Abstract
Optic neuritis (ON) is an inflammatory optic neuropathy that is often a harbinger of central nervous system (CNS) demyelinating disorders. ON is frequently misdiagnosed in the clinical arena, leading to either inappropriate management or diagnostic delays. As a result, patients may fail to achieve optimal recovery. The treatment response to corticosteroids and long term risk of multiple sclerosis was established in the first clinical trials conducted roughly 30 years ago. Spontaneous resolution was observed in the vast majority of patients and intravenous high-dose corticosteroids hastened recovery; half of the patients eventually developed multiple sclerosis. Over the ensuing decades, the number of inflammatory conditions associated with ON has significantly expanded exposing substantial variability in the prognosis, treatment, and management of ON patients. ON subtypes can frequently be distinguished by distinct clinical, serological, and radiological profiles allowing expedited and specialized treatment. Guided by an increased understanding of the immunopathology underlying optic nerve and associated CNS injuries, novel disease management strategies are emerging to minimize vision loss, improve long-term surveillance strategies, and minimize CNS injury and disability. Knowledge regarding the clinical signs and symptoms of different ON subtypes is essential to guide acute therapy, prognosticate recovery, accurately identify underlying CNS inflammatory disorders, and facilitate study design for the next generation of clinical and translational trials.
INTRODUCTION
Optic neuritis (ON) is a term used to describe any inflammatory condition affecting the optic nerve. Because ON is caused by a variety of central nervous system (CNS) and systemic disorders, incidence rates vary from 1.4 to 33 per 100,000 people, depending on diagnostic accuracy, efficient case capture, and population demographics.[1–5] ON, however, is frequently misdiagnosed because of errors in eliciting or interpreting the history and physical examination.[6]
Distinguishing between subtypes of ON is both challenging and important in the current era, as serological and radiographic biomarkers can help refine diagnoses and tailor treatments. Clinical and radiologic features, such as older age, bilateral optic nerve involvement, and location of optic nerve inflammation may signal a specific etiology. Furthermore, treatment algorithms established by the Optic Neuritis Treatment Trial (ONTT)[7], conducted roughly 30 years ago, are not universally applicable.
This narrative review will focus on the salient features that distinguish ON from other common causes of optic neuropathy in adults. Moreover, we will highlight clinical phenotypes of that characterize specific subtypes of autoimmune ON associated with CNS disease—multiple sclerosis and idiopathic (MS-ON; considered together as the phenotypes overlap), myelin oligodendrocyte glycoprotein (MOG-ON), and neuromyelitis optica spectrum disorder (NMOSD-ON). Although it does not seem to cause a retrobulbar ON, we have also included glial fibrillary acidic protein (GFAP-ON) because it is an autoimmune meningoencephalitis with inflammatory optic disc edema (papillitis) that should be considered when evaluating patients with possible ON. Within this context, we will discuss evolving diagnostic algorithms, acute treatment options, long-term surveillance strategies, and prognostic indicators for recovery.
METHODS
We searched Pubmed/Medline and the Cochrane Database of Systematic Reviews for English-language studies using the following terms: optic neuritis, neuromyelitis optica spectrum disorder, aquaporin-4 antibodies, myelin oligodendrocyte glycoprotein antibodies, glial fibrillary acidic protein antibodies. All relevant articles were reviewed. Articles that were not captured in the initial search, but known to the authors were also reviewed. ClinicalTrials.gov was searched to obtain all relevant phase 2 and 3 clinical trials not returned in the search.
CLINICAL PRESENTATION
History and Examination
As the diagnosis of ON remains largely clinical, knowledge regarding the cardinal symptoms and signs of optic nerve dysfunction is key to avoiding diagnostic errors. Table 1 provides clinical characteristics of ON subtypes compared to other important optic neuropathies. With the exception of GFAP, which presents with concurrent meningoencephalitis, ON may be isolated and the initial presentation of MS, NMO, or MOG.[8] It can be challenging to distinguish ON subtypes acutely, because vision loss may be variable at onset, and clinical phenotypes may overlap. Patient demographics may provide initial clues to the underlying etiology. MS-ON predominantly presents in young women (mean age of 32 years; female:male-3:1)[7] while NMOSD-ON presents in slightly older individuals and shows a prominent female bias (mean age of 40 years; female:male-9:1).[9, 10] MOG-ON may present at multiple ages and shows no sex bias. Similarly, GFAP-ON shows no sex bias; the median age is approximately 40 years.[11]
Table 1.
Clinical characteristics of optic neuritis subtypes and other common optic neuropathies.
| Optic neuritis subtypes | Other common optic neuropathiesa | |||||||
|---|---|---|---|---|---|---|---|---|
| MS ON[7, 12]b | MOG ON[11] | NMOSD ON[10, 79] | GFAP ON[8] | NA-AION | LHON | Compressive optic neuropathy | Toxic/Metabolic optic neuropathy | |
| Age | 20–30s | 30s | 40s | 40s | Over 50 | Younger | Variable | Variable |
| Sex | 75% Women | Women = Men | 90% Women | 60% Men | Men > Women | Men > Women | Variable | Variable |
| Onset | Subacute | Subacute | Subacute | Subacute | Acute | Acute to sub-acute | Often chronic and progressive, with subacute awareness of vision loss when central vision in affected | Sub-acute to chronic |
| Pain | Common (92%) | Common (86%) | Variable | Uncommon | Rare | Uncommon | Uncommon (unless orbital involvement) | Uncommon |
| Laterality | Unilateral, rarely bilateral | Unilateral, ~30–40% bilateral | Unilateral, 20–30% bilateral | Bilateral | Unilateral | Bilateral | Unilateral or bilateral | Bilateral |
| Positive Visual Phenomena | Phosphenes in 30% | Unknown | Unknown | Unknown | Uncommon | Uncommon | Uncommon | Uncommon |
| Recurrence | Can occur | Common | Common | Uncertain | Uncommon | Uncommon | Variable with lesion type | Possible partial reversal upon elimination of toxic factors or treatment of metabolic factors |
| Visual acuity at onset | Variable, but approximately 35% 20/200 or worse | Variable, often worse than 20/200 | Severe vision loss worse than 20/200 in ~80% | Vision usually preserved | Variable | Often poor | Variable | Variable |
| RAPD c | Present | Present | Present | Absent | Present | May be absent in early phase | Present | Absent |
| Color Vision | Abnormal | Abnormal | Abnormal | Normal | Abnormal | Abnormal | Abnormal | Abnormal |
| Visual Fields | Diffuse, central scotoma | Variable | Total, quadrant, central, Altitudinal defects | Big blind sports, and variable visual field defects reporter | Arcuate, altitudinal, cecocentral scotoma | Central and cecocentral | Temporal, central, altitudinal | Central, cecocentral scotomas, temporal defects |
| Fundus | Normal to mild optic disc edema in ~35% | Moderate to severe optic disc edema in ~85% | Variable optic disc edema | Moderate to severe optic disc edema | Optic disc edema | Normal or “pseudo”optic disc edema | Normal, swollen or pale optic disc with possible increased cupping | Normal, swollen or pale optic disc |
| OCT d | Mild RNFL increase acutely, GCIPL thinning in early weeks | Significant RNFL thickening acutely, early GCIPL loss | Variable RNFL thickening acutely, with profound GCIPL loss | RNFL thickening acutely with or without GCIPL loss | RNFL thickening (resolves in two months) acutely with early GCIPL loss | RNFL thickening acutely, early GCIPL loss | Variable RNFL loss, hemiretinal GCIPL thinning (pituitary tumors) | Variable RNFL thickening acutely, early GCIPL loss |
| Visual Recovery | Good, 95% 20/40 or better | Good (~20/30) | 20–30% with poor recovery | Good | Variable but some vision loss generally persists | Variable, but generally poor | Variable | Variable |
Legend: MS ON = multiple sclerosis optic neuritis, MOG ON = myelin oligodendrocyte oligoprotein optic neuritis, NMOSD ON = neuromyelitis optica spectrum disorder optic neuritis, GFAP ON = glial fibrillary acidic protein optic neuritis, NA- AION = non-arteritic anterior ischemic optic neuropathy, LHON = Leber hereditary optic neuropathy, RAPD = relative afferent pupillary defect, OCT=optical coherence tomography, RNFL= retinal nerve fiber layer (peripapillary), GCIPL=ganglion cell inner plexiform layer (macular), RPE=retinal pigmented epithelium, INL=inner nuclear layer
Other optic neuropathies that should be considered include infectious (e.g. Lyme, syphilis, tuberculosis, viral), systemic immune (e.g. Lupus), infiltrative (e.g. neoplastic), seronegative (autoimmune, chronic relapsing inflammatory optic neuropathy), and paraneoplastic (e.g. collapsing response-mediator protein-5).[13] Neuroretinitis, either idiopathic or infectious (Bartonella henselae, toxoplasmosis, etc), may also present like ON.
Idiopathic ON has the same features as MS-associated ON.
Relative afferent pupillary defect may be absent in patients with bilateral optic neuropathies or prior optic neuropathy in the fellow eye.
General OCT patterns within the early weeks of vision loss.
While eye pain that worsens with eye movement is a common symptom in most cases of ON, over-emphasizing the significance of pain may be a diagnostic pitfall. Stunkel and colleagues reported that over-reliance on the presence of eye pain or pain with eye movements represented a critical diagnostic error in 12% of patients referred for evaluation of ON.[6] Indeed, pain that is recurrent with stereotyped features, and accompanied by aura would be highly unusual for ON, and more likely representative of a headache disorder. Moreover, pain persisting over many days or longer in the presence of a normal visual examination would not be consistent with ON and should raise clinical suspicion for an alternative process. However, it is important to recognize that pain preceded visual signs and symptoms in 39.5% of ONTT participants.[12] In NMOSD- and GFAP-ON, eye pain may be less prominent due to the mechanisms and location of optic nerve injury. Phosphenes are another common symptom of ON, particularly MS-ON, but are not specific and can occur in other optic neuropathies and retinopathies.[12]
Examination of a patient with acute ON reveals visual acuity loss, visual field deficits, color vision impairment, and a relative afferent pupillary defect (RAPD) in the affected eye. The absence of an RAPD should raise diagnostic concern unless the patient has bilateral involvement or a history of optic neuropathy in the fellow eye.[13] The extent of visual acuity loss may vary across ON subtypes during the acute phase. In MS-ON, high-contrast visual acuity loss is moderate, with the majority of patients having acuity better than 20/200.[7] In contrast, NMOSD-ON and MOG-ON often present with more significant vision loss, worse than 20/400.[11, 14] Visual acuity is largely preserved in GFAP.[8] Bilateral involvement is also more common with MOG-ON than with MS-ON or NMOSD-ON.[15] As with any optic neuropathy, color vision loss is often disproportionately affected relative to high contrast acuity, and therefore, not specific for ON. It may, however, be useful in distinguishing retinal diseases that can mimic ON. Although infrequently utilized in the neurology clinic, perimetry testing may reveal diffuse or discrete patterns of visual field loss. In most adult MS-ON cases, the funduscopic examination is normal, with less than 30% of patients presenting with clinically apparent optic disc edema.[7, 13] Severe optic disc edema and optic disc hemorrhages should raise concern for MOG-ON or GFAP-ON. Although ocular inflammation (e.g. uveitis, perivascular sheathing, retinal vasculitis) can accompany MS-ON, possibly MOG-ON, and GFAP (vitritis), these findings should prompt also consideration of infectious or systemic inflammatory causes of optic nerve and/or retinal dysfunction.[13, 16–18]
The value of optical coherence tomography (OCT) is in distinguishing ON from other causes of vision loss, rather than distinguishing between ON subtypes. Acute optic neuropathies, such as ON, can cause thickening of the peripapillary retinal nerve fiber layer (RNFL), a measure of axonal integrity. If the ganglion cells are permanently injured, the macular ganglion cell inner plexiform layer (GCIPL) will begin to thin followed by thinning of the RNFL. The timing and severity of this progression can provide some clues as to the cause of the optic nerve injury. For example, in patients with monocular vision loss from Leber’s Hereditary Optic Neuropathy, the RNFL will thicken acutely, just as in ON, but thinning of GCIPL occurs earlier than in patients with ON. Likewise, both non-arteritic ischemic optic neuropathy and ON will cause thickening of the RNFL acutely, but the subsequent GCIPL and RNFL thinning tends to conform to the visual field loss in ischemic optic neuropathy and it is more diffuse in ON. OCT can also be quite valuable in distinguishing retinal masqueraders of vision loss by identifying subtle retinal changes that are difficult to appreciate on the dilated funduscopic examination. However, OCT provides little additional diagnostic information about ON subtypes than is available from the fundus examination alone, particularly during the acute phase. Within the context of longitudinal OCT studies, patterns of peripapillary RNFL thickening acutely, thinning of the macular GCIPL, followed by thinning of the RNFL emerge among ON subtypes, but the clinical utility of these patterns is uncertain when caring for individual patients.[19]
PATHOPHYSIOLOGY
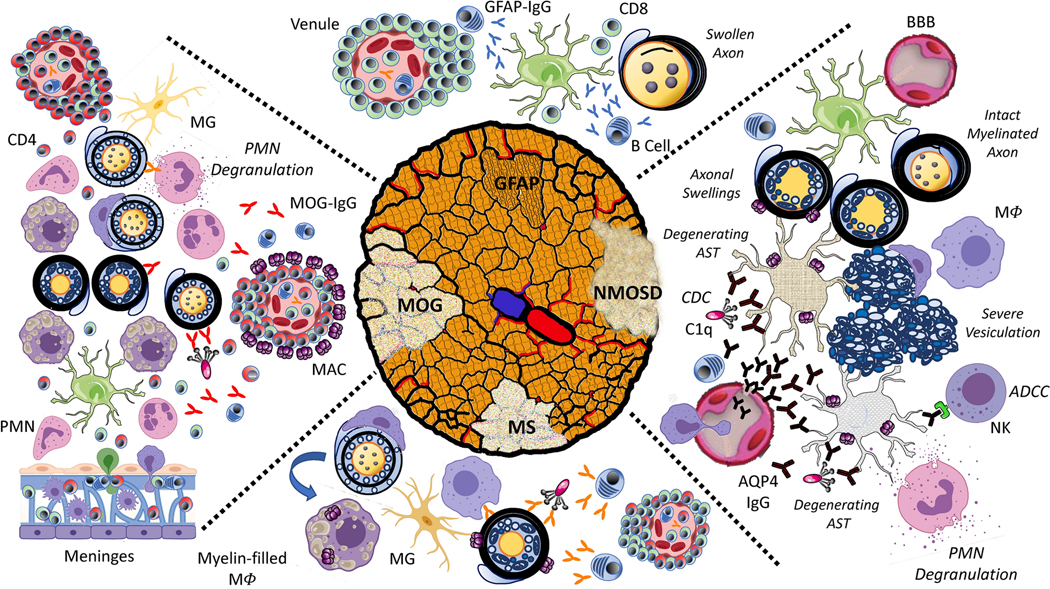
The distinct clinical presentations and outcomes of autoimmune ON subtypes are the direct result of diverse inflammatory pathophysiology.[20] Antigenic targets have been identified in two subtypes of ON, NMOSD and MOG, yet the focus of the immune response remains unknown in the majority of ON cases. While acute histopathology is lacking in human ON, autopsy tissue and animal models have provided insight into the impact of various components of the innate and adaptive immune systems.[21, 22] In NMOSD, aquaporin-4 autoantibodies (AQP4-IgG) [23, 24] targeting CNS astrocytes are sufficient to drive optic nerve, spinal cord, and brain lesions through complement and cell-mediated mechanisms (Figure 1, NMOSD).[25, 26] While bystander injury from AQP4-IgG-mediated complement activation is a source of oligodendrocyte injury in experimental models,[27] acute disruption of glial-neuronal coupling from astrocytopathy may also play an important role[28–33]. In MOG-ON, MOG autoantibodies (MOG-IgG) are likely to directly and indirectly augment optic nerve injury through complement-mediated cytotoxicity, antibody-dependent cell-mediated phagocytosis (ADCP) and cytotoxicity (ADCC), and antigen presentation[33, 34] (Figure 1, MOG). In animal models induced with MOG peptide immunization, optic nerve injury occurs in the absence of MOG-IgG and results from sequential microglial activation, astrogliosis, immune cell infiltration, and neuronal degeneration.[35–37]. This may indicate that there are antibody-dependent and -independent immunopathologies in MOG-ON. Indeed, histopathology of MOG-IgG lesions obtained at biopsy and autopsy reveal mixed features of cellular and humoral immunopathology. Recently, GFAP-IgG antibodies have been identified in patients with optic disc edema and associated visual changes consistent with isolated optic nerve head inflammation (papillitis).[8] Whether GFAP-IgG, directed against an intracellular astrocytic intermediate filament, is truly pathogenic remains to be determined; however, GFAP-specific CD8 T cells have been shown to induce relapsing CNS autoimmune disease in animal models (Figure 1, GFAP).[38] A target antigen has remained elusive in MS, yet myelin autoantibodies derived from MS patients have been shown to induce complement-mediated lysis of target oligodendrocytes.[39] These antibodies may contribute to MS-ON in active MS lesions a similar manner to MOG-IgG in MOG-ON (Figure 1, MS). For infectious (e.g., syphilis, Lyme, Bartonella, tuberculosis) and non-infectious systemic causes of optic nerve inflammation (sarcoidosis, granulomatous polyangiits, systemic lupus erythematosus, Sjogrens syndrome) the mechanisms underlying optic nerve tissue injury are likely complex[13, 20] Novel experimental models are needed to identify the optimal regimens of antimicrobial, antiviral, and immunosuppressive therapies for these conditions.
Figure 1.
Schematic of the immune pathophysiology of neuromyelitis optica spectrum disorder (NMOSD), myelin oligodendrocyte glycoprotein (MOG), glial fibrillary acidic protein (GFAP), and multiple sclerosis (MS) optic neuritis. NMOSD: AQP4-IgG enters through defects in the blood-brain barrier (BBB), bind to astrocytes (AST), and initiate complement dependent cytotoxicity (CDC) by assembling complexes for complement C1q (C1q) binding. AQP4-IgG also activates antibody-dependent cell-mediated cytotoxicity (ADCC) by natural killer (NK) cells and complement products stimulate degranulation of polymorphonuclear cells (PMNs). Membrane attack complex (MAC) may transit to adjacent oligodendrocytes resulting damage represented histopathologically by myelin vesiculation. Degenerating ASTs alter oligodendrocyte physiology resulting in axonal swelling. Myelin debris is removed by infiltrating macrophages (Mϕ). MOG: Perivenous and confluent demyelination are mediated by combined humoral and cellular mechanisms. CD4-lymphocyte and granulocytic infiltrates emerging from venous and meningeal sources result in focal and confluent regions of demyelination highlighted by nascently demyelinated axons with split myelin sheaths and vesiculation, myelin-laden macrophages within active demyelinating regions, and activated microglia (MG) in the periplaque area. Peripherally generated MOG-IgG may contribute to myelin destruction through CDC and ADCC, as well as activated T cell infiltration by facilitating phagocytosis and antigen presentation. Perivenous MAC deposition and diffuse myelin protein loss are histologic features supporting diffuse antibody-mediated myelin destruction. GFAP: GFAP papillitis results from secondary axonal swelling. GFAP-IgG is predominantly generated intrathecally; however, its role in driving disease pathology is unclear. Animal models demonstrate a predominantly perivascular, meningeal, and vascular CD8 T cell infiltrates. MS: Active MS lesions are characterized by the deposition of complement and immunoglobulin. The perivenous inflammatory infiltrates are mainly composed of CD8+ T cells and B cells producing intrathecal IgG in association with activated microglia and macrophages. MAC complexes are observed along myelin sheaths and within myelin-laden Mϕ, suggestive of active CDC.
ACUTE MANAGEMENT
Diagnostic testing
The history and clinical examination are typically sufficient to distinguish ON from other common causes of vision loss. Ideally, patients with ON should be evaluated with orbital and cranial MRI scans to assist with diagnosis, treatment, and long-term management. Orbital imaging can be helpful to distinguish ON from other common optic neuropathies (e.g. compressive optic neuropathy) and help to differentiate ON subtypes within the correct clinical context. The sensitivity of MRI for acute ON is approximately 80–94% when imaging occurs within 30 days of symptom onset.[4, 40–42] Although patterns of optic nerve enhancement can help distinguish different ON subtypes, these features are not exclusive (Table 2). For example, longitudinal involvement of the intraorbital and intracranial optic nerve segments are highly suggestive of MOG-ON, and NMOSD-ON, respectively, but can also occur in MS-ON[43] (Table 2). The variability in MRI sensitivity underscores the importance of using history and clinical examination features to enhance pre-test diagnostic probability. When MRI is performed, the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-up of Early MS recommend that orbital imaging include coronal short tau inversion recovery or fat suppressed T2 images and post-gadolinium fat-suppressed T1 images with section thickness of ≤2 mm with coverage through the chiasm.[44] Concurrent brain MRI may demonstrate lesions diagnostic (or strongly suspicious) for MS, or suggest alternative inflammatory disorders. Additional radiographic testing (Table 2) may be warranted based on the demographics, presentation, and clinical examination.
Table 2.
Serologic testing and MRI features of optic neuritis subtypes
| Multiple sclerosis | Neuromyelitis optica spectrum disorder | Myelin oligogendrocyte glycoprotein | Glial fibrillary acidic protein | |
|---|---|---|---|---|
| Specific laboratory tests | None | Aquaporin 4 antibody, cell based assaya,c | MOG antibody, cell based assayb,c | GFAP antibodyb |
| Acute orbital imaging features d | Short segment of optic nerve T1 gadolinium enhancement. Optic nerve enlargement may also be present. | T1 gadolinium – enhancing lesion extending over more than one half of the optic nerve length or involving the optic chiasm or juxtachiasmal[43, 56] | Short or long segment of T1 gadolinium enhancement, perineural and peribulbar T1 gadolinium enhancement[11] | Optic nerve enhancement is often not present, but other imaging features may suggest the diagnosise [81] |
Legend: MRI= magnetic resonance imaging, MOG=myelin oligodendrocyte glycoprotein, GFAP=glial fibrillary acidic protein
Sensitivity of the cell based assay in serum: 0.76 (95% CI: 0.67–0.82); specificity of cell based assay in serum: 0.99 (95% CI: 0.97–0.99).[82]
Seropositivity currently defines the presence of the disease (100% sensitive). Specificity unknown.
May be falsely negative in the setting of immunosuppressive therapy or plasma exchange.
These features are not exclusive to any specific ON subtype.
Radial, perivascular T1 gadolinium enhancement in the cerebral hemispheres
Recommendations regarding serologic and specific autoantibody testing are evolving (Table 2) and should target the potential underlying condition. Patients with radiographic features suggestive of NMOSD or MOG, should undergo serologic testing for AQP4-IgG and MOG-IgG with a serum cell-based assay. Specific clinical features that suggest NMOSD or MOG and should prompt testing for these conditions include, severe optic disc edema, severe vision loss (worse than 20/200), progressive or bilateral vision loss, relapsing or recurrent ON. Notably, testing for AQP4-IgG in the CSF is not sensitive or cost-effective and, therefore, it is not routinely recommended.[45] Like AQP4-IgG, MOG-IgG is primarily produced in the periphery and therefore, the utility of testing the CSF is likely to be low. In the largest study to date of GFAP, Chen et al recommend CSF GFAP autoantibodies in patients with bilateral optic disc edema with unexplained meningoencephalitis or radial perivascular enhancement on MRI.[8]
Acute Treatment
High-dose corticosteroids, both oral and intravenous (IV), are the most commonly used treatment for acute ON. A meta-analysis of three randomized controlled trials found no benefit in visual acuity recovery at 1 month, 6 months, and 1 year based on the dose or duration of oral treatment.[46] A meta-analysis of two trials comparing placebo to IV corticosteroids of over 3000 mg total also found no significant improvement in visual acuity, contrast sensitivity, or visual field at 6 months.[46] These meta-analyses were strongly influenced by the results of the ONTT, the largest therapeutic trial conducted for acute ON. The ONTT found that the only benefit of corticosteroids was hastened visual recovery within the first 2 weeks, which is the primary indication for treatment. Secondary analyses of the trial data suggest that this early benefit is only about 1–2 lines of Snellen acuity.[47]
An unexpected finding from the ONTT was that subjects receiving lower dose oral prednisone (1mg/kg) were at an increased risk of ON relapse within the first 2 years.[7] Therefore, low-dose oral prednisone is not recommended for treating acute ON. Oral prednisone in doses that are bioequivalent to 1000 mg per day IV doses of methylprednisolone used in the ONTT, may be an option, but the benefit of faster recovery has not been assessed.[48] The risk of ON relapse with high dose oral corticosteroids has also not been studied. Other therapies investigated in phase 2 and 3 randomized, controlled trials are summarized in Table 3. Most recent studies have focused on the potential neuroprotective role of various agents using structural and electrophysiological primary endpoints. None have demonstrated clinical value and should therefore not be used within the context of routine clinical care.
Table 3.
Completed Phase 2 and 3 randomized trials of treatments for acute optic neuritisa
| Citation | Pertinent enrollment criteria | Country, single or multi-center | Design | Analysis plan | Number enrolled | Intervention | Primary outcome | Results (Treated vs Placebo) |
|---|---|---|---|---|---|---|---|---|
| Trials with primary visual outcomes | ||||||||
| Roed, et al (2005)[83] | Inclusion: Clinical diagnosis of ON, age
18–59, symptoms <4 weeks Exclusion: prior ON in same eye, corticosteroid with I month, immunosuppression within 12 months 2000–2003 |
Denmark, single center | Double-blind, parallel arms | Intention-to-treat | 34 treatment 34 placebo |
IVIg 0.4 g/kg (infusions at 0,1,2,30,60 days) | Contrast sensitivity (Arden gratings) at 6 months | Median score of 93 (IQR 77, 94) vs 89 (IQR 77, 107); P=0.16 |
| Tsakiri, et al (2012)[84] | Inclusion: Clinical diagnosis of ON, age
18–59, symptoms <4 weeks Exclusion: prior ON in same eye, corticosteroid with I month, immunosuppression within 6 months 2006–2008 |
Denmark, single center | Double-blind, parallel arms | Intention-to-treat | 32 treatment 32 placebo |
Simvastatin 80 mg | Contrast sensitivity (Arden grating) at 6 months | 93 {95%CI 82, 103) vs 84 (95%CI 76, 92); P=0.06 |
| (unpublished) Results reported on clinicaltrials.gov January 2020 |
Inclusion: at least 1 episode of optic
neuritis in the last 12 months, clinically definite MS, age
18–70, VA of 20/30 or worse Exclusion: use of 4 aminopyridine in the past 4 weeks, seizure, ocular disease 2011–2013 |
USA, single center | Crossover assignment, blinding uncertain | Intention-to-treat and per-protocol | 23, dalfampridine followed by placebo 23, placebo followed by dalfampridine | Placebo or dalfampridine 10mg twice daily for 3 weeks each with 2 week wash out between treatment assignments | 8 primary outcomes listed: VA (logMAR
score and raw letters) at visits 2 and 3 compared to visit 1 using intention to treat and per protocol analyses |
No differences in multiple analyses |
| Trials with structural/physiologic primary outcomes | ||||||||
| Suhs, et al (2012)b[85] | Inclusion: first episode of ON with VA
≤0.5 (decimal system) diagnosed by an ophthalmologist, onset
within 10 days, age 18–50 years Exclusion: ocular disease in either eye, treatment with corticosteroids, erythropoietin, or immunosuppression within 30 days 2006–2011 |
Germany; multicenter (3) | Double-blind, parallel arms | Intention-to-treat with replacement of missing values using the last observation | 21, treatment 19, placebo |
Erythropoietin 33,000 units as add on to methylprednisolone | Reduction in RNFL thickness from baseline at 16 weeks | Median change: 7.5 μm (IQR 1.5, 14.5)
vs 16.0 μm (IQR 8.0, 20.0); P=0.04 |
| Raftopoulos, et al (2016)[86] | Inclusion: first episode of ON confirmed by a
neuro-ophthalmologist, 18–60 years, within 14 days of onset with
VA 6/9 or worse Exclusion: sodium or calcium channel inhibitors in the past 2 weeks, ocular disease, immune therapies within 2 months 2011–2015 |
UK, multicenter (2) | Double-blind, parallel arms | “Modified” intention-to-treat in all patients in which there was baseline and 6 month data | 42 treatment 44, placebo |
Phenytoin 15mg/kg oral load for 3 days followed by 4 or 6 mg/kg daily maintenance dose | Mean difference in RNFL thickness at 6 months in affected eye compared to baseline RNFL thickness in the unaffected eye | 6-month difference of 7.15 μm (95% CI 1.08, 13.22); P=0.021 |
| Cadavid, et al (2017)[87] | Inclusion: first episode of ON, 18–55
years, within 28 days of onset Exclusion: CNS inflammatory disease, ocular disease including high refractive error 2012–2014 |
Australia, Canada, Europe; multicenter (33) | Double-blind, parallel arms | Intention-to-treat | 41, treatment 41, placebo |
Opicinumab (anti-LINGO-1 mAb) 100mg/kg infusion once every 4 weeks for 20 weeks following methylprednisolone 1g/day for 3–5 days | Change in full-field VEP latency at 24 weeks | 17.3 milliseconds (SE 2.5) vs 20.8
milliseconds (SE 2.5); Difference of 3.5 milliseconds (95% CI −10.6, 3.7); P=0.33 |
| McKee, et al (2019)[88] | Inclusion: first episode of ON, 18–55
years, within 28 days of onset with VA 6/9 or worse
Exclusion: sodium or calcium channel inhibitors in the past 2 weeks, ocular disease, immune therapies within 2 months 2013–2015 |
UK, single center | Double-blind, parallel arms | Intention-to-treat | 23, treatment 20, placebo |
Amiloride 10mg daily for 5 months with a 1 month wash out | Mean difference in RNFL thickness at 6 months in affected eye compared to baseline RNFL thickness in the unaffected eye using scanning laser polarimetry | 6-month difference −0.46 (95%CI −5.02, 4.10); P = 0.840 |
Legend: IVIg= intravenous immunoglobulin, RNFL= retinal nerve fiber layer thickness, IQR=interquartile range, UK = United Kingdom, USA = United States of America, ON = optic neuritis, VEP = visual evoked potential
Fingolimod[89], lipoic acid[90], and minocycline (no data) terminated early due to lack of recruitment. Clemestine (NCT02521311) currently recruiting. Phase 4 ACTH trial currently recruiting (NCT01838174)
Phase 3 trial completed, but not published (NCT01962571)
Due to disparate levels of visual recovery observed with ON associated with NMOSD, MOG, GFAP and other inflammatory diseases (see “Prognosis”), acute treatment algorithms are being proposed based on expert opinion and retrospective studies (Table 4). It is important to appreciate that the results of the ONTT may not apply to all ON subtypes since only three trial participants were MOG-IgG positive and none were positive for AQP4-IgG (177 out of 457 participants total had serum available for analysis).[49] Furthermore, IV corticosteroids (e.g. methylprednisolone 1g daily IV for 3 to 5 days) alone may be suboptimal for visual recovery in non-MS-ON variants, particularly NMOSD-ON.[50] Retrospective studies on the acute treatment of NMOSD-ON support the early use of plasma exchange as add-on therapy.[14, 51, 52].
Table 4.
| Treatment phase | Multiple sclerosis/idiopathic[7, 20] | Neuromyelitis Optica Spectrum Disorder[20, 57] | Myelin oligogendrocyte glycoprotein[72] | Glial fibrillary acidic protein[20, 74] |
|---|---|---|---|---|
| Acute | Consider high dose corticosteroids for 3 days | High dose corticosteroids for 3–5 days and plasma exchange | High dose corticosteroids for 3–5 days followed by a 1 to 3 week corticosteroid taper. Consider plasma exchange if no recovery within 1–2 weeks and vision loss is severe | High dose corticosteroids followed by a corticosteroid taper over weeks-to-months. Consider adding plasma exchange or intravenous immunoglobulin |
| Long term | Disease modifying therapy | Immunosuppression | Immunosuppression if poor visual recovery or relapsing disease | May require immunosuppression |
LONGTERM SURVEILLANCE AND MANAGEMENT
Multiple Sclerosis
As noted previously, all patients diagnosed with ON should undergo MRI to evaluate for MS or risk of developing MS. The ONTT found that half of all patients with ON will develop clinically definite MS after 15 years, the highest risk occurring in those patients with at least one white matter lesion of 3 mm or more. As the diagnostic criteria for MS have increased in sensitivity since the completion of the ONTT, the risk of MS following an episode of ON is now likely higher, and the 2017 McDonald Criteria allow for the diagnosis of MS to be made after a single, isolated attack of ON based on MRI criteria for dissemination in space, and MRI or CSF criteria for dissemination in time.[53] Importantly, the radiological lesion in the optic nerve itself, cannot be counted toward meeting MRI criteria for dissemination in space or time. Injectable, infusion, and oral-based disease modifying therapies for the treatment of MS exist to slow disease progression. For patients with isolated ON with normal MR imaging, or for ON patients with MRI lesions that do not meet criteria for MS, screening neurologic examinations and yearly surveillance brain MRI for 5 years may be considered.[54, 55] Impromptu imaging should occur if any new neurologic signs or symptoms arise.
Other CNS autoimmune disorders with optic neuritis
Standard recommendations for long term surveillance or treatment of other disorders that may manifest with ON are currently evolving. The primary goal is to detect the earliest evidence of ongoing CNS inflammation and select therapies that will minimize new attacks and mitigate long term disability.
If diagnostic criteria for NMOSD are met[56], immunosuppression should commence immediately to prevent further neurologic disability. The optimal first line agent and duration of treatment in this context are uncertain,[57] but have historically included rituximab,[58] azathioprine,[59, 60] mycophenolate mofetil,[61] and chronic corticosteroids.[62, 63] Recent Phase 3 clinical trials have resulted in the emergence of 3 new therapeutics that significantly reduce the risk of future NMOSD attacks (drug mechanism; proportion with attacks in the treatment group versus controls): eculizumab (complement C5 inhibition; 3% [3 of 96] vs. 43% [20 of 47], hazard ratio 0.06 [95% CI, 0.02 to 0.20]; p<.001)[64], satralizumab (IL-6 receptor inhibition; 20% [8 of 41] vs. 43% [18 of 42] 0.38, hazard ratio 0.38 [95% CI: 0.16 to 0.88], p=0.02)[65], and inebilizumab (CD-19-targeted B cell depletion; 12% [21 of 174] vs. 39% 22 of 56], 0. 27 [95% CI 0.15 to 0.50]; p<.001 )[66].
MOG-ON may be monophasic, but up to 85% may have relapsing disease.[11, 67–71] Therefore, expert opinion currently suggests that long term immunosuppression should be considered after the first attack if visual recovery is poor or in patients who have experienced multiple attacks.[72] In one recent retrospective multicenter study of 70 MOG-ON patients, the annualized relapse rate was 1.6 prior to initiating immunosuppressive therapy and 0.3 following immunosuppression suggesting that immunosuppression is effective in suppressing relapses.[73] The optimal therapy and duration of treatment remain unclear, but the most commonly used treatments include intravenous immunoglobulin, rituximab, mycophenolate mofetil, and azathioprine.[11, 72, 73]
GFAP may also have monophasic, relapsing, or progressive course.[8, 74] Long term immunosuppressive therapy may be needed for some patients. For relapsing ON and inflammatory optic neuropathies, such as those secondary to rheumatologic conditions, similar steroid sparing agents are often necessary to allow corticosteroid weaning.
PROGNOSIS
For patients with idiopathic or MS-ON, recovery of visual acuity is good.[7, 75] At one year, regardless of treatment, 75% have a visual acuity of 20/20 or better and 95% have 20/40 or better acuity.[47, 75] Only 2.4% of idiopathic or MS-ON patients have a recovered visual acuity of 20/200 or worse.[75] Despite visual improvement, patients are often left with reductions in visual quality of life likely attributable to incomplete recovery of contrast sensitivity or higher order visual function.[76] Racial and gender disparities in visual recovery after idiopathic and MS-ON also exist, with worse outcomes noted among men and non-white patients.[77, 78]
For patients with MOG-ON, the final visual outcomes tend to be favorable. In the largest series to date, 5.7% (5 of 87) of patients were left with a visual acuity of 20/200 or worse, which is slightly worse than the prognosis for idiopathic or MS-ON.[11] Of note, the five patients in the study with poor recovery were treated with IV corticosteroids and one also underwent plasma exchange. For patients with NMOSD-ON, visual recovery is not as robust and worsens with subsequent episodes. Approximately 20–30% of NMOSD patients will remain functionally blind in the affected eye (20/200 or worse) after their initial ON episode whereas approximately 70% of those with a relapsing course will have a visual acuity of 20/200 or worse in the affected eye(s).[79, 80] Vision is typically preserved in patients with GFAP throughout their course. Visual prognosis is uncertain for other inflammatory optic neuropathies.
CONCLUSION
ON is a common cause of vision loss, but the causes, and treatments vary. Knowledge of the clinical signs and symptoms of ON subtypes is essential to guide diagnostic investigations, accurately prognosticate recovery, delineate treatment, and identify CNS inflammatory disorders. Previously, the ONTT experience highlighted the favorable natural history of ON, and informed our understanding regarding the association between ON and future MS risk. In the current era, however, treatment recommendations from the ONTT are not extendable to other ON subtypes, particularly NMOSD-ON and MOG-ON. Understanding the diverse pathophysiology of optic nerve inflammatory injury is likely to yield novel acute and prophylactic therapies for established and emerging ON subtypes in the future.
Acknowledgments
Funding: Dr. De Lott is supported by the National Institutes of Health, Bethesda, MD (K23EY027849). The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of interest/Competing interests: Dr. De Lott is supported by the National Institutes of Health, Bethesda, MD (K23EY027849). Dr. Bennett is supported by the National Institutes of Health (R01EY022936, R01NS115488) and the Guthy Jackson Charitable Foundation. Dr. Bennett reports payment for study design/consultation from MedImmune/Viela Bio, personal fees from AbbVie, Alexion, Chugai, Clene Nanomedicine, Equillium, Frequency Therapeutics, Genentech, Mitsubishi-Tanabe, Reistone-Bio, and Roche, grants and personal fees from Novartis, grants from Mallinckrodt, and has a patent for Aquaporumab issued. Dr. Costello has received payments for consultation from Roche, Alexion, and Frequency Therapeutics.
Footnotes
Ethics approval: Not applicable
Consent to participate: Not applicable
Consent for publication: Not applicable
Availability of data and material: Not applicable
Code availability: Not applicable
ETHICAL STANDARDS
The manuscript does not contain clinical studies or patient data.
Declarations
REFERENCES
- 1.Rodriguez M, et al. , Optic neuritis: a population-based study in Olmsted County, Minnesota. Neurology, 1995. 45(2): p. 244–250. [DOI] [PubMed] [Google Scholar]
- 2.Percy AK, Nobrega FT, and Kurland LT, Optic neuritis and multiple sclerosis. An epidemiologic study. Arch Ophthalmol, 1972. 87(2): p. 135–9. [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Lapiscina EH, et al. , Is the incidence of optic neuritis rising? Evidence from an epidemiological study in Barcelona (Spain), 2008–2012. Journal of neurology, 2014. 261(4): p. 759–767. [DOI] [PubMed] [Google Scholar]
- 4.Soelberg K, et al. , A population-based prospective study of optic neuritis. Multiple sclerosis (Houndmills, Basingstoke, England), 2017. 23(14): p. 1893–1901. [DOI] [PubMed] [Google Scholar]
- 5.Woung LC, et al. , Optic neuritis among National Health Insurance enrollees in Taiwan, 2000–2004. Neuroepidemiology, 2007. 29(3–4): p. 250–4. [DOI] [PubMed] [Google Scholar]
- 6.Stunkel L, et al. , Incidence and Causes of Overdiagnosis of Optic Neuritis. JAMA Ophthalmol, 2018. 136(1): p. 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck RW, et al. , A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med, 1992. 326(9): p. 581–8. [DOI] [PubMed] [Google Scholar]
- 8.Chen JJ, et al. , Optic Disc Edema in Glial Fibrillary Acidic Protein Autoantibody-Positive Meningoencephalitis. J Neuroophthalmol, 2018. 38(3): p. 276–281. [DOI] [PubMed] [Google Scholar]
- 9.Mealy MA, et al. , Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol, 2012. 69(9): p. 1176–80. [DOI] [PubMed] [Google Scholar]
- 10.Papais-Alvarenga RM, et al. , Clinical course of optic neuritis in patients with relapsing neuromyelitis optica. Arch Ophthalmol, 2008. 126(1): p. 12–6. [DOI] [PubMed] [Google Scholar]
- 11.Chen JJ, et al. , Myelin Oligodendrocyte Glycoprotein Antibody-Positive Optic Neuritis: Clinical Characteristics, Radiologic Clues, and Outcome. Am J Ophthalmol, 2018. 195: p. 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The clinical profile of optic neuritis. Experience of the Optic Neuritis Treatment Trial. Optic Neuritis Study Group. Arch Ophthalmol, 1991. 109(12): p. 1673–8. [DOI] [PubMed] [Google Scholar]
- 13.Bennett JL, Optic Neuritis. Continuum (Minneap Minn), 2019. 25(5): p. 1236–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merle H, et al. , Treatment of optic neuritis by plasma exchange (add-on) in neuromyelitis optica. Arch Ophthalmol, 2012. 130(7): p. 858–62. [DOI] [PubMed] [Google Scholar]
- 15.Sato DK, et al. , Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology, 2014. 82(6): p. 474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costello F, Inflammatory optic neuropathies. Continuum (Minneap Minn), 2014. 20(4 Neuro-ophthalmology): p. 816–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor TR, et al. , Prevalence and demographics of multiple sclerosis-associated uveitis: a UK biobank study. Mult Scler Relat Disord, 2020. 43: p. 102209. [DOI] [PubMed] [Google Scholar]
- 18.Ramanathan S, et al. , Uveitis and optic perineuritis in the context of myelin oligodendrocyte glycoprotein antibody seropositivity. Eur J Neurol, 2019. 26(8): p. 1137–e75. [DOI] [PubMed] [Google Scholar]
- 19.Chen J.J. a.C., F., The role of optical coherence tomography in neuro-ophthalmology. Annals of Eye Science, 2018. 3(6). [Google Scholar]
- 20.Horton L and Bennett JL, Acute Management of Optic Neuritis: An Evolving Paradigm. J Neuroophthalmol, 2018. 38(3): p. 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hokari M, et al. , Clinicopathological features in anterior visual pathway in neuromyelitis optica. Ann Neurol, 2016. 79(4): p. 605–24. [DOI] [PubMed] [Google Scholar]
- 22.Tsoi VL, et al. , Immunohistochemical evidence of inducible nitric oxide synthase and nitrotyrosine in a case of clinically isolated optic neuritis. J Neuroophthalmol, 2006. 26(2): p. 87–94. [DOI] [PubMed] [Google Scholar]
- 23.Lennon VA, et al. , A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet, 2004. 364(9451): p. 2106–12. [DOI] [PubMed] [Google Scholar]
- 24.Lennon VA, et al. , IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med, 2005. 202(4): p. 473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asavapanumas N, et al. , Experimental mouse model of optic neuritis with inflammatory demyelination produced by passive transfer of neuromyelitis optica-immunoglobulin G. J Neuroinflammation, 2014. 11: p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett JL, et al. , Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol, 2009. 66(5): p. 617–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan T, Smith AJ, and Verkman AS, Complement-dependent bystander injury to neurons in AQP4-IgG seropositive neuromyelitis optica. J Neuroinflammation, 2018. 15(1): p. 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hillebrand S, et al. , Circulating AQP4-specific auto-antibodies alone can induce neuromyelitis optica spectrum disorder in the rat. Acta Neuropathol, 2019. 137(3): p. 467–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weil MT, et al. , Loss of Myelin Basic Protein Function Triggers Myelin Breakdown in Models of Demyelinating Diseases. Cell Rep, 2016. 16(2): p. 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herwerth M, et al. , In vivo imaging reveals rapid astrocyte depletion and axon damage in a model of neuromyelitis optica-related pathology. Ann Neurol, 2016. 79(5): p. 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Bennett JL, and Verkman AS, Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann Neurol, 2011. 70(6): p. 943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saadoun S, et al. , Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain, 2010. 133(Pt 2): p. 349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peschl P, et al. , Human antibodies against the myelin oligodendrocyte glycoprotein can cause complement-dependent demyelination. J Neuroinflammation, 2017. 14(1): p. 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spadaro M, et al. , Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann Neurol, 2018. 84(2): p. 315–328. [DOI] [PubMed] [Google Scholar]
- 35.Kitley J, et al. , Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology, 2012. 79(12): p. 1273–7. [DOI] [PubMed] [Google Scholar]
- 36.Probstel AK, et al. , Anti-MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype. J Neuroinflammation, 2015. 12: p. 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manogaran P, et al. , Retinal pathology in experimental optic neuritis is characterized by retrograde degeneration and gliosis. Acta Neuropathol Commun, 2019. 7(1): p. 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang B, et al. , Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy: A Novel Meningoencephalomyelitis. JAMA Neurol, 2016. 73(11): p. 1297–1307. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, et al. , Myelin-specific multiple sclerosis antibodies cause complement-dependent oligodendrocyte loss and demyelination. Acta Neuropathol Commun, 2017. 5(1): p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bursztyn LDLLB, Cornblath WT, Sensitivity of Magnetic Resonance Imaging for Acute Demyelinating Optic Neuritis. 2015, North American Neuro-Ophthalmology Society Meeting, San Diego, CA. [Google Scholar]
- 41.Kupersmith MJ, et al. , Contrast-enhanced MRI in acute optic neuritis: relationship to visual performance. Brain, 2002. 125(Pt 4): p. 812–22. [DOI] [PubMed] [Google Scholar]
- 42.McKinney AM, et al. , Accuracy of routine fat-suppressed FLAIR and diffusion-weighted images in detecting clinically evident acute optic neuritis. Acta Radiol, 2013. 54(4): p. 455–61. [DOI] [PubMed] [Google Scholar]
- 43.Storoni M, et al. , Distinguishing optic neuritis in neuromyelitis optica spectrum disease from multiple sclerosis: a novel magnetic resonance imaging scoring system. J Neuroophthalmol, 2013. 33(2): p. 123–7. [DOI] [PubMed] [Google Scholar]
- 44.Traboulsee A, et al. , Revised Recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. AJNR Am J Neuroradiol, 2016. 37(3): p. 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majed M, et al. , Clinical utility of testing AQP4-IgG in CSF: Guidance for physicians. Neurol Neuroimmunol Neuroinflamm, 2016. 3(3): p. e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gal RL, Vedula SS, and Beck R, Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev, 2015(8): p. CD001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Lott LB, et al. , Association of Individual-Level Factors With Visual Outcomes in Optic Neuritis: Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open, 2020. 3(5): p. e204339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrow SA, et al. , Effect of Treating Acute Optic Neuritis With Bioequivalent Oral vs Intravenous Corticosteroids: A Randomized Clinical Trial. JAMA Neurol, 2018. 75(6): p. 690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen JJ, et al. , Prevalence of Myelin Oligodendrocyte Glycoprotein and Aquaporin-4-IgG in Patients in the Optic Neuritis Treatment Trial. JAMA Ophthalmol, 2018. 136(4): p. 419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleiter I, et al. , Neuromyelitis optica: Evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol, 2016. 79(2): p. 206–16. [DOI] [PubMed] [Google Scholar]
- 51.Kleiter I, et al. , Apheresis therapies for NMOSD attacks: A retrospective study of 207 therapeutic interventions. Neurol Neuroimmunol Neuroinflamm, 2018. 5(6): p. e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonnan M, et al. , Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry, 2018. 89(4): p. 346–351. [DOI] [PubMed] [Google Scholar]
- 53.Thompson AJ, et al. , Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol, 2018. 17(2): p. 162–173. [DOI] [PubMed] [Google Scholar]
- 54.Rae-Grant A, et al. , Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology, 2018. 90(17): p. 777–788. [DOI] [PubMed] [Google Scholar]
- 55.Excellence N.I. f.H.a.C. Multiple sclerosis in adults: management. 2014. November, 11, 2019 [cited 2020 November 27]; Available from: https://www.nice.org.uk/guidance/cg186/chapter/Recommendations#diagnosing-ms.
- 56.Wingerchuk DM, et al. , International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology, 2015. 85(2): p. 177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romeo AR and Segal BM, Treatment of neuromyelitis optica spectrum disorders. Curr Opin Rheumatol, 2019. 31(3): p. 250–255. [DOI] [PubMed] [Google Scholar]
- 58.Damato V, Evoli A, and Iorio R, Efficacy and Safety of Rituximab Therapy in Neuromyelitis Optica Spectrum Disorders: A Systematic Review and Meta-analysis. JAMA Neurol, 2016. 73(11): p. 1342–1348. [DOI] [PubMed] [Google Scholar]
- 59.Bichuetti DB, et al. , Treating neuromyelitis optica with azathioprine: 20-year clinical practice. Mult Scler, 2019. 25(8): p. 1150–1161. [DOI] [PubMed] [Google Scholar]
- 60.Costanzi C, et al. , Azathioprine: tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology, 2011. 77(7): p. 659–66. [DOI] [PubMed] [Google Scholar]
- 61.Huang Q, et al. , Low-Dose Mycophenolate Mofetil for Treatment of Neuromyelitis Optica Spectrum Disorders: A Prospective Multicenter Study in South China. Front Immunol, 2018. 9: p. 2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe S, et al. , Low-dose corticosteroids reduce relapses in neuromyelitis optica: a retrospective analysis. Mult Scler, 2007. 13(8): p. 968–74. [DOI] [PubMed] [Google Scholar]
- 63.Berger JR, Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord, 2017. 12: p. 59–63. [DOI] [PubMed] [Google Scholar]
- 64.Pittock SJ, et al. , Eculizumab in Aquaporin-4-Positive Neuromyelitis Optica Spectrum Disorder. N Engl J Med, 2019. 381(7): p. 614–625. [DOI] [PubMed] [Google Scholar]
- 65.Yamamura T, et al. , Trial of Satralizumab in Neuromyelitis Optica Spectrum Disorder. N Engl J Med, 2019. 381(22): p. 2114–2124. [DOI] [PubMed] [Google Scholar]
- 66.Cree BA, et al. , Placebo-controlled study in neuromyelitis optica-Ethical and design considerations. Mult Scler, 2016. 22(7): p. 862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Mol CL, et al. , The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult Scler, 2020. 26(7): p. 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jarius S, et al. , MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation, 2016. 13(1): p. 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jarius S, et al. , MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation, 2016. 13(1): p. 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jurynczyk M, et al. , Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain, 2017. 140(12): p. 3128–3138. [DOI] [PubMed] [Google Scholar]
- 71.Lopez-Chiriboga S, et al. , Long-term Outcomes in Patients With Myelin Oligodendrocyte Glycoprotein Immunoglobulin G-Associated Disorder. JAMA Neurol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen JJ and Bhatti MT, Clinical phenotype, radiological features, and treatment of myelin oligodendrocyte glycoprotein-immunoglobulin G (MOG-IgG) optic neuritis. Curr Opin Neurol, 2020. 33(1): p. 47–54. [DOI] [PubMed] [Google Scholar]
- 73.Chen JJ, et al. , Steroid-sparing maintenance immunotherapy for MOG-IgG associated disorder. Neurology, 2020. 95(2): p. e111–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flanagan EP, et al. , Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: Analysis of 102 patients. Ann Neurol, 2017. 81(2): p. 298–309. [DOI] [PubMed] [Google Scholar]
- 75.Beck RW, Cleary PA, and Backlund JC, The course of visual recovery after optic neuritis. Experience of the Optic Neuritis Treatment Trial. Ophthalmology, 1994. 101(11): p. 1771–8. [DOI] [PubMed] [Google Scholar]
- 76.Sabadia SB, et al. , 20/40 or Better Visual Acuity After Optic Neuritis: Not as Good as We Once Thought? J Neuroophthalmol, 2016. 36(4): p. 369–376. [DOI] [PubMed] [Google Scholar]
- 77.Moss HE, et al. , Association of race/ethnicity with visual outcomes following acute optic neuritis: an analysis of the Optic Neuritis Treatment Trial. JAMA Ophthalmol, 2014. 132(4): p. 421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costello F, et al. , Sex-specific differences in retinal nerve fiber layer thinning after acute optic neuritis. Neurology, 2012. 79(18): p. 1866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wingerchuk DM, et al. , The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology, 1999. 53(5): p. 1107–14. [DOI] [PubMed] [Google Scholar]
- 80.Merle H, et al. , Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology, 2007. 114(4): p. 810–5. [DOI] [PubMed] [Google Scholar]
- 81.White D, et al. , Enlarged and Enhancing Optic Nerves in Advanced Glial Fibrillary Acidic Protein Meningoencephalomyelitis. J Neuroophthalmol, 2019. 39(3): p. 411–415. [DOI] [PubMed] [Google Scholar]
- 82.Ruiz-Gaviria R, et al. , Specificity and sensitivity of aquaporin 4 antibody detection tests in patients with neuromyelitis optica: A meta-analysis. Mult Scler Relat Disord, 2015. 4(4): p. 345–9. [DOI] [PubMed] [Google Scholar]
- 83.Roed HG, et al. , A double-blind, randomized trial of IV immunoglobulin treatment in acute optic neuritis. Neurology, 2005. 64(5): p. 804–10. [DOI] [PubMed] [Google Scholar]
- 84.Tsakiri A, et al. , Simvastatin improves final visual outcome in acute optic neuritis: a randomized study. Mult Scler, 2012. 18(1): p. 72–81. [DOI] [PubMed] [Google Scholar]
- 85.Suhs KW, et al. , A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Annals of Neurology, 2012. 72(2): p. 199–210. [DOI] [PubMed] [Google Scholar]
- 86.Raftopoulos R, et al. , Phenytoin for neuroprotection in patients with acute optic neuritis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol, 2016. 15(3): p. 259–69. [DOI] [PubMed] [Google Scholar]
- 87.Cadavid D, et al. , Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol, 2017. 16(3): p. 189–199. [DOI] [PubMed] [Google Scholar]
- 88.McKee JB, et al. , Amiloride does not protect retinal nerve fibre layer thickness in optic neuritis in a phase 2 randomised controlled trial. Mult Scler, 2019. 25(2): p. 246–255. [DOI] [PubMed] [Google Scholar]
- 89.Albert C, et al. , Fingolimod after a first unilateral episode of acute optic neuritis (MOVING) - preliminary results from a randomized, rater-blind, active-controlled, phase 2 trial. BMC Neurol, 2020. 20(1): p. 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Falardeau J, et al. , Oral lipoic acid as a treatment for acute optic neuritis: a blinded, placebo controlled randomized trial. Mult Scler J Exp Transl Clin, 2019. 5(2): p. 2055217319850193. [DOI] [PMC free article] [PubMed] [Google Scholar]