Highlights
-
•
Giant inguinoscrotal hernias rarely have stomach in, and those that do are often neglected and present in extremis.
-
•
A multitude of surgical approaches currently exists, including a two-stage reduction then interval hernia repair.
-
•
Surgeons must be aware of abdominal compartment syndrome risk, and consider this in their management planning.
Keywords: Inguinoscrotal, Hernia, Gastric, Perforation, Compartment syndrome
Abstract
Introduction
Inguinoscrotal hernias often contain bowel, but it is rare to see it contain part or all of the stomach. These patients tend to present in extremis.
Presentation of case
This is the case of a 74 year old gentleman who presented in obstruction and acutely unwell from giant bilateral inguinoscrotal hernias. CT scan confirmed the left hernia contained the majority of the bowel and stomach. He underwent laparotomy and repair of the left sided hernia. Intraoperatively he was also found to have a gastric perforation and underwent distal gastrectomy. 7 days post operatively he returned to theatre for repair of his right sided hernia. The patient made a full recovery.
Discussion
Review of similar literature highlights numerous surgical methods in repairing these hernias. A two-stage approach appears to mitigate the risk of abdominal compartment syndrome, whilst also allowing for an interval hernia repair in a non-hostile environment. Gastric perforation repair technique also varies, with majority of literature reporting primary repair.
Conclusion
We hope our approach to management can help guide others faced with similar challenging cases. Moreover, it highlights some operative challenges including dealing with associated gastric perforation and mitigating the risk of abdominal compartment syndrome.
1. Introduction
Inguinoscrotal hernias regularly contain bowel, however it is rare to see an inguinoscrotal hernia containing all or part of the stomach. Moreover, when these patients present acutely they tend to be in extremis. This report outlines an approach to such a case and in turn can hopefully guide other teams faced with similar challenging cases. In particular, it highlights operative difficulties such as managing associated gastric perforation and mitigating the risks of abdominal compartment syndrome.
2. Presentation of case
The 74-year old male patient in this case presented with a 24-h history of multiple episodes of dark vomitus and generalised abdominal tenderness. This was on a background of bilateral, longstanding, large, irreducible inguinoscrotal hernias for which he had not sought medical attention. Further past medical history included hypertension and type two diabetes mellitus. He was independent with all activities of daily living and his only regular medication was an ACE-inhibitor.
Initial examination revealed the abdomen was tender but not peritonitic. Bowel sounds were present but quiet on auscultation. Additionally, the inguinoscrotal hernias were tender and not reducible.
The patient’s observations on admission were within normal parameters apart from a tachycardia of 117bpm. Bloodwork demonstrated only a mildly raised CRP and mild acute kidney injury (creatinine 137 μmol/L). However, his lactate on venous blood gas was raised at 3.9.
An erect chest radiograph performed was normal.
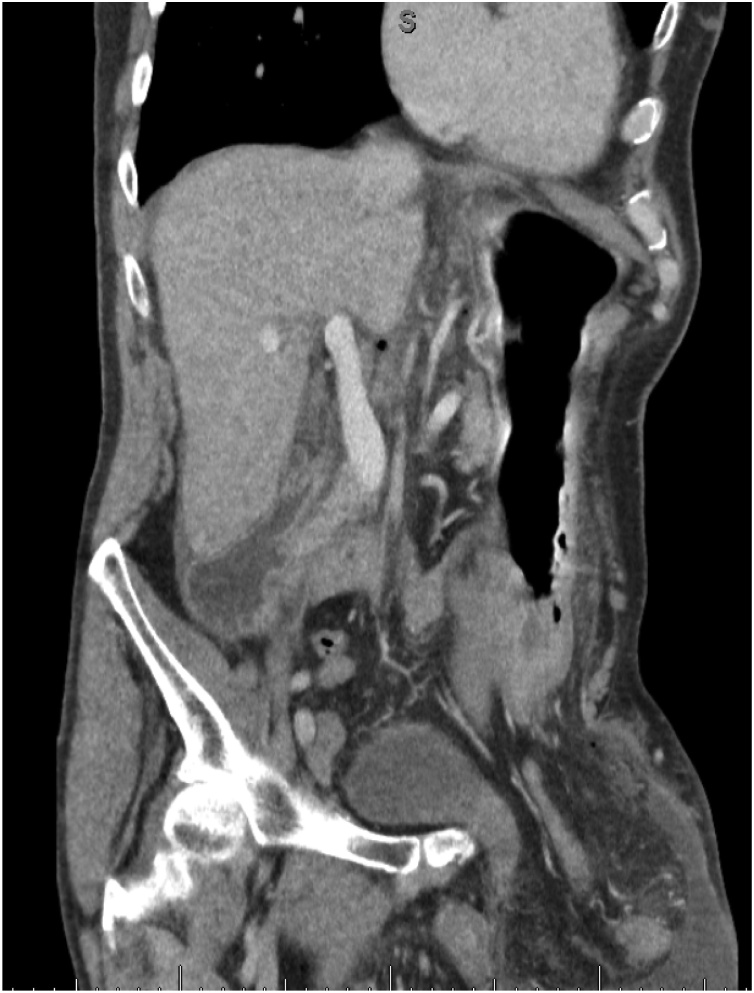
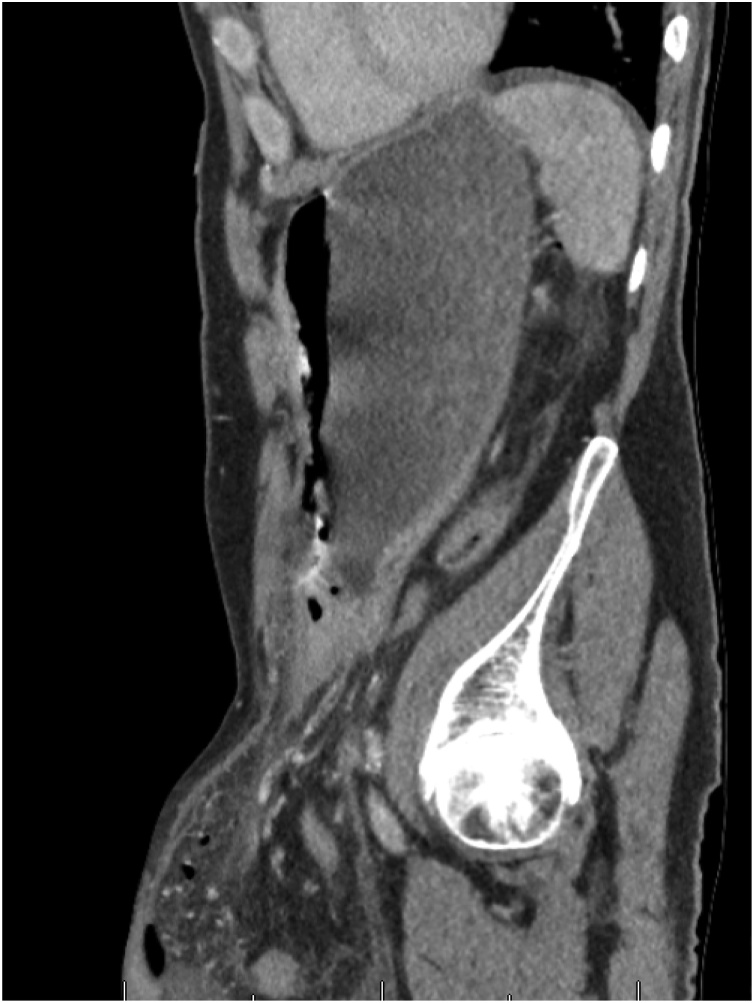
Subsequent CT scan of the abdomen and pelvis showed massive bilateral inguinal hernias with the majority of small bowel and stomach extending into the left side and air fluid level within it suggestive of perforation. Coronal and sagittal sections can be seen in Fig. 1, Fig. 2 respectively.
Fig. 1.
Coronal plane CT image.
Fig. 2.
Axial plane CT image.
The patient was resuscitated and underwent a midline laparotomy. The stomach within the left inguinal hernia was found to be necrotic and had perforated at the antrum. The decision was made to carry out a distal gastrectomy. A gastro-jejunostomy was formed with Echilon 60 and the enterotomy was then closed with Stratifix. An inguinal incision was then made, and the left hernia repaired using a Bassini technique, preserving the cord. However, the right was not repaired in order to reduce the risk of abdominal compartment syndrome. The patient was managed post-operatively in ITU.
The patient recovered well post-operatively but failed to open his bowels or pass flatus and CT abdomen and pelvis on day 7 showed a tight necked loop of obstructed small bowel in the right inguinal hernia.
He was immediately returned to theatre and underwent open mesh repair of the right inguinal hernia, with the return of the small bowel to the abdominal cavity relieving the obstruction. Corrugated drains were inserted, and the patient was again managed post-operatively on ITU. He had a 4 week inpatient stay after this owing to an episode of atrial fibrillation and 12 × 10 cm right groin collection requiring ultrasound guided drainage.
Nonetheless at follow up one month post discharge he had made a remarkable recovery with well healed wounds and he was completely asymptomatic, with no recurrence.
At 6 month review the patient continued to show no signs of recurrence.
3. Discussion
This a rare case and as such its value lies in adding to the limited body of published material on the management of giant inguinoscrotal hernias with gastric contents.
Of note in this case is how the risk of raised intra-abdominal pressure was managed. It has long been known that the reduction and repair of large hernias involves the risk of suddenly increasing abdominal pressure and compromising respiration, due to loss of domain [1]. Many techniques have been used to mitigate this risk which can be seen in Table 1. These include a one stage complete repair, two stage technique with initial perforation repair and delayed hernia repair, delayed primary closure, pre-operative progressive pneumoperitoneum, abdominal component separation and resection of bowel [[1], [2], [3], [4], [5]]. In this case we opted for a two stage repair, only repairing the immediately offending left hernia and returning to repair the right side at a later date to reduce the risk of abdominal compartment syndrome. However, this had the unintended consequence of leading to post operative obstruction from the unrepaired side. One stage complete repair with close observation post operatively may be a superior method, as utilised elsewhere with no ensuing abdominal compartment syndrome [6].
Table 1.
Table detailing operative approaches in prevention of abdominal compartment syndrome.
| Author | Summary of case | Treatment strategy for prevention of abdominal compartment syndrome | Authors comments |
|---|---|---|---|
| Sayad et al. 2018 | Giant inguinoscrotal hernia with associated gastric perforation. Primary suture repair of gastric perforation. Elective inguinal hernia repair 3 months later. | Two stage closure | “we were able to avoid an initial repair of a complex hernia… where the intra-abdominal pressure is already increased secondary to the peritonitis and the ileus.” |
| Staubitz et al. 2017 | Right sided inguinoscrotal hernia containing 2/3rds small bowel, colon. Midline laparotomy with inguinal hernia mesh repair. Abdomen closed using component separation. | Component separation. | Patient declined pneumoperitoneum. They opted to avoid bowel resection given risks associated with anastomosis. Abdomen closed using component separation as contents were “too voluminous to allow direct tension-free suturing of the lower part of the laparotomy wound” |
| Oprea et al. 2014 | Study assessing use of pneumoperitoneum in 17 patients with loss of domain secondary to giant abdominal wall defects. | Pneumoperitoneum | Minimal risk to patient. Of less utilisation in acute presentations. |
| Lajevardi et al. 2015 | Giant inguinoscrotal hernia with associated 10 cm lesser curvature gastric perforation. One stage repair approach taken with primary gastric repair, and hernia repair with pre-peritoneal mesh. Subtotal colectomy also performed with ileo-sigmoid anastomosis. |
Bowel resection | Subtotal colectomy performed to allow primary abdominal wall closure and reduce volvulus risk. |
| Fitz et al. 2016 | Bilateral giant inguinoscrotal hernia, containing distal stomach duodenum and head of pancreas on right. Left side contained almost entire small bowel and colon. 11 cm perforation on lesser curvature of stomach. Initial laparotomy and primary repair of gastric perforation followed by relook laparotomy and Bassini repair of hernia. |
Delayed primary closure | “Due to the chronic nature of the hernias, there was a substantial loss of domain which prohibited abdominal closure. The large bilateral inguinal hernia sacs were reduced into the peritoneal cavity and temporarily sutured to the abdominal sidewall to prevent re-herniation, and a wound vacuum-assisted closure device was placed” |
In this case the patient was acutely unwell and required distal gastrectomy due to perforation of the stomach. It is thought that the descent of the greater omentum into the large hernial sac draws the stomach down [7], which in turn stresses the peritoneal attachments and vasculature putting it at high risk of perforation. Gastric perforation has been regularly reported in other cases of inguinoscrotal hernias containing stomach and seems to be a recurrent issue. However, it has been invariably managed with primary repair rather than gastrectomy as in this case [4,5,8,9].
Midline laparotomy with inguinal incision for repair of hernia defect, were the incisions of choice in the operative management of our patient. Similar literature of giant inguinoscrotal hernias with associated gastric perforation highlights a varied approach.
Since the turn of the millennium there have been four reported cases of giant inguinoscrotal hernia with associated gastric perforation. Two of these opted for a two-stage approach to management. Sayad et al. employed a two stage approach with initial laparotomy and primary suture repair of gastric perforation, followed by open inguinal hernia repair at a 3-month interval [2]. Fitz et al. used a two stage approach, initially laparotomy with primary suture repair of the gastric perforation with the hernias reduced and temporarily sutured to the abdominal side wall. A wound vacuum-assisted closure device was placed, due to difficulty closing the abdominal wall. One week later the patient underwent relook laparotomy and Bassini repair of the hernia [5]. Lajevardi et al. opted for a one stage approach with primary gastric repair and hernia mesh repair, using midline laparotomy only [4]. Doggar et al. opted for a similar approach to us. They utilised a midline laparotomy and performed gastrectomy with end-to-end anastomosis. During the same operation they made an inguinal incision and repaired the hernial defect [10].
All four cases report initial success but do not comment on long term follow up outcomes. We are the only case to document successful results from surgical management of giant inguinoscrotal hernia containing stomach, with long-term follow up to the length of six months.
We feel midline laparotomy allows for optimal management and repair of the gastric perforation, alongside the standard open approach for inguinal hernia repair.
As seen from review of the literature, there is currently a breadth of approaches to the management of giant inguinoscrotal hernias with and without gastric perforation, despite a brevity of literature. Indeed, a formal review of similar cases would be helpful in forming a consensus on optimum management. This case was reported in line with the SCARE guideline [11].
4. Conclusion
These hernia often present in extremis, and can be complicated by gastric perforation and require gastrectomy or primary repair. Patients are at risk of raised intra-abdominal pressure post-operatively due to loss of domain, and surgeons should be aware of this risk when planning operative management.
Conflicts of interest
None.
Sources of funding
On acceptance, Imperial College Open Access Fund may contribute to fees. This had no impact on writing of the manuscript or submission.
Ethical approval
Ethical approval was not required for this manuscript.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Study concept and design: Daniel Campioni-Norman and Joseph Heylen
Data collection and interpretation: Daniel Campioni-Norman and Joseph Heylen
Interpretation and organisation of figures: Daniel Campioni-Norman and Joseph Heylen
Writing the paper: Daniel Campioni-Norman and Joseph Heylen
Critical revision of the manuscript for important intellectual content: Daniel Campioni-Norman and Joseph Heylen
Registration of research studies
N/A.
Guarantor
Mr Daniel Campioni-Norman.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Staubitz J., Gassmann P., Kauff D., Lang H. Surgical treatment strategies for giant inguinoscrotal hernia - a case report with review of literature. BMC Surg. 2017;19(17):135. doi: 10.1186/s12893-017-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sayad P., Tan A.Z. A case report of a gastric perforation in a giant inguinoscrotal hernia: a two-step approach. Int. J. Surg. Case Rep. 2019;1(55):174–178. doi: 10.1016/j.ijscr.2019.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.V. Oprea, O. Matei, D. Gheorghescu, D. Leuca, F. Buia, M. Rosianu, Dinca M. Chirurgia 2014 September - October; 109:664-669. [PubMed]
- 4.Lajevardi S.S., Gundara J.S., Collins S.A., Samra J.S. Acute gastric rupture in a giant inguinoscrotal hernia. J. Gastrointest. Surg. 2015;19(12):2283–2285. doi: 10.1007/s11605-015-2916-y. [DOI] [PubMed] [Google Scholar]
- 5.Fitz E., Chihara R., Stanton-Maxey K.J. Gastric perforation associated with bilateral inguinal hernias. J. Am. Coll. Surg. 2016;222 doi: 10.1016/j.jamcollsurg.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Junge K., Otto J., Oral H. A rare cause of gastric outlet stenosis- scrotal hernia. Dtsch. Arztebl. Int. [Internet] 2019;116(29–30):507. doi: 10.3238/arztebl.2019.0507b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DAVEY W.W., STRANGE S.L. The stomach as a content of inguinal and femoral herniae. Br. J. Surg. [Internet] 1954;41(170):651–658. doi: 10.1002/bjs.18004117023. [DOI] [PubMed] [Google Scholar]
- 8.Padmanabhan A.I., Lowenfels A.B. Letter: an unusual hernia. JAMA. 1976;235(26):2813. doi: 10.1001/jama.235.26.2813c. [DOI] [PubMed] [Google Scholar]
- 9.Gue S. Spontaneous rupture of stomach. A rare complication of inguinal hernia. Br. J. Surg. 1970;57(2):154–155. doi: 10.1002/bjs.1800570218. [DOI] [PubMed] [Google Scholar]
- 10.Dogar M.A., Chaudhary A. Inguinal hernia containing stomach, transverse colon and small bowel. Pakistan J. Med. Health Sci. 2011;5(3) [Google Scholar]
- 11.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 Statement: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]