Highlights
-
•
Checkpoint inhibitor therapy affecting PD-L1 as treatment for advanced solid tumors.
-
•
Success in trial pembrolizumab therapy in multiresistant metastatic choriocarcinoma.
-
•
Long-term remission after pembrolizumab therapy in multiresistant choriocarcinoma.
-
•
Only six reported cases, one with comparable follow-up and outcome.
Keywords: Choriocarcinoma, Pembrolizumab, GTN, β-HCG
Abbreviations: β-HCG, beta-human chorionic gonadotropin; CPI, checkpoint inhibitor; CT, computed tomography; cMRI, cerebral magnetic resonance imaging; WHO, Worl Health Organization; ECOG, Eastern Cooperative Oncology Group; ETT, epithelioid trophoblastic tumor; GTD, gestational trophoblastic disease; GTN, gestational trophoblastic neoplasia; PD-1/PDL-1, programmed cell death-1/ programmed cell death ligand-1; PSTT, placental site trophoblastic tumor
1. Introduction
Choriocarcinoma is considered the most malignant subtype of gestational trophoblastic disease (GTD) with a high invasive and metastatic potential (Seckl et al., 2000). Incidence is reported to be approximately 1 in 40 000 pregnancies in North America and Europe (Lurain, 2010).
Based on the FIGO-staging for gestational trophoblastic neoplasia, patients with choriocarcinoma can be divided into high- and low-risk gestational trophoblastic neoplasia (GTN) groups (FIGO staging for gestational trophoblastic neoplasia, 2000). Whereas the treatment of low-risk GTN is single-agent chemotherapy, high-risk GTN are treated with multiple agent chemotherapy. >95% of patients with high-risk GTN experience complete response after chemotherapy (Ngan et al., 2018). However, 0.5–5.0% succumb to the disease, mainly due to late diagnosis or multidrug resistance (Powles et al., 2007).
As the recent introduction of checkpoint inhibitor (CPI) therapy targeting programmed cell death ligand 1 (PD-L1) provided a new therapeutic strategy for several advanced solid tumors (Herbst et al., 2016), the anti-PD-inhibitor pembrolizumab appears to be a promising therapeutic option for the subset of patients with choriocarcinoma experiencing chemotherapy resistance (Huang et al., 2017, Ghorani et al., 2017, Goldfarb et al., 2020, Clair et al., 2020).
2. Case presentation
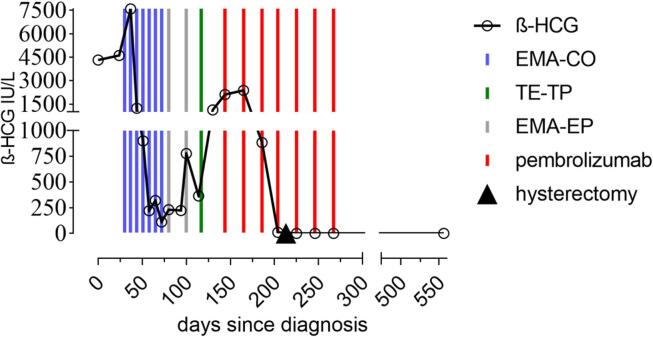
In August 2018, a thirty-one-year-old woman presented at our clinic after being diagnosed with choriocarcinoma at a secondary care center. Six months before, she had delivered at term by cesarean section after uneventful pregnancy. Due to postpartal vaginal bleeding and alleged placenta residuals, the patient was treated with dilation and curettage twice. The Beta-human chorionic gonadotropin (β-HCG) levels were 28872 IU/L (June 2018) and 4323 IU/L (August 2018), post-curettage respectively (Fig. 1). The histology of the tissue gained from the second curettage revealed choriocarcinoma and the patient was transferred to our institution.
Fig. 1.
Beta-human chorionic gonadotropin (β -HCG) trend with presentation of treatment.
Histology was confirmed at our institution: Predominantly solid formations of cytotrophoblasts, intermediate trophoblasts and syncytiotrophoblasts were found. Tumor cells showed a pronounced nuclear polymorphism and focal-microscopically up to 14 partially pluripolar mitoses. On top of hemorrhagic necroses, there was also lymphatic invasion. In the immunohistochemical examination tumor cells diffusely and particularly strongly expressed β-HCG and cytokeratin AE1/3. The proliferation marker MIB-1 amounted up to 90%. The intermediate trophoblast cells stained MUC4 positive. In some cases, focal low-grade expression of alpha inhibin, GATA3 and CK18 as well as SMA in myometrial smooth muscle cells was also observed. Melan A, S100 and HPL were negative.
Staging commenced on 6th August 2018 by computed tomography (CT) and cerebral magnetic resonance imaging (cMRI). CT demonstrated the presence of multiple (n = 14) pulmonary and two (n = 2) vaginal metastases. Considering these findings, the WHO risk score was 10 (Table1).
Table 1.
WHO prognostic scoring system for GTN with highlighted scoring according to our patient, total score ≤ 6 low-risk, ≥7 high-risk.
|
Modified WHO prognostic scoring system | ||||
|---|---|---|---|---|
| Scores | 0 | 1 | 2 | 4 |
| Age | <40 | >40 | – | – |
| Antecedent pregnancy | mole | abortion | term | – |
| Interval months from index pregnancy | <4 | 4–6 | 7–12 | >12 |
| Pretreatment serum β -HCG | <103 | 103–104 | 104–105 | >105 |
| Largest tumor size (including uterus) | <3 | 3–4 cm | ≥5 cm | – |
| Site of metastases | lung | spleen, kidney | gastro-intestinal | liver, brain |
| Number of metastases | – | 1–4 | 5–8 | >8 |
| Previous failed chemotherapy | – | – | Single drug | ≥2 drugs |
After the case was taken over by our clinic, a chemotherapy regimen of etoposide, methotrexate, actinomycin- D, cyclophosphamide and vincristine (EMA-CO) was induced. From 14th August to 26th September the patient received four cycles of EMA-CO. After an initial drop from 7584 IU/l to 1234 IU/l β-HCG, levels reached a plateau at 221 IU/l. (Fig. 1). Due to an alleged chemotherapy resistance, treatment was modified to etoposide, methotrexate, actinomycin-D, followed by etoposide and cisplatin (EMA-EP) on 3rd October (Fig. 1).
As β-HCG levels were observed to rise from 231 IU/l on 3rd October to 776 IU/l on 23rd October, treatment was adapted to paclitaxel/cisplatin followed by paclitaxel/etoposide starting from 9th November.
The patient, however, experienced disease progression and β-HCG level increased to 2115 IU/L. In the meantime, computed tomography showed progression of disease. The intrauterine mass showed a progression in size from 1,5 × 1,2 × 1,4 cm before to 3,5 × 2,5 × 3,3 cm. Also the size of the vaginal metastases increased from 1,2 × 1,2 × 1,2 cm to 1,8 × 2,4 × 2,3 cm. In an additional immunohistochemical work-up on PDL1, 90% of tumor cells and 5% of immune cells expressed complete membrane positivity.
Based on these findings, an experimental therapy with pembrolizumab 200 mg q3w was induced on 6th December after consulting Michael Seckl for advice (Department of Medical Oncology, Charing Cross Hospital, London, UK). The therapy was well tolerated without relevant side effects. A steady decline of β-HCG-levels and respective radiological response, regarding significant size reduction of the primary, the pulmonal and vaginal metastases was observed (Fig. 1, Fig. 2).
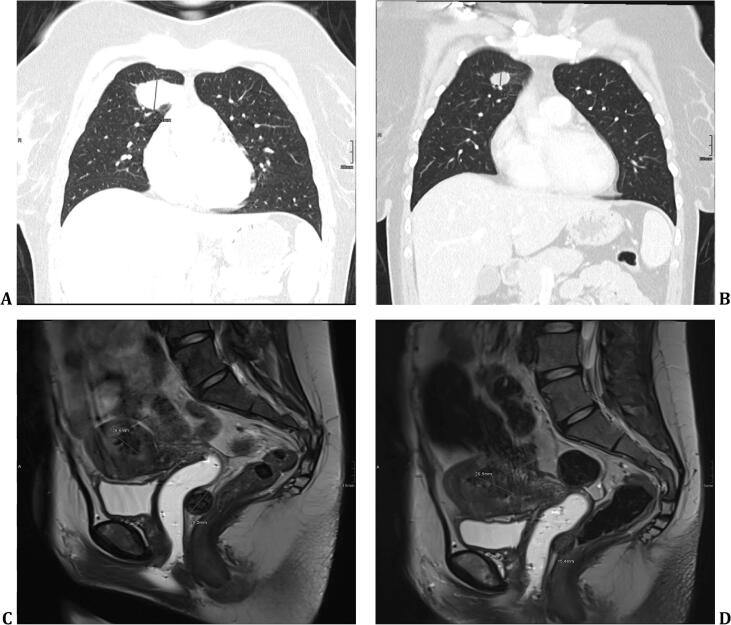
Fig. 2.
Computed tomography demonstrating a 26,5-mm nodule in right superior lobe before initiation of pembrolizumab (A) and after four cycles (B). Choriocarcinoma and vaginal metastasis with a diameter of 20,2 mm before the first cycle of pembrolizumab (C) and after four cycles (D) demonstrating decrease in size to 15,4 mm.
After completion of four cycles of pembrolizumab, a salvage-hysterectomy with partial colpectomy was conducted on 13th February. Final histology reported no vital tumor cells. After surgery, therapy with pembrolizumab was continued for three additional consolidation cycles (total of 7 cycles until 8th April 2019).
The β-HCG levels dropped below the positivity threshold of 0.1 IU/L on 29th April 2019 and the patient remained in good clinical condition (Eastern Cooperative Oncology Group (ECOG) Score 0). The patient is enrolled in regular follow-up in our institution and latest visit in April 2021 showed complete clinical remission and negative β-HCG levels 24 months after end of treatment and 33 months after initial diagnosis.
3. Discussion
The expression of PD-L1 in tumor cells, however, can be observed in varying extent and has been associated with CPI therapy response (Keir et al., 2008). In addition to expression in tumor cells, evidence suggests that PD-L1 plays an essential role in fetomaternal tolerance (Guleria et al., 2005). The placental trophoblast is substantial in the suppression of maternal immunological rejection against the fetus and shows in nearly all trophoblastic lineages expression of PD-L1 (Lu et al., 2019). As GTN immunostaining shows intense PD-L1 immunoreactivity, this type of tumors appear to be a promising target for CPI therapy (Veras et al., 2017).
In line, activity of pembrolizumab in chemotherapy-resistant GTN has been investigated in small cohorts and reported to be very successful in this patient group (Huang et al., 2017, Ghorani et al., 2017, Choi et al., 2019, Goldfarb et al., 2020, Clair et al., 2020). However, only five cases of pembrolizumab treatment for chemotherapy-resistant choriocarcinoma have been reported to date. Few patients with other forms of GTN (ETT, PSTT) have also been treated with pembrolizumab (Table 2). Also, a Chinese preliminary study of PD-1 inhibitor treatment in eight patients with drug-resistant recurrent GTN showed a high effective rate and relatively mild side effects (Cheng et al., 2020).
Table 2.
Overview of cases to date.
| Author | Patient characteristics | Agent, dosage and number of cycles | Outcome and previously described PFS |
|---|---|---|---|
| Huang et al. (Huang et al., 2017) | 26-year-old woman with metastasized chemotherapy-resistant choriocarcinoma | Two cycles 200 mg q3w followed by two cycles 100 mg q3w pembrolizumab | Full remission |
| Ghorani et al. (Ghorani et al., 2017) | 39-year-old and a 37-year-old patient with metastasized chemotherapy-resistant choriocarcinoma | Nine and seven cycles of 2 mg/kg q3w pembrolizumab | Full remission for 24 and five months |
| Min Chul Choi et al. (Choi et al., 2019) | 39-year-old patient with a PSTT and a 49-year-old patient with an ETT, both metastasized and chemotherapy-resistant | Nine and eleven cycles of 200 mg q3w pembrolizumab | Full remission |
| Goldfarb et al. (Goldfarb et al., 2020) | 50-year-old woman with metastasized chemotherapy-resistant choriocarcinoma | Six cycles of pembrolizumab | Progression after 22 months, re-induction with pembrolizumab |
| Clair et al. (Clair et al., 2020) | 30-year-old woman with metastasized chemotherapy-resistant choriocarcinoma | Ten cycles of 200 mg q3w pembrolizumab | Full remission for 31 months |
The suggestion that the immune system and thus also immunotherapy plays an essential role in GTN is collaborated by the only prospective study of immunotherapy in GTN to date (You et al., 2020). The TROPHIMMUN trial assessed avelumab, which is an anti-programmed death ligand-1 (anti-PD-L1) immunoglobulin G1 monoclonal antibody, in woman with chemotherapy-resistant GTN. In this study approximately 50% of patients could be cured with avelumab.
Apart from that, side effects observed less frequently and less severely with CPI than with chemotherapies in the TROPHIMMUN trial and other trials from different tumors (André et al., 2020).
Furthermore, an ongoing phase II trial (NCT04303884) investigates – exactly fitting to our patient case - the clinical efficacy of patients with GTN resistant to multi-agents chemotherapy who treat with pembrolizumab.
4. Conclusion
Similar to a few described cases so far and moving into center of interest, in our case, the patient́s tumor demonstrated strong PD-L1 expression. Despite frustrating response to cytotoxic chemotherapy, she experienced full remission after pembrolizumab and has durable response of currently more than two years after end of treatment. Except for Clair et al. (Clair et al., 2020) no one has yet reported such a long progression-free follow-up. Even though reported observations appear promising for patients with metastasized chemotherapy resistant GTN, sizeable prospective multicenter trials will be necessary to substantiate current evidence on CPI treatment in this particular setting.
Previous presentations
None.
Disclaimers
The Authors have nothing to disclaim.
Author contribution
VP and TB performed the literature search, investigation and screened the data. NP extracted the imaging. VP wrote the draft manuscript. TB, AR, CG, SP critically revised the manuscript for important intellectual content. SP supervised the project. All authors gave final approval of the version submitted and any revised version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- André T., Shiu K.-K., Kim T.W., Jensen B.V., Jensen L.H., Punt C. Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N. Engl. J. Med. [Internet] 2020;383(23):2207–2218. doi: 10.1056/NEJMoa2017699. Available from: www.nejm.org/doi/10.1056/NEJMoa2017699, Dec 3 [cited 2021 Jun 15] [DOI] [PubMed] [Google Scholar]
- Cheng, H.Y., Yang, J.J., Zhao, J., Ren, T., Feng, F.Z., Wan, X.R., et al., 2020. [Preliminary study of PD-1 inhibitor in the treatment of drug-resistant recurrent gestational trophoblastic neoplasia]. Zhonghua Fu Chan Ke Za Zhi [Internet]. Jun 25 [cited 2021 Jun 15];55(6):390–4. Available from: www.ncbi.nlm.nih.gov/pubmed/32842245. [DOI] [PubMed]
- Choi M.C., Oh J., Lee C. Effective anti-programmed cell death 1 treatment for chemoresistant gestational trophoblastic neoplasia. Eur. J. Cancer [Internet] 2019 doi: 10.1016/j.ejca.2019.08.024. Nov [cited 2020 Apr 3];121:94–7. Available from: linkinghub.elsevier.com/retrieve/pii/S0959804919304848. [DOI] [PubMed] [Google Scholar]
- Clair K.H., Gallegos N., Bristow R.E. Successful treatment of metastatic refractory gestational choriocarcinoma with pembrolizumab: A case for immune checkpoint salvage therapy in trophoblastic tumors. Gynecol. Oncol. Rep. [Internet] 2020 doi: 10.1016/j.gore.2020.100625. Nov [cited 2021 Mar 24];34:100625. Available from: www.ncbi.nlm.nih.gov/pubmed/32964090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIGO staging for gestational trophoblastic neoplasia 2000. Int J Gynecol Obstet [Internet]. 2002 Jun 1 [cited 2020 Apr 5];77(3):285–7. Available from: doi.wiley.com/10.1016/S0020-7292%2802%2900063-2. [DOI] [PubMed]
- Ghorani Ehsan, Kaurb Baljeet, Fisher Rosemary, Short Dee, Joneborg Ulrika, Carlson Joseph W., Akarca Ayse, Marafioti Teresa, Quezada Sergio A., Naveed Sarwar, M.J.S., 2017. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia [Internet]. [cited 2019 Dec 26]. Available from: www.thelancet.com. [DOI] [PubMed]
- Goldfarb J.A., Dinoi G., Mariani A., Langstraat C.L. A case of multi-agent drug resistant choriocarcinoma treated with Pembrolizumab. Gynecol. Oncol. Rep. [Internet] 2020;32 doi: 10.1016/j.gore.2020.100574. May [cited 2021 Mar 24]. Available from: www.ncbi.nlm.nih.gov/pubmed/32395603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria I., Khosroshahi A., Ansari M.J., Habicht A., Azuma M., Yagita H. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. [Internet] 2005;202(2):231–237. doi: 10.1084/jem.20050019. Available from: www.ncbi.nlm.nih.gov/pubmed/16027236, Jul 18 [cited 2021 Jun 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst, R.S., Baas, P., Kim, D.-W., Felip, E., Pérez-Gracia, J.L., Han, J.-Y., et al., 2016. Articles Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. www.thelancet.com [Internet]. [cited 2019 Dec 26];387. Available from: dx.doi.org/10.1016/. [DOI] [PubMed]
- Huang M., Pinto A., Castillo R.P., Slomovitz B.M. Complete serologic response to pembrolizumab in a woman with chemoresistant metastatic choriocarcinoma. J. Clin. Oncol. [Internet] 2017;35(27):3172–3174. doi: 10.1200/JCO.2017.74.4052. Sep 20 [cited 2019 Dec 26]. Available from: ascopubs.org/doi/10.1200/JCO.2017.74.4052. [DOI] [PubMed] [Google Scholar]
- Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. [Internet] 2008 doi: 10.1146/annurev.immunol.26.021607.090331. Apr [cited 2019 Dec 26];26(1):677–704. Available from: www.ncbi.nlm.nih.gov/pubmed/18173375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Teng X., Fu G., Bao L., Tang J., Shi H. Analysis of PD-L1 expression in trophoblastic tissues and tumors. Hum. Pathol. [Internet] 2019 doi: 10.1016/j.humpath.2018.10.001. [cited 2021 Jun 15];84:202–12. Available from: www.ncbi.nlm.nih.gov/pubmed/30339966. [DOI] [PubMed] [Google Scholar]
- Lurain J.R. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. AJOG [Internet] 2010 doi: 10.1016/j.ajog.2010.06.073. [cited 2019 Dec 26]; Available from: www.AJOG.org. [DOI] [PubMed] [Google Scholar]
- Ngan H.Y.S., Seckl M.J., Berkowitz R.S., Xiang Y., Golfier F., Sekharan P.K. Update on the diagnosis and management of gestational trophoblastic disease. Int. J. Gynecol. Obstet. [Internet] 2018 doi: 10.1002/ijgo.12615. Oct [cited 2019 Dec 26];143:79–85. Available from: [DOI] [PubMed] [Google Scholar]
- Powles T., Savage P.M., Stebbing J., Short D., Young A., Bower M. A comparison of patients with relapsed and chemo-refractory gestational trophoblastic neoplasia. Br. J. Cancer [Internet] 2007;96(5):732–737. doi: 10.1038/sj.bjc.6603608. Mar 13 [cited 2019 Dec 26], Available from: www.nature.com/articles/6603608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl M.J., Fisher R.A., Salerno G., Rees H., Paradinas F.J., Foskett M. Choriocarcinoma and partial hydatidiform moles. Lancet. 2000;356(9223):36–39. doi: 10.1016/S0140-6736(00)02432-6. [Internet]. Jul 1 [cited 2019 Dec 26]. Available from: www.sciencedirect.com/science/article/pii/S0140673600024326?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Veras E., Kurman R.J., Wang T.-L., Shih I.-M. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int. J. Gynecol. Pathol. [Internet] 2017;36(2):146–153. doi: 10.1097/PGP.0000000000000305. Mar [cited 2021 Mar 24], Available from: www.ncbi.nlm.nih.gov/pubmed/27362903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You B., Bolze P.-A., Lotz J.-P., Massardier J., Gladieff L., Joly F. Avelumab in patients with gestational trophoblastic tumors with resistance to single-agent chemotherapy: Cohort A of the TROPHIMMUN phase II trial. J. Clin. Oncol. [Internet] 2020;38(27):3129–3137. doi: 10.1200/JCO.20.00803. Available from: www.ncbi.nlm.nih.gov/pubmed/32716740. [cited 2021 Jun 16] [DOI] [PMC free article] [PubMed] [Google Scholar]