microencapsulation and coating procedures
Internal coating step
A 1.5% (w/v) alginate solution (Sigma-Aldrich, Missouri, USA) was initially prepared for inner encapsulation. Initially, 9 log CFU/mL of the respective LAB strain was mixed with 20 mL of alginate solution at a 1:5 (v/v) ratio and was then ready for the further microencapsulation step14. For the extrusion method, the alginate mixtures were added dropwise through a 3 mL-syringe into 100 mL of CaCl2 (1 mol/L) (Merck KGaA, Darmstadt, Germany) and left for 30 min for gelation to achieve the alginate beads39,49. For the emulsion method, the alginate beads were settled by adding the alginate mixtures to 100 mL of soybean oil (Sigma-Aldrich, Missouri, USA) containing 0.2% (v/v) of Tween 80 while stirring with a magnetic stirrer. Next, 100 mL of CaCl2 (1 mol/L) was added into the mixture to solidify the alginate beads, which were then harvested by centrifugation at 350g for 10 min at 4 °C40. Alginate beads obtained from the extrusion and emulsion methods were rinsed with and then kept in 0.1% (w/v) peptone water (Becton, Dickinson and Company, Maryland, USA) at 4 °C14. For the spray drying method, alginate mixtures were atomized through the spray dryer (Mini Spray Dryer B-290, Buchi, Flawil, Switzerland) with an inlet temperature of 130 °C, and the alginate beads were then collected from the collecting vessel23.
External coating step
A 0.5% (w/v) chitosan solution (Union Chemical 1986, Bangkok, Thailand) in 100 mL of 1% (v/v) acetic acid (Merck KGaA, Darmstadt, Germany) was prepared for the outer microcapsule coating, and the solution was filtered through a nylon cloth to separate the insoluble material17. The alginate beads obtained from the extrusion and emulsion methods were immersed into the chitosan solution and shaken at 100 rpm for 40 min. The obtained alginate-chitosan coated beads were then washed with and stored in 0.1% (w/v) peptone water at room temperature14,49. Meanwhile, 1 g of the beads obtained from the spray drying method was added to 100 mL of the above 0.5% (w/v) chitosan solution and then atomized through a spray dryer. The alginate-chitosan double-coated beads were then collected from the collecting vessel and kept at room temperature23.
Size and morphology of the microcapsules
The size and morphology of the obtained probiotic microcapsules were examined using light microscopy and scanning electron microscopy (SEM). The capsules were placed on a specimen aluminum stub with the help of double-sided sticky tape and coated in a sputter coater for 2 min at an accelerating voltage of 15 kV24,25.
Efficacy evaluation of the microencapsulations
The EY
Calculation of the EY percentage was modified from that previously reported28 as follows: Eq. (1), where N0 and N represented the number of viable CFUs before and after the encapsulation process, respectively.
| 1 |
Viability test
The obtained microencapsulated products from the three methods, along with free cells, were stored at room temperature for up to 6 months. Evaluation of the LAB viability was performed at 0, 30, 90, and 180 days after encapsulation. For decapsulation, 1 g of the respective alginate-chitosan coated bead preparation obtained from the extrusion or emulsion method was liquefied in 9 mL sodium citrate (0.1 mol/L, pH 6.0) (Merck KGaA, Darmstadt, Germany) and stirred for 10 min8. Meanwhile, 1 g of the obtained spray-dried beads and 1 mL of free cells were suspended in 9 mL of 0.1% (w/v) peptone water23. The liquefied suspensions were decimally diluted in 0.1% (w/v) peptone water and plated on MRS agar by the drop plate method. Viable cells were enumerated as the number of colonies after incubation at 37 °C for 48 h and converted to CFU/mL or g50. The reduction in viability was characterized in terms of the log (N/N0), where N0 and N represented the initial and actual viable CFUs, respectively39.
Thermotolerance test
For the thermal-tolerance evaluation, 1 mL of preheated 0.1% (w/v) peptone water at 60, 70, 80, and 100 °C was mixed with 1 g of alginate-chitosan coated beads or 1 mL of free cells, and then incubated at that temperature set for various times as follows: 60 °C for 30, 45, and 60 min; 70 °C for 15 and 30 min; 80 °C for 1 and 5 min; and 100 °C for 10 and 30 s. The double-coated beads were decapsulated, and their cell viability was determined as described previously8,23,39,50.
Confirmation of probiotic properties after encapsulation
Acid and bile tolerance test
The probiotic properties were determined as previously reported46,51. After six months storage, 1 g of alginate-chitosan coated beads obtained from the extrusion, emulsion, or spray drying methods were decapsulated as described previously8,23. Then 10 µL of the liquefied suspension was cultivated on MRS agar at 37 °C for 18–24 h. Fresh LAB strains were used as control (100% viability). A full loop of LAB cells was inoculated into the MRS broth and incubated at 37 °C for 24 h. The overnight cultures of microencapsulated products and fresh cells were harvested by centrifugation at 10,000g for 10 min at 4 °C.
For the acid tolerance test, overnight cultures at approximately 1 × 108 CFU/mL were re-suspended in 1 equivalent/L MRS broth adjusted to pH 2.0 with HCl (Carlo Erba Reagents, Val de Reuil, France). For the bile tolerance determination, 1 × 108 CFU/mL of the cultures were inoculated in 0.3% (w/v) Oxgall powder (Sigma-Aldrich, Missouri, USA) supplemented MRS broth (pH 6.5). After incubation at 37 °C for 12 h, viable bacterial counts were measured using the spread plate method. The culture suspension was diluted serially and plated on MRS agar, then enumerated after incubation at 37 °C for 48 h. Microencapsulated products with ≥ 104 CFU/mL indicated the persistency of acid-bile tolerance.
Antibacterial effect
Evaluation of the antibacterial activity was performed using the agar well diffusion assay as reported45,52. In brief, 1 g of 6-month stored alginate-chitosan coated beads prepared by the three methods were decapsulated and prepared as an overnight culture as described in the previous section “Acid and bile tolerance test”. Likewise, acid and bile tolerance tests were also performed from frozen stocks (− 80 °C) of the respective free cell LAB. Each overnight culture was centrifuged at 7000g for 5 min at 4 °C, and the supernatant was harvested and filtered through a sterile filter (0.22 μm pore-size) (Corning, New York, USA) to achieve a cell free supernatant (CFS).
Three strains of indicator pathogens, comprised of enterotoxigenic E. coli (ETEC) VP10Ltb + , enterohaemorrhagic E. coli (EHEC) T2R2-26-HB2, and Salmonella choleraesuis 86-1, were grown at 37 °C overnight on Luria–Bertani (LB) broth (Becton, Dickinson and Company, Maryland, USA) at 108 CFU/mL. Two mL of each indicator pathogen were thoroughly mixed with 18 mL of LB agar [1.2% (w/v), 45 °C] and poured into a Petri-dish. The agar was left for 30 min, and then 6-mm diameter wells were punched with a sterile tip. Thereafter 50 µL of the respective CFS was added into each well. In addition, each CFS was also adjusted to pH 7.0 with 4 mol/L NaOH (pH 7.0) (Carlo Erba Reagents, Val de Reuil, France) and likewise assayed, to rule out an acidic effect45. The plates were incubated at 37 °C for 24–48 h, and the antibacterial activity was recorded as the growth-free inhibition zone around each well. Inhibition zones were measured from the edge of each well. The CFS from the free cell LAB and MRS medium without an inoculating strain were used as positive and negative controls, respectively.
Data analysis
Numerical data were presented as the mean ± standard deviation (SD) of replicated determinations. The average microcapsule diameter, EY percentage, log reduction of viability, and viable bacterial cell count among the three different methods (extrusion, emulsion, and spray drying) were compared. All of the experiments were performed in triplicate. Those parameters were analyzed by one-way ANOVA, and comparison of means were tested by Tukey’s multiple range tests using the SPSS version 22.0 software (IBM, NY, USA). The effects were considered to be significant at P < 0.05.
Acknowledgements
We are gratefully thankful to the Department of Chemistry, Faculty of Science, Chulalongkorn University for providing basic knowledge, encapsulation material, and research facilities. This study was supported by grants from Research and Researchers for Industries (PHD58I0078), Agricultural Research Development Agency (Public Organization (CRP5905021240 and CRP6205031210), the 90th Anniversary of Chulalongkorn University Scholarship (Ratchadaphiseksomphot Endowment Fund), the CHE-TRF Senior Research Fund (RTA6280013) and Pathogen Bank, Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand. We finally thank Professor David J. Hampson of Murdoch University, Australia, for kindly providing editorial assistance and advice during the preparation of this manuscript.
Author contributions
P.P. and N.P.* conceived and designed the experiments. All of the experimental designs were revised by N.P. and N.M. P.P. and P.A. performed the experiments. The data were analyzed and interpreted by P.P., P.A., W.S. and N.P.* P.P. wrote a draft of the manuscript, A.S. and N.P.* critically revised the drafted manuscript. All authors reviewed the manuscript and read and approved the final manuscript. *Indicated the Corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAO/WHO. Guidelines for the evaluation of probiotics in food: report of a Joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. Food and Agriculture Organization of the United Nations/World Health Organization, London, Ontario. https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (2002).
- 2.Ishibashi N, Shimamura S. Bifidobacteria: Research and development in Japan. Food Technol. 1993;46:126–136. [Google Scholar]
- 3.Lee YK, Salminen S. The coming of age of probiotics. Trend Food Sci. Technol. 1995;6:241–245. doi: 10.1016/S0924-2244(00)89085-8. [DOI] [Google Scholar]
- 4.Robinson RK. Survival of Lactobacillus acidophilus in fermented products. Suid Afrikaanse Tydskrif vir Suiwelkunde. 1987;19:25–27. [Google Scholar]
- 5.de Lange CFM, Pluske J, Gong J, Nyachoti CM. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 2010;134:124–134. doi: 10.1016/j.livsci.2010.06.117. [DOI] [Google Scholar]
- 6.de Vos P, Faas MM, Spasojevic M, Sikkema J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010;20:292–302. doi: 10.1016/j.idairyj.2009.11.008. [DOI] [Google Scholar]
- 7.Kechagia M, et al. Health benefits of probiotics: A review. ISRN Nutr. 2013;2013:1–7. doi: 10.5402/2013/481651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guimarães RR, Vendramini ALDA, Santos ACD, Leite SGF, Miguel MAL. Development of probiotic beads similar to fish eggs. J. Funct. Food. 2013;5:968–973. doi: 10.1016/j.jff.2013.01.002. [DOI] [Google Scholar]
- 9.Kailasapathy K. Microencapsulation of probiotic bacteria: Technology and potential applications. Curr. Issues Intest. Microbiol. 2002;3:39–48. [PubMed] [Google Scholar]
- 10.Krasaekoopt W, Bhandari B, Deeth H. Evaluation of encapsulation techniques of probiotics for yoghurt. Inter Dairy J. 2003;13:3–13. doi: 10.1016/S0958-6946(02)00155-3. [DOI] [Google Scholar]
- 11.Muthukumarasamy P, Allan-Wojtas P, Holley RA. stability of Lactobacillus reuteri in different types of microcapsules. J. Food Sci. 2006;71:M20–M24. doi: 10.1111/j.1365-2621.2006.tb12395.x. [DOI] [Google Scholar]
- 12.Burgain J, Gaiani C, Linder M, Scher J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011;104:467–483. doi: 10.1016/j.jfoodeng.2010.12.031. [DOI] [Google Scholar]
- 13.Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release. 2012;162:56–67. doi: 10.1016/j.jconrel.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Krasaekoopt W, Bhandari B, Deeth H. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy J. 2004;14:737–743. doi: 10.1016/j.idairyj.2004.01.004. [DOI] [Google Scholar]
- 15.Lee JS, Cha DS, Park HJ. Survival of freeze-dried Lactobacillus bulgaricus KFRI 673 in chitosan-coated calcium alginate microparticles. J. Agric. Food Chem. 2004;52:7300–7305. doi: 10.1021/jf040235k. [DOI] [PubMed] [Google Scholar]
- 16.Shu XZ, Zhu KJ. The release behavior of brilliant blue from calcium-alginate gel beads coated by chitosan: The preparation method effect. Eur. J. Pharm. Biopharm. 2002;53:193–201. doi: 10.1016/S0939-6411(01)00247-8. [DOI] [PubMed] [Google Scholar]
- 17.Chávarri M, et al. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010;142:185–189. doi: 10.1016/j.ijfoodmicro.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Koo SM, Cho YH, Huh CS, Baek YJ, Park J. Improvement of the stability of Lactobacillus casei YIT 9018 by microencapsulation using alginate and chitosan. J. Microbiol. Biotechnol. 2001;11:376–383. [Google Scholar]
- 19.Liliana SC, Vladimir VC. Probiotic encapsulation. Afr. J. Microbiol. Res. 2015;7:4743–4753. doi: 10.5897/AJMR2013.5718. [DOI] [Google Scholar]
- 20.Huang S, et al. Spray drying of probiotics and other food-grade bacteria: A review. Trends Food Sci. Technol. 2017;63:1–17. doi: 10.1016/j.tifs.2017.02.007. [DOI] [Google Scholar]
- 21.Iqbal M, Zafar N, Fessi H, Elaissari A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int. J. Pharm. 2015;496:173–190. doi: 10.1016/j.ijpharm.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 22.Ivanovska TP, Smilkov K, Zhivikj Z, Tozi LP, Mladenovska K. Comparative evaluation of viability of encapsulated Lactobacillus casei using two different methods of microencapsulation. Int. J. Pharm. Phytopharm. Res. eIJPPR. 2014;4:20–24. [Google Scholar]
- 23.Flores-Belmont IA, Palou E, López-Malo A, Jiménez-Munguía MT. Simple and double microencapsulation of Lactobacillus acidophilus with chitosan using spray drying. Int. J. Food Stud. 2015;4:188–200. doi: 10.7455/ijfs/4.2.2015.a7. [DOI] [Google Scholar]
- 24.Khosravi Zanjani MA, Ghiassi Tarzi B, Sharifan A, Mohammadi N. Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in simulated human gastro-intestinal condition. Iran. J. Pharm. Res. IJPR. 2014;13:843–852. [PMC free article] [PubMed] [Google Scholar]
- 25.Mokarram RR, Mortazavi SA, Najafi MBH, Shahidi F. The influence of multi stage alginate coating on survivability of potential probiotic bacteria in simulated gastric and intestinal juice. Food Res. Int. 2009;42:1040–1045. doi: 10.1016/j.foodres.2009.04.023. [DOI] [Google Scholar]
- 26.Avila-Reyes SV, Garcia-Suarez FJ, Jiménez MT, San Martín-Gonzalez MF, Bello-Perez LA. Protection of L. rhamnosus by spray-drying using two prebiotics colloids to enhance the viability. Carbohydr. Polym. 2014;102:423–430. doi: 10.1016/j.carbpol.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 27.Corona-Hernandez RI, et al. Structural stability and viability of microencapsulated probiotic bacteria: A review. Compr. Rev. Food Sci. Food Saf. 2013;12:614–628. doi: 10.1111/1541-4337.12030. [DOI] [PubMed] [Google Scholar]
- 28.Maciel GM, Chaves KS, Grosso CR, Gigante ML. Microencapsulation of Lactobacillus acidophilus La-5 by spray-drying using sweet whey and skim milk as encapsulating materials. J. Dairy Sci. 2014;97:1991–1998. doi: 10.3168/jds.2013-7463. [DOI] [PubMed] [Google Scholar]
- 29.Champagne CP, Fustier P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007;18:184–190. doi: 10.1016/j.copbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Jantzen M, Gopel A, Beermann C. Direct spray drying and microencapsulation of probiotic Lactobacillus reuteri from slurry fermentation with whey. J. Appl. Microbiol. 2013;115:1029–1036. doi: 10.1111/jam.12293. [DOI] [PubMed] [Google Scholar]
- 31.Holkem AT, et al. Development and characterization of alginate microcapsules containing Bifidobacterium BB-12 produced by emulsification/internal gelation followed by freeze drying. LWT Food Sci. Technol. 2016;71:302–308. doi: 10.1016/j.lwt.2016.04.012. [DOI] [Google Scholar]
- 32.Yeung TW, Arroyo-Maya IJ, McClements DJ, Sela DA. Microencapsulation of probiotics in hydrogel particles: Enhancing Lactococcus lactis subsp. cremoris LM0230 viability using calcium alginate beads. Food Funct. 2016;7:1797–1804. doi: 10.1039/c5fo00801h. [DOI] [PubMed] [Google Scholar]
- 33.Barbosa J, Borges S, Teixeira P. Effect of different conditions of growth and storage on the cell counts of two lactic acid bacteria after spray drying in orange juice. Beverages. 2016;2:8. doi: 10.3390/beverages2020008. [DOI] [Google Scholar]
- 34.Gardiner GE, et al. Comparative survival rates of human-derived probiotic Lactobacillus paracasei and L. salivarius strains during heat treatment and spray drying. Appl. Environ. Microbiol. 2000;66:2605–2612. doi: 10.1128/aem.66.6.2605-2612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JU, et al. Encapsulation of probiotic Lactobacillus acidophilus by ionic gelation with electrostatic extrusion for enhancement of survival under simulated gastric conditions and during refrigerated storage. Int. J. Food Sci. Technol. 2017;52:519–530. doi: 10.1111/ijfs.13308. [DOI] [Google Scholar]
- 36.Mortazavian AM, et al. Viability of calcium-alginate-microencapsulated probiotic bacteria in Iranian yogurt drink (Doogh) during refrigerated storage and under simulated gastrointestinal conditions. Aust. J. Dairy Technol. 2008;63:24–29. [Google Scholar]
- 37.Sohail A, Turner MS, Coombes A, Bhandari B. The viability of Lactobacillus rhamnosus GG and Lactobacillus acidophilus NCFM following double encapsulation in alginate and maltodextrin. Food Bioprocess Technol. 2013;6:2763–2769. doi: 10.1007/s11947-012-0938-y. [DOI] [Google Scholar]
- 38.Nunes GL, et al. Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT Food Sci. Technol. 2018;89:128–133. doi: 10.1016/j.lwt.2017.10.032. [DOI] [Google Scholar]
- 39.Cheow WS, Hadinoto K. Biofilm-like Lactobacillus rhamnosus probiotics encapsulated in alginate and carrageenan microcapsules exhibiting enhanced thermotolerance and freeze-drying resistance. Biomacromol. 2013;14:3214–3222. doi: 10.1021/bm400853d. [DOI] [PubMed] [Google Scholar]
- 40.Mandal S, Puniya AK, Singh K. Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. Int. Dairy J. 2006;16:1190–1195. doi: 10.1016/j.idairyj.2005.10.005. [DOI] [Google Scholar]
- 41.Teoh L, Mirhosseini H, Shuhaimi M, Yazid A, Manap A. Tolerance of free and encapsulated probiotics towards heat treatment and high sodium concentration. J. Food Agric. Environ. 2011;9:69–73. [Google Scholar]
- 42.Papadimitriou K, et al. Stress physiology of lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2016;80:837–890. doi: 10.1128/MMBR.00076-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weimer, B. C. In Stress Responses of Lactic Acid Bacteria (eds. Effie, T. & Konstantinos, P.) 129–144 (Springer US, 2011).
- 44.Atia A, et al. A prebiotic matrix for encapsulation of probiotics: Physicochemical and microbiological study. J. Microencapsul. 2016;33:89–101. doi: 10.3109/02652048.2015.1134688. [DOI] [PubMed] [Google Scholar]
- 45.Sirichokchatchawan W, et al. Autochthonous lactic acid bacteria isolated from pig faeces in Thailand show probiotic properties and antibacterial activity against enteric pathogenic bacteria. Microb. Pathog. 2018;119:208–215. doi: 10.1016/j.micpath.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 46.Sirichokchatchawan W, Tanasupawat S, Niyomtham W, Prapasarakul N. Identification and antimicrobial susceptibility of lactic acid bacteria from fecal samples of indigenous and commercial pigs. Thai J. Vet. Med. 2017;47:329–338. [Google Scholar]
- 47.Sirichokchatchawan W, Temeeyasen G, Nilubol D, Prapasarakul N. Protective effects of cell-free supernatant and live lactic acid bacteria isolated from Thai pigs against a pandemic strain of porcine epidemic diarrhea virus. Probiotics Antimicrob. Proteins. 2018;10:383–390. doi: 10.1007/s12602-017-9281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prasad J, Gill H, Smart J, Gopal PK. Selection and characterisation of Lactobacillus and Bifidobacterium strains for use as probiotics. Int. Dairy J. 1999;8:993–1002. doi: 10.1016/S0958-6946(99)00024-2. [DOI] [Google Scholar]
- 49.Zhou Y, Martins E, Groboillot A, Champagne CP, Neufeld RJ. Spectrophotometric quantification of lactic bacteria in alginate and control of cell release with chitosan coating. J. Appl. Microbiol. 1998;84:342–348. doi: 10.1046/j.1365-2672.1998.00348.x. [DOI] [Google Scholar]
- 50.Ivanova E, Chipeva V, Ivanova I, Dousset X, Poncelet D. Encapsulation of Lactic acid bacteria in calcium alginate beads for bacteriocin production. J. Cult. Collect. 2003;3:53–58. [Google Scholar]
- 51.Federici S, et al. Identification and functional traits of lactic acid bacteria isolated from Ciauscolo salami produced in Central Italy. Meat Sci. 2014;98:575–584. doi: 10.1016/j.meatsci.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Lin WH, Hwang CF, Chen LW, Tsen HY. Viable counts, characteristic evaluation for commercial lactic acid bacteria products. Food Microbiol. 2006;23:74–81. doi: 10.1016/j.fm.2005.01.013. [DOI] [PubMed] [Google Scholar]