Abstract
Purpose
To analyse the clinical outcome in patients with meibomian gland dysfunction (MGD) who underwent intense pulsed light (IPL) plus low-level light therapy (LLL).
Materials and Methods
The prospective non-comparative study included identified by MGD patients with altered interferometry and lower loss area of the meibomian glands (LAMG), who underwent IPL plus LLL, between July 2020 and August 2020. A multimodal assessment was performed before, 2–3 weeks, and 6 months after treatment. The main outcome was lipid layer thickness (LLT) and the secondary outcomes were the ocular surface disease index (OSDI) score, presence of corneal fluorescein staining (CFS), blink rate (BR), Schirmer test (ST), tear meniscus height (TMH), tear osmolarity (OSM), non-invasive break-up time (NIBUT) and LAMG.
Results
This study included 62 eyes of 31 patients, 61.3% female, with a mean age of 66.94±9.08 years at the time of IPL plus LLL treatment. LLT (<0.001) grades improved 6 months after treatment. The mean OSDI score improved (p<0.001) from 45.02±21.17 (severe symptoms) to 22.35±17.68 (moderate symptoms) at 2–3 weeks and 8.24±17.9.91 (normal) at 6 months after treatment. CFS was identified in 51.6% (32/62) before and in 45.2% (28/62) 6 months (p=0.293) after treatment. ST (p=0.014) grades improved; OSM grades mild worsened (p<0.001); TMH, NIBUT and LAMG grades did not modify 6 months after treatment. No patient suffered any adverse effects.
Conclusion
IPL combined with LLL was effective and safe, improving the lipid layer thickness in MGD and decreasing the level of symptoms.
Keywords: meibomian gland dysfunction, dry eye disease, intense pulsed light, level low light treatment, lipid layer thickness, OSDI
Introduction
Dry eye disease (DED) is a multifactorial disease of the ocular surface in which there is a loss of the tear film homeostasis and ocular symptoms.1 Meibomian gland dysfunction (MGD), considered the major cause of DED,2 can modify negatively the quality and quantity of tear film, and subsequently, ocular surface damage can occur.3
For diagnosis and assessment of dry eye, the evaluation of patient-reported symptoms, tear film stability (eg non-invasive break-up time, tear osmolarity), tear volume (eg tear meniscus height and Schirmer test), tear film composition (eg tear osmolarity), damage to ocular surface (eg ocular surface staining) and eyelid aspects (eg interferometry, meibography, and blink/lid closure analysis) is recommended.4 Regarding symptomatology, the ocular surface disease index (OSDI) is one of the most widely used and validated questionnaires for measuring the subjective severity of DED, analyzing the frequency of symptoms, environmental triggers, and vision-related quality of life.5,6
Diagnostic labels influence subsequent management and treatment. It is believed that most patients with DED suffer from different combinations of MGD (evaporative type) and tear underproduction (aqueous deficient type),7 often making effective therapy a challenge. The classic treatment options for MGD (eg warm compresses, eyelid hygiene, anti-inflammatory and antibiotic agents, dietary supplements) are unsatisfactory.8
New potential therapeutic options have been explored. Intense pulsed light therapy (IPL) is a light-emitting system and has been reported as an effective and safe procedure in MGD.9 The emission wavelength of IPL discharged from Xenon flash lamps, ranging from 400 to 1200 nm, is within the visible light and infrared radiation wavelength of the electromagnetic spectrum. The produced broad wavelength is considered advantageous, as it can be absorbed by a variety of chromophores, eg, melanin (400–750 nm) and hemoglobin (578 nm), to develop heat.9 Low-level light therapy (LLL) is a different kind of photobiomodulation. LLL therapy can be defined as the use of low-power monochromatic light from light-emitting diodes (LEDs) in the red to near-infrared wavelengths (λ= 600–1100 nm) to modulate a biological function or induce a therapeutic effect in a nondestructive and nonthermal manner, different from IPL. LLLT differs from the conventional effects of high-energy photon delivery commonly associated with lasers, which are mediated by a greater release of energy and result in heating and tissue destruction. The effects of LLLT implicate conversion of luminous energy to metabolic energy with subsequent modulation of the biological functioning of cells.10 LLL seems to have potential benefits in retinal disease, stroke, neurotrauma, neurodegeneration, memory, and mood disorders10 and improving the tear break up time in MGD.11
According to the authors’ knowledge, there is only one published study in the literature about the effects of IPL plus LLL on clinical measures of dry eye related to severe MGD.12 Our study aimed to evaluate the clinical outcomes of the same treatment in MGD, including patients with altered interferometry but with lower loss area of the meibomian glands, varying degrees of dry eye severity, in a multimodal assessment.
Materials and Methods
Study Design
A prospective study including patients with MGD who underwent IPL plus LLL in the Ophthalmology Department of Centro Hospitalar Universitário do Porto (CHUPorto), between July 2020 and August 2020. This study was conducted following the tenets of the Declaration of Helsinki (1964). The authors ensured that all patients’ anonymity was carefully protected. Informed consent for procedures and the use of data for publication was signed for all patients. Approval was obtained from the “Departamento de Ensino, Formação e Investigação” (DEFI).
Participants
The inclusion criteria were as follows: adult patients; clinical signs of MGD (meibomian orifice plugging, eyelid margin foaminess, and hyperemia/telangiectasias) with low loss area of the meibomian glands and with altered interferometry; ability to cooperate in treatment and follow-up visits; absence of contraindications for IPL and LLL treatment; IPL plus LLL treatment during 3 sessions separated by 1 week between them, with complete clinical evaluation (defined in the parameters section) made before and after 2–3 weeks and 6 months of treatment, according to the study protocol.
The contraindications for IPL and LLL treatment in our study were as follows: pregnancy, epilepsy, piercings in the treatment area, photosensitizing drugs, skin cancer history in the treatment area, keloid history, psoriasis, vitiligo, lupus disease, or another connective tissue disease, herpes zoster infection, skin photo Fitzpatrick type VI and toxin botulinum 1 week before and 2 weeks after treatment.
Patients who missed a treatment session or clinical evaluation were included in “loss to follow-up” and they were excluded from outcome analysis.
Treatment Protocol
All treatments were carried out by four doctors (AM, PMB, JHM, and IB). In all patients, Eye-light® and the MY MASK-E® (Espansione Marketing S.p.A., Bologna, Italy) were used for IPL and LLL treatment, respectively (Table 1). The level of energy delivered is automatically customized for each patient, and the device’s algorithm determined it according to two parameters introduced in the device by the doctor: the degree of skin pigmentation (subjectively evaluated with skin Fitzpatrick scale) and the severity of dry eye (determined by ME-CHECK® Screening). ME-CHECK is a device that analyzes the automatic calculation of meibomian gland patency percentage and the automatic OSDI-6 questionnaire, assigning a severity level of dry eye in the end.
Table 1.
Technical Specifications of the IPL and LLL Treatment
| Parameters | IPL (Eye-Light®) | LLL (MY MASK-E®) |
|---|---|---|
| Protective device | Yes | No |
| Patient position | Reclined in an armchair | Supine position |
| Type of application | 5 painless light shots | One mask |
| Treatment duration | 5 minutes | 15 minutes |
| Anatomic target | Lower lid | Upper and lower lids |
| Type of laser | High power (short, hot pulses of light) | Low power (long wavelength, red LED light) |
| Interaction with tissue | Photo-thermal | Photobiomodulation |
| Energy level delivered | Automatically customized treatments based on the the degree of skin pigmentation (subjectively evaluated with skin Fitzpatrick scale) and the severity of dry eye (determined by ME-CHECK® Screening). | |
Abbreviations: IPL, intense pulsed light; LLL, low level light; LED, light emitting diode.
Treatment for all patients started with IPL followed by LLL application. During the IPL treatment, patients’ eyes were covered with a protective device, as recommended in the manufacturer’s user manual, and patients were reclined in an armchair. IPL consisted of five painless light shots: three were placed along the inferior orbital rim with the device in the vertical position, the 4th delivered vertically behind the lateral canthus and the 5th was delivered with the device horizontal along the inferior orbital rim. This sequence was repeated for the contralateral eye and it took less than 5 minutes overall. Then, LLL treatment was performed with no protective device and with patients in the supine position. Patients were instructed to keep their eyes closed, ensuring application to the upper and lower lids. One mask was placed on the patients’ periorbital area and it took 15 minutes. After treatment, sunscreen was applied to the treatment area and all patients were instructed to maintain their artificial eye drops, gels, or ointments until the next evaluation. About 77% of cases had already used it: 42% without and 35 with preservatives. Nothing was changed. For those who weren´t using drops, gels or ointments, nothing was added. Combined treatment was performed during three sessions separated by 1 week between them.
Parameters
The following variables were analyzed: demographic characteristics; systemic and ocular comorbidities; and complete clinical evaluation made before, 2–3 weeks, and 6 months after IPL plus LLL treatment.
The complete clinical evaluation included:
- The symptom analysis:
- -the validated questionnaire OSDI 12: the final OSDI score, ranges from 0 to 100, with higher values indicating greater severity [normal (<12), mild (13–22), moderate (23–32), or severe (33–100)].
- -the subjective end report answered by each patient after treatment as “better”, “same” and “worse”.
- The ocular surface analysis:
- -Slip lamp evaluation of the ocular surface: identify whether corneal fluorescein staining (CFS) or other changes associated with dry eye were present.
- -Quality of tear film: Schirmer test, tear osmolarity, non-invasive break-up time (NIBUT), blink rate, lipid layer thickness, loss area of the meibomian glands, and tear meniscus height.
We used Schirmer test I to evaluated tear production. The tear osmolarity was measured by TearLab® Osmolarity System (Tearlab, San Diego, CA, USA). The NIBUT, blink rate, lipid layer thickness through auto-interferometry, loss area of the meibomian glands through meibography, and tear meniscus height were measured by IDRA® Ocular Surface Analyser (SBM SISTEMI, Italy). The blink rate is a parameter that determines the quality of blinking. It takes into account blink frequency, count of full blinks, count of partial blinks, and inter-blink duration.
The main outcome was lipid layer thickness and the secondary outcomes were as follows: the OSDI score, presence of CFS, blink rate, Schirmer test, tear meniscus height, tear osmolarity, non-invasive break-up time, and loss area of the meibomian glands.
Statistical Analysis
Statistical analysis was performed using the SPSS program (SPSS Statistics, version 22.0 for Windows, SPSS Inc., IBM, Somers, NY). The normality of the variables was evaluated by the Kolmogorov–Smirnov test. For pre and post-treatment analysis, the Wilcoxon test and paired sample t-test were used. The comparison between independent continuous variables was performed using the Mann–Whitney test and T-Student test. The Fisher exact test was used for nominal scaled data. Spearman’s bivariate correlation test was applied to study correlations. P values less than 0.05 were considered statistically significant.
Results
Demographic Data
Sixty-four eyes of thirty-two patients were initially included. Two eyes of one patient belong to “loss to follow-up” due to one missed session and they were not included in the analysis. Therefore, we analysed 62 eyes of 31 patients, 38.70% male and 61.29% female, aged 51 to 81 years, with a mean age of 66.94±9.08 years (y) at the time of IPL and LLP treatment (Table 2). Regarding systemic factors, 93.55% of cases were diabetic patients (86.21% of type 2) and 64.52% had systemic arterial hypertension.
Table 2.
Demographic Data (n=62 Eyes/31 Patients)
| Gender | 38.70% Male/ 61.29% Female |
| Age | 66.94±9.08 [51 to 81] |
| Systemic Pathology | |
| Diabetes Mellitus | 93.55% (29/31) |
| Systemic arterial hypertension | 64.52% (20/31) |
| Previous ocular surgery | |
| Cataract surgery | 17.74% (11/62) |
| Pars plana vitrectomy | 3.23% (2/62) |
| Anti-angiogenic intravitreous injections | 8.06% (5/62) |
| Eye medications | |
| Lowering ocular hypertension drops | 19.35% (12/62) |
| Artificial eye drops, gels, or ointments | 77.42% (48/62) |
| -Preservative-free | 41.94% (26/62) |
| -Contain preservative | 35.48% (22/62) |
Concerning ocular history, 17.74% had undergone cataract surgery, 3.23% pars plana vitrectomy, and 8.06% anti-angiogenic intravitreous injections previously. Lowering ocular hypertension (OHT) drops were present in 19.35% of cases. The previously most often reported symptoms were as follows: foreign body sensation (59.50%), itching (47.60%), watering (28.60%), and burning (19.00%).
Clinical Outcomes
Questionnaire
The mean OSDI score improved (p<0.001) from 45.02±21.17 (severe symptoms) to 22.35±17.68 (moderate symptoms) at 2–3 weeks and 8.24±17.9.91 (normal) at 6 months after IPL plus LLL treatment (Table 3). Regarding OSDI score, 91.93% and 100% of cases improved OSDI score at 2–3 weeks and 6 months after treatment, respectively. Overall, there was a significant improvement of OSDI grades at 2–3 weeks (p<0.001) and 6 months (p<0.001) after treatment. At 2–3 weeks after treatment, OSDI grade improved in 53.22%, remained similar in 43.55%, and worsened in 3.23% of eyes. At 6 months after treatment, OSDI grade improved in 89.66%, remained similar in 10.34%, and worsened in 0% of eyes. Within the group of eyes with severe symptoms before treatment: 60.47% improved to normal, 20.93% to mild, and 18.60% to moderate category. All eyes with moderate or mild symptoms improved to the normal category. All 6 eyes with normal category maintained the normal category. The OSDI grade frequencies distribution can be better visualized in Figure 1.
Table 3.
Outcomes Before and After IPL+LLL Treatment (n=62)
| Variables | Before IPL+LLL* | 2–3 Weeks After IPL+LLL* | P value | 6 Months After IPL+LLL* | P value |
|---|---|---|---|---|---|
| OSDI score | 45.02±21.17 | 22.35±17.68 | <0.001 | 8.24±9.91 | <0.001 |
| Corneal fluorescein staining | 51.6 | 54.84 | <0.001 | 45.2 | 0.293 |
| NIBUT, sec | 10.18±4.55 | 9.73±3.30 | 0.751 | 9.85±2.41 | 0.792 |
| Blink rate, % | 88.77±22.94 | 98.55±4.42 | 0.004 | 63.22±17.04 | <0.001 |
| Lipid layer thickness, nm | 47.42±23.64 | 66.37±30.29 | <0.001 | 73.93±27.03 | <0.001 |
| Loss area of the MG, % | 10.87±15.77 | 9.11±12.37 | 0.722 | 16.71±16.77 | 0.044 |
| Tear meniscus height, mm | 0.33±0.15 | 0.28±0.16 | 0.001 | 0.33±0.26 | 0.891 |
| Tear osmolarity, mOsm/L | 298.08±11.25 | 306.74±11.81 | <0.001 | 315.49±16.86 | <0.001 |
| Schirmer, mm | 9.63±5.50 | 11.26±6.83 | 0.014 | 11.36±5.60 | 0.008 |
Notes: *values represent “mean±SD”, excepting corneal staining that was represented as percentage. Bold text represents a statistically significant p-value.
Abbreviations: IPL, intense pulsed light; LLL, low level light; OSDI, ocular surface disease index; NIBUT, non-invasive break up time; MG, meibomian glands; SD, standard deviation.
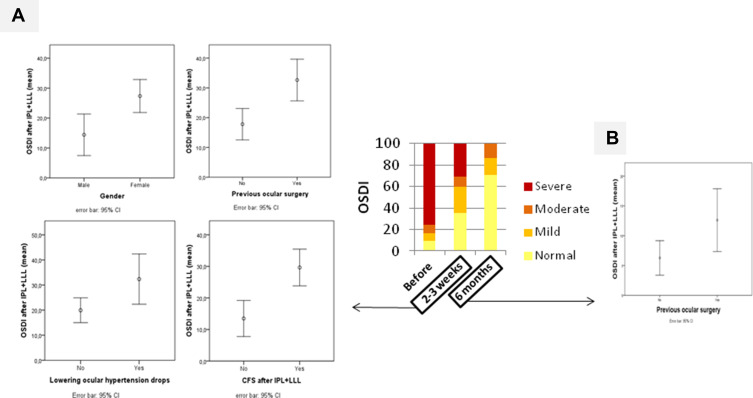
Figure 1.
(A) Mean OSDI at 2–3 weeks after IPL and LLL treatment according to presence of gender (p=0.004), previous ocular surgery (p=0.002), lowering ocular hypertension drops (p=0.028) and corneal fluorescein staining (CFS) after treatment (p<0.001). (B) Mean OSDI at 6 months after IPL and LLP treatment according to presence of previous ocular surgery (p=0.021). Gender, lowering ocular hypertension drops and CFS only correlate with OSDI at 2–3 weeks after IPL and LLL treatment.
Abbreviations: IPL, intense pulsed light therapy; LLL, level low light therapy; OSDI, ocular surface disease index; CI, confidence interval.
Concerning the subjective end report, at 2–3 weeks after treatment 80.64% of cases answered “better” after treatment and 19.35% answered “same”. At 6 months after treatment, all answered better than before treatment.
OSDI score at 2–3 weeks and 6 months after treatment correlated positively with OSDI score before treatment, and severity of dry eye by ME-CHECK before treatment (Table 4). The age and quality of tear film measurements didn´t show a statistically significant correlation with the OSDI score after treatment (Table 4). At 2–3 weeks after treatment, the mean OSDI score was higher in females (p=0.004), in those who had previous ocular surgery (p=0.002), in those who were under lowering ocular hypertension drops (p=0.028), and in those who presented with CFS after treatment (p<0.001) (Figure 1A). At 6 months after treatment, previous ocular surgery was the only factor that correlated with higher OSDI after treatment (Figure 1B).
Table 4.
Correlations with OSDI in Different Times
| Variables | OSDI Score 2–3 Weeks After IPL+LLL | OSDI Score 6 Months After IPL+LLL | ||
|---|---|---|---|---|
| R | P value | R | P value | |
| Severity of dry eye by OSDI 12* | 0.603 | <0.001 | 0.346 | 0.008 |
| Severity of dry eye by ME-CHECK®* | 0.533 | <0.001 | 0.472 | <0.001 |
| Corneal fluorescein stainingꭞ | 0.487 | <0.001 | 0.220 | 0.096 |
| Previous ocular surgery | 0.424 | 0.001 | 0.302 | 0.021 |
| Female gender | 0.388 | 0.002 | 0.202 | 0.129 |
| Lowering ocular hypertension drops | 0.277 | 0.030 | −0.082 | 0.541 |
| Previous cataract surgery | 0.271 | 0.033 | 0.198 | 0.136 |
| Schirmer testꭞ | −0.245 | 0.055 | −0.197 | 0.138 |
| Blink rateꭞ | −0.216 | 0.091 | −0.154 | 0.247 |
| Lipid layer thicknessꭞ | 0.214 | 0.094 | −0.042 | 0.756 |
| Degree of skin pigmentation | −0.212 | 0.097 | 0.142 | 0.287 |
| Tear meniscus heightꭞ | −0.152 | 0.238 | −0.125 | 0.351 |
| Non-invasive break up timeꭞ | 0.137 | 0.292 | 0.121 | 0.366 |
| Area loss of the MGꭞ | 0.102 | 0.429 | 0.037 | 0.785 |
| Age | 0.075 | 0.564 | −0.126 | 0.345 |
| Tear osmolarityꭞ | −0.070 | 0.587 | −0.005 | 0.968 |
Notes: *Before treatment. ꭞAfter treatment. Bold text represents a statistically significant p-value.
Abbreviations: OSDI, ocular surface disease index; IPL, intense pulsed light therapy; LLL, low level light therapy; MG, meibomian glands.
Ocular Surface Evaluation
CFS was identified in 51.61% before, in 54.84% (p<0.001) at 2–3 weeks, and in 45.16% (p=0.293) at 6 months after treatment.
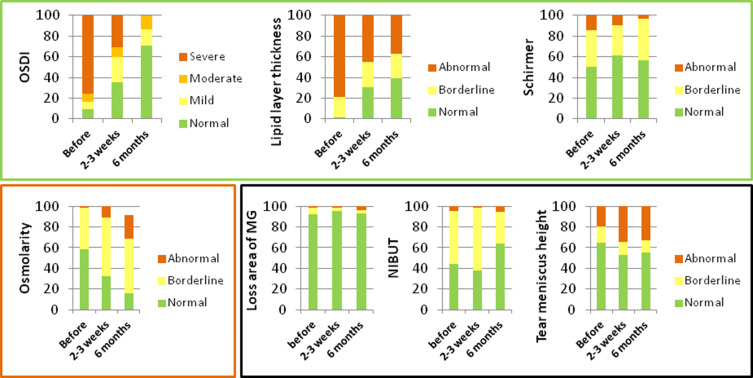
The variations of mean values and grade frequencies of quality of tear film parameters at 2–3 weeks and 6 months after treatment are represented in Table 3 and Figure 2, respectively. The mean lipid layer thickness (p<0.001) and Schirmer test (p=0.014) improved at 2–3 weeks after treatment. These improvements were maintained significant at 6 months after treatment (p<0.001 and p=0.008) and they were also expressed by improvement of grade frequencies of both parameters illustrated in Figure 2 (p<0.001 and p=0.023). The mean tear meniscus height decreased (p=0.001) at 2–3 weeks after treatment, but returned (p=0.891) to similar values at 6 months after treatment. The grade frequencies of tear meniscus height also showed no statistically significant changes (p=0.153) at 6 months after treatment. The mean and grade frequencies of NIBUT showed no statistically significant changes at 2–3 weeks (p=0.751 and p=0.695, respectively) and at 6 months (p=0.792 and p=0.076, respectively) after treatment. The mean loss area of the meibomian glands showed no statistically significant differences at 2–3 weeks (p=0.722) and increased at 6 months after treatment (p=0.044), but not enough to change the grade frequencies (p=0.0773). The mean and grade frequencies of osmolarity increased at 2–3 weeks (p<0.001 and p=0.003, respectively) and at 6 months (p<0.001 and p<0.001, respectively) after treatment. The mean and grade frequencies of blink rate increased at 2–3 weeks (p=0.004 and p=0.012, respectively), but decreased at 6 months (p<0.001 and p<0.001, respectively) after treatment.
Figure 2.
OSDI, lipid layer thickness, Schirmer, osmolarity, loss area of MG, NIBUT and tear meniscus height grade frequencies at before, 2–3 weeks and 6 months after IPL and LLL treatment. OSDI grade: normal (0–12), mild (13–22), moderate (23–32) and severe (33–100). Lipid grade: abnormal (<60), borderline (60–80) and normal (>80). Schirmer grade: abnormal (<5), borderline (5–10) and normal (>10). Loss area of MG grade: abnormal (>60), borderline (40–60) and normal (<40). Osmolarity grade: abnormal (>320), borderline (300–320) and normal (<300). NIBUT grade: abnormal (<5), borderline (5–10) and normal (>10). Tear meniscus height grade: abnormal (<0.22), borderline (>0.44) and normal (0.22–0.44). Green square include variables that improved grade’s frequency: OSDI (p<0.001), lipid layer thickness (p<0.001) and Schirmer (p=0.023). Black square include variables that didn´t change grade’s frequency: loss area of MG (p=0.773), NIBUT (p=0.076) and tear meniscus height (p=0.153). Red square included variables that worsened grade’s frequency: osmolarity (p<0.001).
Abbreviations: NIBUT, non-invasive break up time; MG, meibomius gland; IPL, intense pulsed light therapy; LLL, level low light therapy.
There were no reports of adverse events, such as skin burns or pain during or after the procedures.
Discussion
The results of the present study suggest a therapeutic potential for IPL plus LLL therapy in the management of MGD. Comparisons with other studies have some limitations because of differences in evaluation assessments, protocols, and devices.
Regarding reported symptoms, 91.93% and 100% of cases improved OSDI score, at 2–3 weeks and 6 months after treatment respectively. This improvement was evident in all categories of symptoms. Severe symptoms were present in 75.80% of cases before treatment, 30.6% at 2–3 weeks, and 0% at 6 months after treatment, better than 29.1% reported by Stonecipher et al12 after treatment. Overall, the improvements in OSDI have been reported in treatments with only IPL, with or without manual expression of the meibomian glands,9 with some exceptions.13
Concerning ocular surface analysis, there were improvements in aqueous tear production evaluated by the Schirmer test and in the outflow of meibum from the glands evaluated by lipid layer thickness on the tear film surface. This effect on the aqueous tear production wasn´t reported in the only other IPL with LLL study12 and wasn´t usually verified in IPL without LLL studies,14–19 although it was already reported.20 In our study, this improvement of lachrymal gland secretion occurred at 2–3 weeks and 6 months after treatment. Although the mean tear meniscus height was decreased at 2–3 weeks, this can be explained by the better blink rate. At 6 months, the mean tear meniscus height was increased with worsened blink rate. We hypothesized that lachrymal gland secretion might improve through the indirect LLL effect because previous studies, in general, suggested that IPL does not affect lachrymal glands.21 Unlike IPL, LLL has been shown to influence the lachrymal gland, for example, reducing the number of neutrophils or changing inflammatory cytokines in the lachrymal gland.22 Additionally, LLL is the only treatment that was applied directly to the upper lid (Table 1). The improvement in lipid layer thickness was frequently described in IPL without LLL treatment,8,13,15,17,18,23 but other studies are showing no effect.19,24
The NIBUT didn´t change after IPL plus LLL, remaining with normal values at 6 months after treatment. This can be explained by the small percentage of patients having abnormal values before treatment. The mean tear meniscus height was significantly lower 2–3 weeks after treatment; however, it remained within normal values. This may be due to the observed improvement in the quality of blinking (leading to less tear accumulation) or in the quality of film tear (leading to a decrease in the water production reflex). At 6 months after treatment, the mean tear meniscus height returned to basal value, when the blink rate decreased. The loss area of meibomian glands didn´t change at 2–3 weeks but increased at 6 months after treatment, although remained within normal values too, whereby authors considered that the combined treatment was safe. This parameter is not consistently reported in IPL studies without LLL,9 so no comparison is possible.
The mean tear osmolarity was higher 2–3 weeks and 6 months after treatment. This can be explained by the higher increment on the lipid layer (solute) compared to the increment on the aqueous layer (solvent). In IPL without LLL studies, the tear osmolarity has been reported as lowered23,24 or unchanged.8,13,14,19 According to important studies,25–27 it was expected that DED symptoms and OSDI score would worsen with increasing osmolarity, but this wasn´t the case. This unusual finding confirms that dry eye is a multifactorial and complex disease.
Lastly, corneal staining didn´t change in our study as it was been reported in some IPL without LLL studies,16,19,20,28 although not in others.14,17,18,24,29–33 The fact that 93.5% of cases were diabetic patients can be a confounding factor in this last parameter since they can have an underlying diabetic keratopathy component, leading to a greater propensity to the CFS. Also, CFS can reflect previous changes in the functional unit of the ocular surface of a long duration, which may require more time for its disappearance. We didn´t graduate the CFS, so the analysis is also limited. We didn´t know if the degree of CFS has increased, decreased or didn´t change.
Although we determined the level of energy applied for each patient according to the ME-CHECK Screening, we didn´t use it for determining the number of session treatments, as suggested by the manufacturer. In fact, according to this evaluation, the 19 eyes which maintained in the category severe and the 6 which improved from severe to moderate category at 2–3 weeks after treatment would need at least 1 or 2 additional treatment sessions. However, with our scheme of 3 initial sessions, we prevented unnecessary treatments for 16 eyes that achieved category normal at 2–3 weeks after treatment and that would also have an indication for 1 or 2 more treatments by ME-CHECK Screening. At 6 months after treatment, no eye had severe symptoms, without any additional treatments.
To our knowledge, this is the second study documenting the effects of combined IPL and LLL therapies. Two of the strengths of this study are the inclusion of different severities of DED with MGD, not included in the first study,12 and the multimodal assessment (allowing us to understand the best treatment capabilities). Our sample had a small percentage of loss area of meibomian glands before treatment, which may have contributed to the good results. The operator-dependent variability on IDRA® is not considered as an important factor, because the assessment was performed by only 2 technicians (DA and DJ). The prospective design allowed us to control the same therapeutic scheme and the same evaluation timings. The major limitation of this study is being a non-comparative study, being impossible to distinguish the effects by IPL and LLL separately and to evaluate the placebo effect. Additionally, this study is not randomized and has not a control group. The ideal would be to treat one eye and the other being the control for the same patient, avoiding the tendency in dry eye studies of subjective improvement with any treatment. However, the mask used for LLL (MY MASK-E®) treatment makes it impossible to treat only one eye. In addition, the two eyes are not always the same on the same person. Comparative studies with larger sample sizes are needed to validate these results.
Overall, our study demonstrated that IPL plus LLL is effective and safe to improve the lipid layer thickness in MGD, decreasing the level of symptoms.
Acknowledgments
The authors want to acknowledge all the support granted by the Head of the Ophthalmology Department of Centro Hospitalar e Universitário do Porto, Prof. Dr. Pedro Menéres.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification Report. Ocul Surf. 2017;15(3):276–283. doi: 10.1016/j.jtos.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 2.Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52(4):1994–2005. doi: 10.1167/iovs.10-6997e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology. 2017;124(11S):S20–6. doi: 10.1016/j.ophtha.2017.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539–574. [DOI] [PubMed] [Google Scholar]
- 5.Baudouin C, Aragona P, Van Setten G, et al. Diagnosing the severity of dry eye: a clear and practical algorithm. Br J Ophthalmol. 2014;98(9):1168–1176. doi: 10.1136/bjophthalmol-2013-304619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615 [DOI] [PubMed] [Google Scholar]
- 7.Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. [DOI] [PubMed] [Google Scholar]
- 8.Craig JP, Chen Y-H, Turnbull PRK. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2015;56(3):1965–1970. doi: 10.1167/iovs.14-15764 [DOI] [PubMed] [Google Scholar]
- 9.Tashbayev B, Yazdani M, Arita R, Fineide F, Utheim TP. Intense pulsed light treatment in meibomian gland dysfunction: a concise review. Ocul Surf. 2020;18(4):583–594. doi: 10.1016/j.jtos.2020.06.002 [DOI] [PubMed] [Google Scholar]
- 10.Rojas JC, Gonzalez-Lima F. Low-level light therapy of the eye and brain. Eye Brain. 2011;14(3):49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toyos R. The effects of a red light technology on dry eye due to meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2017;58(8):2236. [Google Scholar]
- 12.Stonecipher K, Abell TG, Chotiner B, Chotiner E, Potvin R. Combined low level light therapy and intense pulsed light therapy for the treatment of meibomian gland dysfunction. Clin Ophthalmol. 2019;13:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigo L, Giannaccare G, Sebastiani S, Pellegrini M, Carones F. Intense pulsed light for the treatment of dry eye owing to meibomian gland dysfunction. J Vis Exp. 2019; 146. [DOI] [PubMed] [Google Scholar]
- 14.Albietz JM, Schmid KL. Intense pulsed light treatment and meibomian gland expression for moderate to advanced meibomian gland dysfunction. Clin Exp Optom. 2018;101(1):23–33. doi: 10.1111/cxo.12541 [DOI] [PubMed] [Google Scholar]
- 15.Arita R, Mizoguchi T, Fukuoka S, Morishige N. Multicenter study of intense pulsed light therapy for patients with refractory meibomian gland dysfunction. Cornea. 2018;37(12):1566–1571. doi: 10.1097/ICO.0000000000001687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin Y, Liu N, Gong L, Song N. Changes in the meibomian gland after exposure to intense pulsed light in Meibomian Gland Dysfunction (MGD) Patients. Curr Eye Res. 2018;43(3):308–313. doi: 10.1080/02713683.2017.1406525 [DOI] [PubMed] [Google Scholar]
- 17.Arita R, Fukuoka S, Morishige N. Therapeutic efficacy of intense pulsed light in patients with refractory meibomian gland dysfunction. Ocul Surf. 2019;17(1):104–110. doi: 10.1016/j.jtos.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 18.Cheng S-N, Jiang F-G, Chen H, Gao H, Huang Y-K. Intense pulsed light therapy for patients with meibomian gland dysfunction and ocular demodex infestation. Curr Med Sci. 2019;39(5):800–809. doi: 10.1007/s11596-019-2108-1 [DOI] [PubMed] [Google Scholar]
- 19.Piyacomn Y, Kasetsuwan N, Reinprayoon U, Satitpitakul V, Tesapirat L. Efficacy and safety of intense pulsed light in patients with meibomian gland dysfunction-a randomized, double-masked, sham-controlled Clinical Trial. Cornea. 2020;39(3):325–332. doi: 10.1097/ICO.0000000000002204 [DOI] [PubMed] [Google Scholar]
- 20.Karaca EE, Evren Kemer Ö, Özek D. Intense regulated pulse light for the meibomian gland dysfunction. Eur J Ophthalmol. 2020;30(2):289–292. doi: 10.1177/1120672118817687 [DOI] [PubMed] [Google Scholar]
- 21.Arita R, Fukuoka S, Mizoguchi T, Morishige N. Multicenter study of intense pulsed light for patients with refractory aqueous-deficient dry eye accompanied by mild meibomian gland dysfunction. J Clin Med. 2020;9(11):3467. doi: 10.3390/jcm9113467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goo H, Kim H, Ahn J, Cho K. Effects of low-level light therapy at 740 nm on dry eye disease in vivo. Med Lasers. 2019;8(2):50–58. doi: 10.25289/ML.2019.8.2.50 [DOI] [Google Scholar]
- 23.Vigo L, Taroni L, Bernabei F, et al. Ocular surface workup in patients with meibomian gland dysfunction treated with intense regulated pulsed light. Diagnostics (Basel). 2019;9(4). doi: 10.3390/diagnostics9040147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dell SJ, Gaster RN, Barbarino SC, Cunningham DN. Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin Ophthalmol. 2017;11:817–827. doi: 10.2147/OPTH.S130706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511–538. [DOI] [PubMed] [Google Scholar]
- 26.Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II Report executive summary. Ocul Surf. 2017;15(4):802–812. doi: 10.1016/j.jtos.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 27.Suzuki M, Massingale ML, Ye F, et al. Tear osmolarity as a biomarker for dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51(9):4557–4561. doi: 10.1167/iovs.09-4596 [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Lv H, Song H, et al. Evaluation of the safety and effectiveness of intense pulsed light in the treatment of meibomian gland dysfunction. J Ophthalmol. 2016;2016:1910694. doi: 10.1155/2016/1910694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rong B, Tang Y, Tu P, et al. Intense pulsed light applied directly on eyelids combined with meibomian gland expression to treat meibomian gland dysfunction. Photomed Laser Surg. 2018;36(6):326–332. doi: 10.1089/pho.2017.4402 [DOI] [PubMed] [Google Scholar]
- 30.Seo KY, Kang SM, Ha DY, Chin HS, Jung JW. Long-term effects of intense pulsed light treatment on the ocular surface in patients with rosacea-associated meibomian gland dysfunction. Cont Lens Anterior Eye. 2018;41(5):430–435. doi: 10.1016/j.clae.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 31.Rong B, Tang Y, Liu R, et al. Long-term effects of intense pulsed light combined with meibomian gland expression in the treatment of meibomian gland dysfunction. Photomed Laser Surg. 2018;36(10):562–567. doi: 10.1089/pho.2018.4499 [DOI] [PubMed] [Google Scholar]
- 32.Choi M, Han SJ, Ji YW, et al. Meibum expressibility improvement as a therapeutic target of intense pulsed light treatment in meibomian gland dysfunction and its association with tear inflammatory cytokines. Sci Rep. 2019;9(1):7648. doi: 10.1038/s41598-019-44000-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Y-F, Liu R-J, Li Y-X, et al. Comparison of anti-inflammatory effects of intense pulsed light with tobramycin/dexamethasone plus warm compress on dry eye associated meibomian gland dysfunction. Int J Ophthalmol. 2019;12(11):1708–1713. doi: 10.18240/ijo.2019.11.07 [DOI] [PMC free article] [PubMed] [Google Scholar]