Abstract
The pathomechanisms and treatment strategy for rare presentations of reversible cerebral vasoconstriction syndrome (RCVS) with anti-phospholipid syndrome (APS) remain to be determined. We report a 67-year-old woman with APS who presented with ischemic stroke due to RCVS. She was treated with low-dose cilostazol and lomerizine hydrochloride, which resulted in functional improvement and recovery of vasoconstriction within 12 weeks. Her plasma endothelin-1 level was decreased after relief of vasoconstriction, compared with the pre-treatment condition. Increased plasma endothelin-1 may be related to the underlying pathomechanism of RCVS with APS, against which cilostazol and lomerizine hydrochloride could be effective.
Keywords: Cilostazol, Lomerizine, Anti-phospholipid antibody syndrome, Reversible cerebral vasoconstriction syndrome, Endothelin-1
Highlights
-
•
We described a case of reversible cerebral vasoconstriction syndrome (RCVS) with anti-phospholipid syndrome (APS).
-
•
Treatment with low-dose cilostazol and lomerizine hydrochloride could therapeutic for RCVS with APS.
-
•
These treatments may alleviate the endothelin-1-related cerebral vascular dysfunction in RCVS/APS.
1. Introduction
Anti-phospholipid syndrome (APS) affects the central nervous system in various situations, and reversible cerebral vasoconstriction syndrome (RCVS), a neurovascular disorder with headache and radiologically reversible vasoconstriction, is considered to be comorbid with APS [[1], [2], [3]]. Previous reports have shown that anti-phospholipid antibody may activate endothelial cells, which causes the release of vasoconstrictors such as endothelin-1 (ET-1) [4]. Here we present a case of RCVS with APS who was treated with low-dose cilostazol and lomerizine hydrochloride. The plasma ET-1 levels were evaluated at pre- and post- treatment.
2. Case presentation
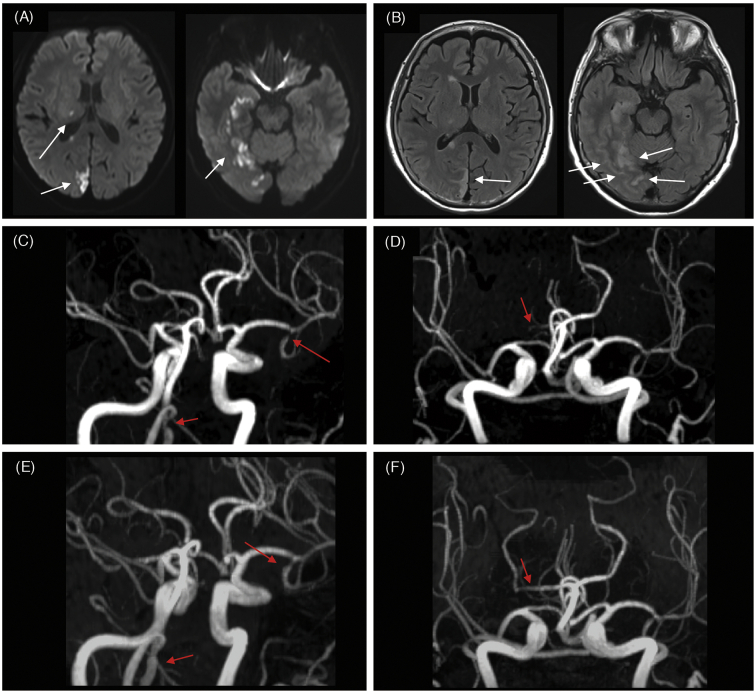
A 67-year-old woman experienced acute blindness of her left visual field and paresthesia of her left hand, mouth, and foot, accompanied by mild headache. She was admitted to our hospital seven days after symptom onset. She had a history of systemic lupus erythematosus (SLE) for 20 years without corticosteroid medication and a history of miscarriage. Physical examination showed erythema on her face and chilblain-like lesions on her fingers. Neurological examination demonstrated a left homonymous hemianopia and left cheiro-oral syndrome. Blood tests showed an increased erythrocyte sedimentation rate at 66 mm/h. Anti-dsDNA antibody was elevated at 159 U/ml, and anti-ssDNA was also elevated at 47 U/ml. Anti-U1RNP antibody was elevated at 190 U/ml. As for anti-phospholipid antibodies, anti-cardiolipin β2 glycoprotein 1 (β2GPI) antibody was elevated to 13 U/ml, and IgG-anti cardiolipin antibody was also elevated to 34.6 mg/dl, which remained high after the interval of more than 12 weeks. The lupus anticoagulant test was 0.99, which was within the normal limit of 1.3. The plasma D-dimer level was less than 0.5 μg/ml. The plasma ET-1 level was 1.9 pg/ml (which is above the level of control patient examined by the same assay; 1.74 pg/ml). Cerebrospinal fluid analysis (CSF) showed increased red blood cells without apparent increase in white blood cells, and the protein level in CSF was 44.7 mg/dl. On admission, 3-T magnetic resonance imaging (MRI) showed multiple cortical and juxta-cortical hyperintense lesions from her right medial temporal lobe to the occipital lobe and right thalamus on diffusion-weighted images (Fig. 1A). Fluid-attenuated inversion recovery images showed hyperintense lesions in sulcal spaces and in the temporo-occipital lobes, which was suggestive of cortical subarachnoid hemorrhage (SAH) (Fig. 1B). Furthermore, magnetic resonance angiography (MRA) showed narrowing of the left second segment of the middle cerebral artery (MCA), the right vertebral artery (VA), and the second segment of the right posterior cerebral artery (PCA) (Fig. 1C, D). A 3D fast spin-echo-based sequence images showed no evidence of thickened vessel walls of affected arteries or hyperintense lesions within vessel walls. According to her clinical signs/symptoms and blood tests, she was initially diagnosed as having APS and SLE based on the recent criteria which were proposed on 2006 and 2012, respectively [5,6]. Furthermore, the RCVS2 score of the present case was 5, which indicates the diagnosis of RCVS with a specificity of 99% and a sensitivity of 90% [7]. Subsequently, she was diagnosed as ischemic stroke mainly due to severe narrowing of the right PCA, which was suspected to be caused by RCVS with APS. She was treated with low-dose cilostazol (100 mg daily) in combination with lomerizine hydrochloride. In two weeks, her symptoms including facial erythema and chilblain-like lesion were relieved without administration of the medication for SLE, and MRA images performed on day 42 showed substantial recovery of stenotic vascular lesions (Fig. 1E, F). The plasma ET-1 level was decreased to 1.53 pg/ml three months after the onset of the symptoms, compared with the pre-treatment condition.
Fig. 1.
Axial view of magnetic resonance images and magnetic resonance angiography performed on day 1 after admission. (A) Diffusion-weighted images show the ischemic lesions in the right temporal areas, right thalamus, and right occipital cortex (white arrows). In the right temporal and right occipital lobes, lesions are located at cortical and juxta-cortical areas. (B) Fluid-attenuated inversion recovery images show the cortical subarachnoid hemorrhage as intrasulcal hyperintensity areas, located at the right occipital area and temporal area (white arrows). (C, D) Magnetic resonance angiography shows narrowing of multiple vessels. The second segment of the left second segment of the middle cerebral artery, right vertebral artery, and right posterior cerebral artery are narrowed (red arrows). (E, F) Magnetic resonance angiography performed on day 42 after admission shows recovery of the narrowed left middle cerebral artery, right vertebral artery, and right posterior cerebral artery (red arrows). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
We described a case of RCVS with APS, which was treated with low-dose cilostazol and lomerizine hydrochloride. In APS patients, autoantibodies related to APS affect the coagulation cascade and activate endothelial cells to release vasoactive agents, which would play a pivotal role in the pathogenesis of RCVS. In this context, we evaluated the plasma level of ET-1, as one of vasoactive agents, and the present case showed a decreased plasma level of ET-1 after the vasoconstriction resolved.
ET-1 is one of the strong vasoactive agents which are derived from endothelial cells, and regulates the smooth muscle tone of vessels. ET-1 has been shown to modulate the smooth muscle tone of vessels with increased Ca2+ influx [8]. Previous study demonstrated that plasma ET-1 levels were elevated in patients with APS, and that plasma ET-1 levels had a correlation with arterial thrombosis in APS patients. An in vitro study also reported that the incubation of human endothelial cells with anti-β2GPI antibodies induced increased expression of mRNA for ET-1 [4]. Thus, ET-1 is considered to have a substantial role in APS-related vascular reactions. However, no report has evaluated plasma ET-1 levels in an RCVS patient with APS. The present study showed decreased plasma ET-1 levels after treatment, which suggested the potential contribution of ET-1 in the underlying pathomechanism of RCVS with APS.
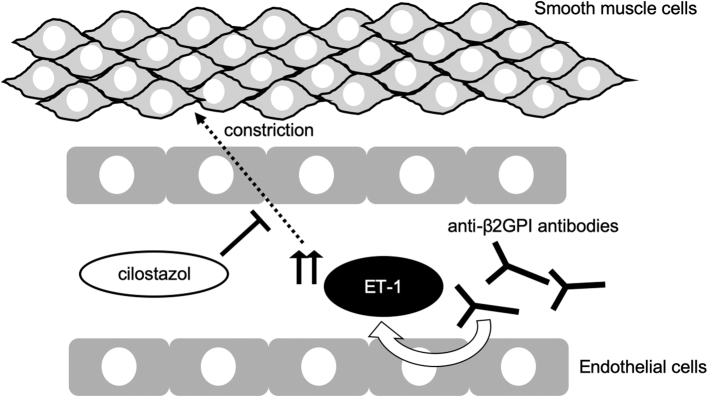
From the viewpoint of therapeutic aspect, the patient was treated with cilostazol. Cilostazol, a selective inhibitor of type III phosphodiesterase, increases cyclic adenosine monophosphate in platelets and vascular smooth muscle cells, inhibits platelet aggregation, and exerts multiple effects on the vasculature. A recent study showed that cilostazol prevented ET-1-induced smooth muscle constriction in vitro, suggesting the potential effect of cilostazol on ET-1-related vasoconstriction (Fig. 2) [8]. The present case, treated with low-dose cilostazol, showed recovery of stenotic vessels with functional improvement. Although a careful interpretation is necessary, a possible explanation for the recovery is cilostazol-mediated beneficial effects on vascular dysfunction in RCVS with APS. As possible side effects of cilostazol, attention should be paid to headache and hemorrhage. Importantly, RCVS has a high rate of hemorrhagic complications (around 30–50%), including SAH and intracranial hemorrhage [9]. Low dose cilostazol (100 mg/day) causes less frequency of headache and hemorrhage than high-dose cilostazol (200 mg/day) [10]. Since the present case showed mild headache with cortical SAH, low-dose cilostazol was chosen for her treatment, and the patient showed a good clinical outcome without any worsening of her headache or hemorrhage. Although it is necessary to clarify the effectiveness and safety of cilostazol for RCVS with APS in randomized clinical trials, low-dose cilostazol might be recommended for RCVS/APS patients presenting with headache and/or SAH. The present case was also treated with a brain-specific vasodilator, lomerizine hydrochloride, which acts at the calcium channel and has vasoactive effects specifically for cerebral vessels, which is an established treatment option for migraine [11,12]. Whether lomerizine hydrochloride has additive or synergistic effects with cilostazol in RCVS/APS needs to be further elucidated.
Fig. 2.
The schema represents the relationships between anti-phospholipid syndrome, endothelin-1 (ET-1), and cilostazol. The endothelial cells with anti-β2GPI antibodies induce increased expression of mRNA for ET-1, leading to vasoconstriction. Cilostazol prevents ET-1-induced smooth muscle constriction.
In conclusion, treatment with low-dose cilostazol and lomerizine hydrochloride would be one of the recommended therapeutic options for RCVS patients with APS. These treatments may alleviate the ET-1-related cerebral vascular dysfunction in RCVS/APS.
Funding
The authors received no financial support for the research, authorship and publication of this article.
Declaration of Competing Interest
The authors declared no potential conflicts of interest with respect to the research, authorship and publication of this article.
References
- 1.Gupta S., Zivadinov R., Ramasamy D., Ambrus J.L., Jr. Reversible cerebral vasoconstriction syndrome (RCVS) in antiphospholipid antibody syndrome (APLA): the role of centrally acting vasodilators. Case series and review of literature. Clin. Rheumatol. 2014;33:1829–1833. doi: 10.1007/s10067-013-2434-9. [DOI] [PubMed] [Google Scholar]
- 2.Uenaka T., Hamaguchi H., Sekiguchi K., Kowa H., Kanda F., Toda T. Reversible cerebral vasoconstriction syndrome in a stroke patient with systemic lupus erythematosus and antiphospholipid antibody. Rinsho Shinkeigaku. 2013;53:283–286. doi: 10.5692/clinicalneurol.53.283. [DOI] [PubMed] [Google Scholar]
- 3.Chung S.W., Lee K.M., Heo S.H., Ra R., Hong S.J., Yang H.I. A systemic lupus erythematosus patient with thunderclap headache: reversible cerebral vasoconstriction syndrome. Lupus. 2019;28:898–902. doi: 10.1177/0961203319845485. [DOI] [PubMed] [Google Scholar]
- 4.Atsumi T., Khamashta M.A., Haworth R.S., Brooks G., Amengual O., Ichikawa K. Arterial disease and thrombosis in the antiphospholipid syndrome: a pathogenic role for endothelin 1. Arthritis Rheum. 1998;41:800–807. doi: 10.1002/1529-0131(199805)41:5<800::AID-ART5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Miyakis S., Lockshin M.D., Atsumi T., Branch D.W., Brey R.L., Cervera R. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J. Thromb. Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 6.Petri M., Orbai A.M., Alarcon G.S., Gordon C., Merrill J.T., Fortin P.R. Derivation and validation of systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum-Us. 2011;63 doi: 10.1002/art.34473. S669-S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocha E.A., Topcuoglu M.A., Silva G.S., Singhal A.B. RCVS2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology. 2019;92 doi: 10.1212/WNL.0000000000006917. e639-e47. [DOI] [PubMed] [Google Scholar]
- 8.Kawanabe Y., Takahashi M., Jin X., Abdul-Majeed S., Nauli A.M., Sari Y. Cilostazol prevents endothelin-induced smooth muscle constriction and proliferation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012;11:906–917. doi: 10.1016/S1474-4422(12)70135-7. [DOI] [PubMed] [Google Scholar]
- 10.Shinohara Y., Katayama Y., Uchiyama S., Yamaguchi T., Handa S., Matsuoka K. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010;9:959–968. doi: 10.1016/S1474-4422(10)70198-8. [DOI] [PubMed] [Google Scholar]
- 11.Calabrese L.H., Dodick D.W., Schwedt T.J., Singhal A.B. Narrative review: reversible cerebral vasoconstriction syndromes. Ann. Intern. Med. 2007;146:34–44. doi: 10.7326/0003-4819-146-1-200701020-00007. [DOI] [PubMed] [Google Scholar]
- 12.Hara H., Shimazawa M., Sasaoka M., Yamada C., Iwakura Y., Sakai T. Selective effects of lomerizine, a novel diphenylmethylpiperazine Ca2+ channel blocker, on cerebral blood flow in rats and dogs. Clin. Exp. Pharmacol. Physiol. 1999;26:870–876. doi: 10.1046/j.1440-1681.1999.03154.x. [DOI] [PubMed] [Google Scholar]