Abstract
Backgrounds and aims
Rheumatoid arthritis (RA), is immune-inflammatory disease which is associated with great pain and disability. Overproduction of pro-inflammatory cytokines and oxidative stress play an important role in RA pathogenesis and related outcomes. The aim of this study was to evaluate the effects of propolis on inflammatory biomarkers and oxidative stress status in RA patients.
Methods/design
Randomized, placebo-controlled, and double-blind clinical trial aiming to recruit 48 patients with RA. Block randomization will be used. An intervention group will receive 500 mg/twice a day propolis capsules for 3 months and control group will receive the placebo for the same dose and duration. The oxidative stress status (malondialdehyde (MDA), total antioxidant capacity (TAC), total oxidant status (TOS), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx)), and inflammatory biomarkers (interleukin-17 (IL-17), Tumor necrosis factor alpha (TNF-α), High-sensitivity C-reactive protein (hs-CRP)), lipid profile (total cholesterol (TC), high density lipoprotein (HDL-c), low density lipoprotein (LDL-c), and triglyceride (TG)) and also physical activity, anthropometric indices, clinical and nutritional status will be measured at beginning and end of this study. The primary analysis will be based on theintention-to-treat principle.
Discussion
If this randomized clinical trial shows the reduction in inflammatory cytokines and oxidative stress and improves clinical outcome, it would provide evidence for other clinical trials to evaluate the efficacy of propolis supplementation in RA patients.
Keywords: Inflammation, Oxidative stress, Propolis, Rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is inflammatory disease which firstly targets synovial tissues, lead to considerable pain and disability in patients. The prevalence of RA in adults is about 0.5–1.0% [1,2]. It has a considerable negative effects on the quality of life (QOL), and the ability to do daily activities [3]. The clinical studies have been shown that oxidative stress play an important role in the etiology of RA, also evidences demonstrated the increased level of oxidative stress biomarkers and decreased antioxidants status in RA patients [[4], [5], [6], [7], [8]] The reactive oxygen species (ROS) can cause inflammatory responses by activating nuclear factor kappa-B (NF-kB) in in RA [4]. Therefore, supplementation with antioxidant agents may help to relieve symptoms and improve QOL. Today's pharmacologic agents such as non-steroidal anti-inflammatory drugs, corticosteroids, and disease-modifying anti-rheumatic drugs (DMARDs) are available to alleviate RA symptoms, but these agents have some side effects [9]. Therefore, attention to adjuvant treatments, especially dietary supplements has been increased.
Propolis is a resinous hive product that is made by honeybees [10]. The composition of propolis is very complex and dependent on the plant sources [11,12]. Analysis of propolis samples from different regions has identified at least 300 different compounds. The beneficial effects of propolis are mostly attributed to the phenolic components such as flavonoids (flavonols, flavones, flavonones, dihydroflavonols), terpenes, beta-steroids, aromatic aldehydes, and alcohols [13,14]. Literature showed that propolis has positive effects on atherogenesis, diabetes, cancer, oxidative and inflammatory status [[15], [16], [17], [18], [19], [20]]. Based on the current evidences, antioxidant features of propolis is responsible for its therapeutic benefits [16,19]. It improves the antioxidant status and prevents the production of proinflammatory cytokines by inhibiting NF-kB [[21], [22], [23]].
To the best of our knowledge, there is no clinical trial that assessed the effects of propolis on biochemical and clinical status in RA, so we hypothesized that propolis would decrease serum inflammatory biomarkers, improve oxidative stress status, and clinical status. Hence, this study has been designed to investigate the efficacy of propolis administration in RA patients.
2. Methods/design
2.1. Trial design
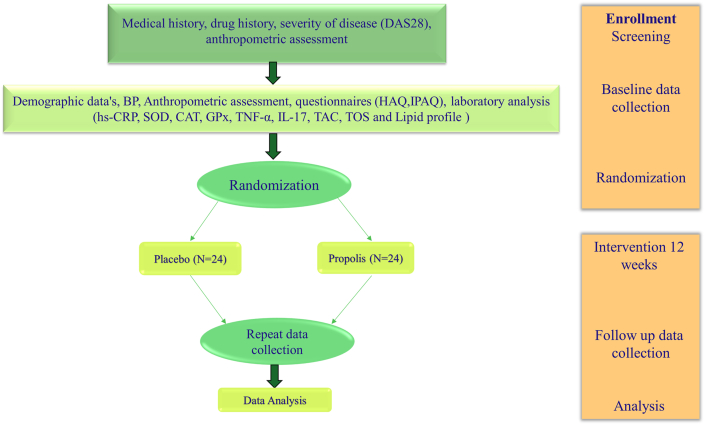
This RCT protocol was written according to the CONSORT SPIRIT 2013 guidelines [24]. In the present double-blind, randomized, placebo-controlled parallel study we will include 48 women with RA. The study design is presented in Fig. 1. Subjects will be selected from patients referring to the Imam Reza hospital related to Mashhad University of Medical Sciences (MUMS) from Aguste 2020. Patients will be screening based on inclusion and exclusion criteria by an experienced rheumatologist.
Fig. 1.
Trial protocol
2.2. Eligibility criteria
Eligibility criteria for RA patients will be done as follows. Participants will be allocated into two groups by block randomization.
2.3. Inclusion criteria
-
-
Diagnosis of the disease by a rheumatologist based on the criteria of the American College of Rheumatology [25].
-
-
Women in the age range of 20–70 years.
-
-
Identical drug advisement during study in both of groups.
-
-
Patients with moderate and severe disease activity
-
-
Participants with a history of antioxidant supplements use in three months leading to the study were also excluded
-
-
The tendency to cooperate and sign informed written consent
2.4. Exclusion criteria
-
-
Pregnancy and lactation
-
-
Taking oral contraceptive pills
-
-
A history of chronic diseases (i.e. cardiovascular disease (CVD), diabetes, cancer, liver or kidney disease)
-
-
Having other autoimmune and inflammatory diseases
-
-
Abnormal hormonal and thyroid disorders
-
-
Alcohol consumption and hookah smoke
-
-
Smoking and being exposed to secondhand smoke (Active or passive smokers)
-
-
Consumption of any steroidal drugs
2.5. Baseline assessment
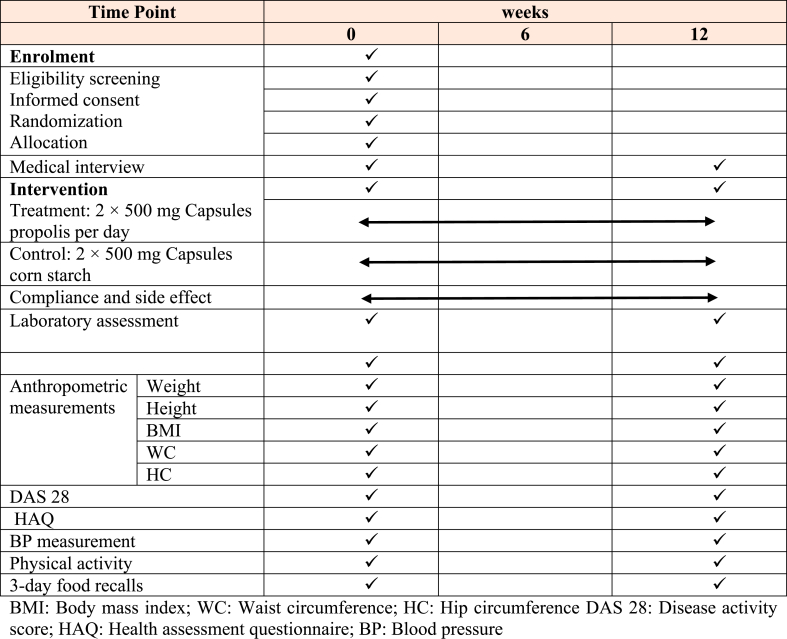
The study timeline is presented in Table 1. The baseline assessment related clinical status (DAS 28) will be performed by rheumatologist, then the research dietitian, who will collect demographic details, medical and social history (living situation, marital status, current occupation), Health activity questionnaire (HAQ), physical activity questionnaire, dietary intake (3 day record) assessment, anthropometric measurement (Weight, Height, body mass index (BMI), waist circumference (WC) and hip circumference (HC).
Table 1.
Timeline and applied tests.
2.6. Randomization and interventions
Eligibility of patients will be determined at the first screening visit. Block randomization method will be used for this trial study. Participants will be allocated randomly (1:1) to intervention and control groups based on random block procedure consisting of four subjects per block.
Blocking will be complemented according to patient's baseline characteristics such as severity of disease (moderate or severe), menstrual status (yes or no), BMI (BMI: <30 and ≥ 30 kg/m2) and type of intervention (propolis or placebo). The intervention group will receive 500 mg propolis (100 mg polyphenol compounds and 67 mg flavonoids) capsules orally twice a day and the control group will receive 500 mg placebo (corn starch) orally twice a day. The drug treatment protocol in the control group will be the same in both of groups. Also, Participants will be instructed to continue their usual diet, physical activity, and medication during the intervention.
Anthropometric indices (Body weight, BMI, WC, HC), and blood pressure will be assessed at beginning and end of intervention. The dietary intakes of patients will be assessed based on 3-day food records and convert to analytical data by Nutritionist IV program modified for Iranian foods. To evaluate the physical activity, we will be used the International Physical Activity Questionnaire (IPAQ) [26].
2.7. Ethics approval
The study has been approved by the ethics committee of Mashhad university of Medical Sciences (ethical code: IR. MUMS.MEDICAL.REC.1399.145) and was registered in the Iranian Registry of Clinical Trials website (IRCT ID: IRCT20190407043194N2).
2.8. Safety consideration
During the screening and follow-up any abnormalities detected will be discussed with rheumatologist involved in the study. Also, if we see any side effects after consumption of propolis, the patient will be excluded from the study. All participants will be informed of their blood test results as the study will be completed.
2.9. Power calculation and sample size estimates
To determine the sample size in this study, we used the previous study [27]. Also, we used from sample size formula suggested for randomized clinical trials. We considered the Type I error of 5% (α = 0.05) and Type II error of 20% (β = 0.2; power = 80%) and serum levels of MDA as a primary outcome, and we calculated the sample size of 18 persons for each group. With 30% dropout, finally twenty-four patients will consider for each group.
2.10. Blinding
Patients and researcher team will remain blinded to complete treatment period of 3 months. Treatment allocations will only be revealed at the end of study. The placebo capsule is similar to the study drug for taste, color, size and both of supplements manufacture with identical company (Shahdineh Golha pharmaceutical Company, Isfahan, Iran).
2.11. Applied tests during the study
2.11.1. Medical interview
At baseline and after the 3 months intervention, we will assess patients and collect data of the number of tender and swollen joints on the basis of the 28-joint count, Visual Analogue Scale (0–100 mm) for DAS-28 and pain.
2.12. Assessment of anthropometric measurements
Weight and height will be measured at the beginning and the end of the study without shoes and minimal clothing by a trained attendant. BMI will be calculated using the height and weight measurements (weight in kg/[squared height in meters]). WC will be measured at the midpoint of the lowest rib and iliac crest.
2.13. Laboratory assessment
Seven ml of venous blood will be obtained from all of patients after 10–12 h fasting at the beginning and after 3 months of intervention in the Sadra lab. The serum and plasma will be separated by centrifugation at 3000 rpm for 10 min, then the serum samples will be stored at −70 °C after centrifugation until assay. Finally, Serum hs-CRP, SOD, CAT, GPx, TNF-α, IL-17, TAC, and TOS concentrations will be quantified by the use of a commercial ELISA kit. Spectrophotometric method will be used to determine serum MDA by using the thiobarbituric acid reactive substance method [28]. Serum levels of triglycerides (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C) will be measured enzymatically. Low-density lipoprotein cholesterol (LDL-C) will be calculated by the Friedwald formula as follows [29]:
LDL cholesterol = (Total cholesterol-HDL cholesterol) – (Triglyceride/5)
2.14. Statistical methods
All our analyses will be based on the intention-to-treat (ITT) approach. Data will be analyzed by using the SPSS software, version 23 (SPSS Inc, Chicago, IL, USA). Quantitative data will be presented as mean ± standard deviation (SD), and qualitative data are will demonstrated as frequency and percent. The Kolmogorov-Smirnov test will be used to assess the normality of data. Paired t-test will be used for before and after intervention comparisons. Analysis of covariance (ANCOVA) will be applied to identify any differences between two treatment groups after adjusting for confounding variables. Results will be considered statistically significant at p-value less than 0.05.
2.15. Outcome measures
The primary outcome of this trial is the change in serum levels and inflammatory (IL-17, TNF-α, TAC ، TOS, and MDA markers between two groups.
2.16. Secondary outcomes include
-
1
Comparison of DAS 28 score changes in two group after 3 months intervention
-
2
Comparison of HAQ score changes in two group after 3 months intervention
-
3
Comparison of Antioxidant enzymes (Superoxide dismutase (SOD), catalase (CAT), Glutathione peroxidase (GPx) changes in two group after 3 months intervention.
-
4
Comparison of Lipid profile (LDL, HDL, TC, and Triglycerides) mean changes in two group after 3 months intervention
3. Discussion
This study is the first clinical trial that will assess the effect of propolis supplementation on inflammation biomarkers and oxidative stress status in women with RA. RA is result the continuous deterioration of cells and tissues [30]. Both oxidative stress and inflammation are considered as main causes in RA pathogenesis [31]. Previous studies have been shown that there is the pro-oxidant/antioxidant imbalance in RA [[32], [33], [34]]. Reducing inflammation and oxidative stress associate with a reduction in the risk of RA. Pro-inflammatory cytokines such as TNF-α and IL-6 play an important role in the pathophysiology of RA [35]. TNF-α and IL-6 are main mediators of cell migration and inflammation in RA [36]. If propolis supplementation reduces inflammation and oxidative stress and improve antioxidant status in this trial, using of propolis as an herbal medicine and nutritional agent can apply for reducing symptom and ameliorating clinical status in RA patients.
Propolis has strong anti-inflammatory function [37]. It able directly and indirectly decrease pro-inflammatory cytokines [38]. Based on the previous clinical trial studies propolis has numerous useful chemical composition with wide phytochemical and antioxidant characteristics that has beneficial effects in human health [16,17,39,40]. Recent meta-analysis that investigated the effect of propolis on inflammatory biomarkers have shown that propolis significantly reduced IL-6, CRP, and TNF-a [41]. Also according to experimental studies propolis prohibit leukotriene and prostaglandin production [42]. The effect of propolis on cyclooxygenase (COX) may be due to flavonoids, which have been shown to suppressed prostaglandin endoperoxide synthase [43]. It also blocks the activation of COX-1, COX-2 [44]. We hope that propolis will improve outcomes by this effect.
4. Conclusion
We describe the protocol for a clinical trial design evaluating the effects of propolis supplementation on inflammatory biomarkers, oxidative stress status, and clinical symptoms of RA patients. We expect that oral supplementation of 1000 mg/day of propolis for 3 months, will improve clinical outcomes and decrease the inflammation and oxidative stress in the RA patients. The result of the current study, positive or negative, could provide a step change in the evidence guiding current and future policies regarding the use or not of propolis as complementary treatment in RA patients.
Trial registration
This trial is registered at clinicaltrials.gov (ID: IRCT20190407043194N2) on July 22, 2020.
Trial status
The trial enrollment started on 5 Aguste 2020 and currently is recruiting patients. Follow-up and collection labour data of patients expected to take about 6 months.
Authors’ contributions
ENE, MK, MHJ, MS and MN conceived and designed the study. HT is responsible for statistics analysis. NP and MM collaborated to perform of the clinical trial.
Funding
This clinical trial is funded by Mashhad University of Medical Sciences.
Declaration of competing interest
None declared.
Acknowledgements
This work was supported by the Mashhad University of Medical Sciences.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2021.100807.
Abbreviations
- RA
Rheumatoid Arthritis
- ROS
Reactive Oxygen Species
- NF-kB
Nuclear Factor Kappa-B
- DMARDs
Disease-Modifying Anti-Rheumatic Drugs
- DAS 28
Disease Activity Score 28
- HAQ
Health Activity Questionnaire
- BMI
Body Mass Index
- IPAQ
Physical Activity Questionnaire
- BP
Blood Pressure
- WC
waist Circumference
- HC
Hip Circumference
- Hs-CRP
High-Sensitivity C - reactive protein
- SOD
Superoxide Dismutase
- CAT
Catalase
- GPx
Glutathione peroxidase
- TNF-α
Tumor Necrosis Factor alpha
- TAC
Total Antioxidant Capacity
- TOS
Total Oxidant Status
- MDA
Malondialdehyde
- TG
Triglycerides
- TC
Total cholesterol
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- IL-17
Interleukin 17
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Gabriel S.E., Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res. Ther. 2009;11(3):229. doi: 10.1186/ar2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver J.E., Silman A.J. Why are women predisposed to autoimmune rheumatic diseases? Arthritis Res. Ther. 2009;11(5):252. doi: 10.1186/ar2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertin P., Fagnani F., Duburcq A., Woronoff A.-S., Chauvin P., Cukierman G., Tropé-Chirol S., Joubert J.-M., Kobelt G. Impact of rheumatoid arthritis on career progression, productivity, and employability: the PRET study. Joint Bone Spine. 2016;83(1):47–52. doi: 10.1016/j.jbspin.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Filippin L.I., Vercelino R., Marroni N., Xavier R.M. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin. Exp. Immunol. 2008;152(3):415–422. doi: 10.1111/j.1365-2249.2008.03634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mateen S., Moin S., Khan A.Q., Zafar A., Fatima N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PloS One. 2016;11(4) doi: 10.1371/journal.pone.0152925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taysi S., Polat F., Gul M., Sari R., Bakan E. Lipid peroxidation, some extracellular antioxidants, and antioxidant enzymes in serum of patients with rheumatoid arthritis. Rheumatol. Int. 2002;21(5):200–204. doi: 10.1007/s00296-001-0163-x. [DOI] [PubMed] [Google Scholar]
- 7.Aryaeian N., Djalali M., Shahram F., Jazayeri S., Chamari M., Nazari S. Beta-carotene, vitamin E, MDA, glutathione reductase and arylesterase activity levels in patients with active rheumatoid arthritis. Iran. J. Public Health. 2011;40(2):102. [PMC free article] [PubMed] [Google Scholar]
- 8.Baskol G., Demir H., Baskol M., Kilic E., Ates F., Karakukcu C., Ustdal M. Investigation of protein oxidation and lipid peroxidation in patients with rheumatoid arthritis. Cell Biochem. Funct.: Cellular biochemistry and its modulation by active agents or disease. 2006;24(4):307–311. doi: 10.1002/cbf.1257. [DOI] [PubMed] [Google Scholar]
- 9.O'Dell J.R. Therapeutic strategies for rheumatoid arthritis. N. Engl. J. Med. 2004;350(25):2591–2602. doi: 10.1056/NEJMra040226. [DOI] [PubMed] [Google Scholar]
- 10.Farooqui T., Farooqui A.A. Molecular mechanism underlying the therapeutic activities of propolis: a critical review. Curr. Nutr. Food Sci. 2010;6(3):186–199. [Google Scholar]
- 11.Salatino A., Fernandes-Silva C.C., Righi A.A., Salatino M.L.F. Propolis research and the chemistry of plant products. Nat. Prod. Rep. 2011;28(5):925–936. doi: 10.1039/c0np00072h. [DOI] [PubMed] [Google Scholar]
- 12.Pahlavani N., Malekahmadi M., Firouzi S., Rostami D., Sedaghat A., Moghaddam A.B., Ferns G.A., Navashenaq J.G., Reazvani R., Safarian M. Molecular and cellular mechanisms of the effects of Propolis in inflammation, oxidative stress and glycemic control in chronic diseases. Nutr. Metabol. 2020;17(1):1–12. doi: 10.1186/s12986-020-00485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mani F., Damasceno H., Novelli E., Martins E., Sforcin J. Propolis: effect of different concentrations, extracts and intake period on seric biochemical variables. J. Ethnopharmacol. 2006;105(1–2):95–98. doi: 10.1016/j.jep.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Viuda‐Martos M., Ruiz‐Navajas Y., Fernández‐López J., Pérez‐Álvarez J. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008;73(9):R117–R124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 15.Karimian J., Hadi A., Pourmasoumi M., Najafgholizadeh A., Ghavami A. The efficacy of propolis on markers of glycemic control in adults with type 2 diabetes mellitus: a systematic review and meta‐analysis. Phytother Res. 2019;33(6):1616–1626. doi: 10.1002/ptr.6356. [DOI] [PubMed] [Google Scholar]
- 16.Zakerkish M., Jenabi M., Zaeemzadeh N., Hemmati A.A., Neisi N. The effect of Iranian propolis on glucose metabolism, lipid profile, insulin resistance, renal function and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Sci. Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-43838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afsharpour F., Javadi M., Hashemipour S., Koushan Y. Propolis supplementation improves glycemic and antioxidant status in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Compl. Ther. Med. 2019;43:283–288. doi: 10.1016/j.ctim.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Gao W., Pu L., Wei J., Yao Z., Wang Y., Shi T., Zhao L., Jiao C., Guo C. Serum antioxidant parameters are significantly increased in patients with type 2 diabetes mellitus after consumption of Chinese propolis: a randomized controlled trial based on fasting serum glucose level. Diabetes therapy : research, treatment and education of diabetes and related disorders. 2018;9(1):101–111. doi: 10.1007/s13300-017-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mujica V., Orrego R., Pérez J., Romero P., Ovalle P., Zúñiga-Hernández J., Arredondo M., Leiva E. 2017. The role of propolis in oxidative stress and lipid metabolism: a randomized controlled trial, Evidence-Based Complementary and Alternative Medicine 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pahlavani N., Sedaghat A., Moghaddam A.B., Kiapey S.S.M., Navashenaq J.G., Jarahi L., Reazvani R., Norouzy A., Nematy M., Safarian M. Effects of propolis and melatonin on oxidative stress, inflammation, and clinical status in patients with primary sepsis: study protocol and review on previous studies. Clinical nutrition ESPEN. 2019;33:125–131. doi: 10.1016/j.clnesp.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Yerra V.G., Negi G., Sharma S.S., Kumar A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox biology. 2013;1(1):394–397. doi: 10.1016/j.redox.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Búfalo M.C., Ferreira I., Costa G., Francisco V., Liberal J., Cruz M.T., Lopes M.C., Batista M.T., Sforcin J.M. Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-κB and MAPK activation in macrophages. J. Ethnopharmacol. 2013;149(1):84–92. doi: 10.1016/j.jep.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z., Zhu A., Takayama F., Okada R., Liu Y., Harada Y., Wu S., Nakanishi H. 2013. Brazilian green propolis suppresses the hypoxia-induced neuroinflammatory responses by inhibiting NF-κB activation in microglia, Oxidative Medicine and Cellular Longevity 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan A.-W., Tetzlaff J.M., Altman D.G., Laupacis A., Gøtzsche P.C., Krleža-Jerić K., Hróbjartsson A., Mann H., Dickersin K., Berlin J.A. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann. Intern. Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., III, Birnbaum N.S., Burmester G.R., Bykerk V.P., Cohen M.D.J.A., rheumatism 2010 rheumatoid arthritis classification criteria. an American College of Rheumatology/European League Against Rheumatism collaborative initiative. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 26.Wolin K.Y., Heil D.P., Askew S., Matthews C.E., Bennett G.G. Validation of the international physical activity questionnaire-short among blacks. J. Phys. Activ. Health. 2008;5(5):746–760. doi: 10.1123/jpah.5.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helli B., Mowla K., Mohammadshahi M., M.J.J.o.t.A.C.o.N. Jalali Effect of sesamin supplementation on cardiovascular risk factors in women with rheumatoid arthritis. 2016;35(4):300–307. doi: 10.1080/07315724.2015.1005198. [DOI] [PubMed] [Google Scholar]
- 28.D.R.J.F.r.b. Janero medicine, Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. 1990;9(6):515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 29.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 30.McInnes I.B., Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 31.Vaghef-Mehrabany E., Homayouni-Rad A., Alipour B., Sharif S.-K., Vaghef-Mehrabany L., S.J.J.o.t.A.C.o.N. Alipour-Ajiry Effects of probiotic supplementation on oxidative stress indices in women with rheumatoid arthritis: a randomized double-blind clinical trial. 2016;35(4):291–299. doi: 10.1080/07315724.2014.959208. [DOI] [PubMed] [Google Scholar]
- 32.Ozkan Y., Yardým-Akaydýn S., Sepici A., Keskin E., Sepici V., Simsek B. Oxidative status in rheumatoid arthritis. Clin. Rheumatol. 2007;26(1):64–68. doi: 10.1007/s10067-006-0244-z. [DOI] [PubMed] [Google Scholar]
- 33.Jaswal S., Mehta H.C., Sood A.K., Kaur J. Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin. Chim. Acta. 2003;338(1):123–129. doi: 10.1016/j.cccn.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Cimen M., Çimen Ö., Kacmaz M., Öztürk H., Yorgancioğlu R., Durak I. Oxidant/antioxidant status of the erythrocytes from patients with rheumatoid arthritis. Clin. Rheumatol. 2000;19(4):275–277. doi: 10.1007/pl00011172. [DOI] [PubMed] [Google Scholar]
- 35.Smolen J.S., Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat. Rev. Drug Discov. 2003;2(6):473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- 36.Smolen J.S., Aletaha D., Koeller M., Weisman M.H., Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370(9602):1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 37.Freires I.A., de Alencar S.M., Rosalen P.L. A pharmacological perspective on the use of Brazilian Red Propolis and its isolated compounds against human diseases. Eur. J. Med. Chem. 2016;110:267–279. doi: 10.1016/j.ejmech.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 38.Al Ghamdi A.A., Badr G., Hozzein W.N., Allam A., Al-Waili N.S., Al-Wadaan M.A., Garraud O. Oral supplementation of diabetic mice with propolis restores the proliferation capacity and chemotaxis of B and T lymphocytes towards CCL21 and CXCL12 by modulating the lipid profile, the pro-inflammatory cytokine levels and oxidative stress. BMC Immunol. 2015;16(1):54. doi: 10.1186/s12865-015-0117-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Anjum S.I., Ullah A., Khan K.A., Attaullah M., Khan H., Ali H., Bashir M.A., Tahir M., Ansari M.J., Ghramh H.A. Composition and functional properties of propolis (bee glue): a review. Saudi J. Biol. Sci. 2019;26(7):1695–1703. doi: 10.1016/j.sjbs.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farooqui T., Farooqui A.A.J.F.B. Beneficial effects of propolis on human health and neurological diseases. 2012;4:779–793. doi: 10.2741/e418. [DOI] [PubMed] [Google Scholar]
- 41.Shang H., Bhagavathula A.S., Aldhaleei W.A., Rahmani J., Karam G., Rinaldi G., Clark C., Salehisahlabadi A., Yuan Q. Effect of propolis supplementation on C-reactive protein levels and other inflammatory factors: a systematic review and meta-analysis of randomized controlled trials. J. King Saud Univ. Sci. 2020;32(2):1694–1701. [Google Scholar]
- 42.Mirzoeva O., Calder P. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostagl. Leukot. Essent. Fat. Acids. 1996;55(6):441–449. doi: 10.1016/s0952-3278(96)90129-5. [DOI] [PubMed] [Google Scholar]
- 43.Mirzoeva O., Calder P.J.P., Leukotrienes, Acids E.F. The effect of propolis and its components on eicosanoid production during the inflammatory response. 1996;55(6):441–449. doi: 10.1016/s0952-3278(96)90129-5. [DOI] [PubMed] [Google Scholar]
- 44.Orban Z., Mitsiades N., Burke T.R., Jr., Tsokos M., Chrousos G.P.J.N. Caffeic acid phenethyl ester induces leukocyte apoptosis. modulates nuclear factor-kappa B and suppresses acute inflammation. 2000;7(2):99–105. doi: 10.1159/000026427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.