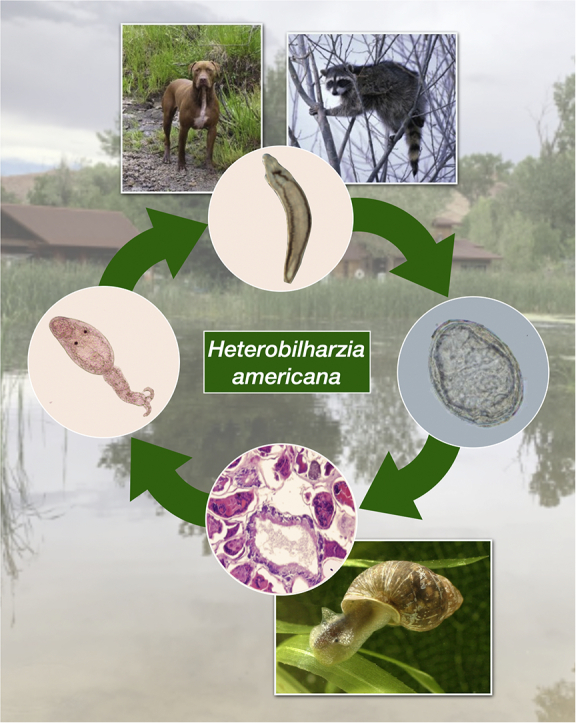
Abstract
Parasites with complex life cycles engaging multiple host species living among different environments well-exemplify the value of a cross-cutting One Health approach to understanding fundamental concerns like disease emergence or spread. Here we provide new information regarding a pathogenic schistosome trematode parasite of both wild and domestic mammals that has recently expanded its known range from mesic/wet environments of the southeastern United States to the arid southwest. In 2018, 12 dogs living near a man-made pond in Moab, Utah, were found positive for Heterobilharzia americana, the most westerly report of this endemic North American schistosome, and the first from Utah. Raccoon scats collected near the pond were positive for H. americana eggs, and snails living near the pond's water line identified as Galba humilis shed H. americana cercariae, the first indication of natural infections in this widespread North American snail species. The susceptibility of G. humilis to H. americana was confirmed experimentally. Our studies support the existence of two variants of H. americana and emphasize the need for further investigations of lymnaeids and their compatibility with H. americana, to better define the future potential for its spread. Capture of a new species of intermediate host vector snail and construction of man-made habitats suitable for this snail have created the potential for a much more widespread animal health problem, especially for dogs and horses. H. americana will prove difficult to control because of the role of raccoons in maintaining transmission and the amphibious habits of the snail hosts of this pathogenic schistosome.
Keywords: Emerging infectious disease, Range expansion, Schistosomiasis, Heterobilharzia, Galba humilis, Vector biology
Graphical abstract
Highlights
-
•
The pathogenic canine schistosome Heterobilharzia americana expands its known range.
-
•
First evidence of new snail vector, Galba humilis.
-
•
Galba humilis is widely distributed in North America, enabling further spread.
-
•
Raccoons are also important hosts and their increasing abundance also favors spread.
1. Introduction
The OneHealth concept recognizes that the health concerns of humans and wild and domestic animals and plants are intertwined and that changing environmental circumstances might have unforeseen effects leading to unexpected emergence of infectious diseases with far-reaching implications [1,2]. Along with the spillover of zoonotic pathogens like SARS-CoV-2 into new hosts [3], another factor driving infectious disease emergence is expansion of pathogens or their hosts or vectors into new environments. The range expansions of several tick species [4], and the meningeal worm Parelaphostrongylus tenuis [5] provide familiar examples. Here we discuss the expansion of an endemic pathogenic schistosome of wild and domestic mammals into new North America environments.
Heterobilharzia americana is one of two indigenous North American species of schistosome trematodes infecting mammals. It is a parasite of raccoons but is noteworthy for the broad range of other wild and domestic mammals it can infect, especially dogs [6]. In humans, cercariae of H. americana cause severe dermatitis but humans do not develop patent infections. However, H. americana can survive for 45 days in rhesus monkeys [7]. The possibility of some degree of visceral development of H. americana in primates should not be ruled out. H. americana is endemic in U.S states bordering the Gulf of Mexico and the southeastern Atlantic, to the Carolinas. Also notable has been its sporadic recovery from mammals in Kansas [8], Oklahoma [6,9], Tennessee [10], Indiana [11], and Arkansas [6].
The life cycle of H. americana, typical for digenetic trematodes of the Family Schistosomatidae, involves a mammalian definitive host in which paired male and female worms are found in mesenteric blood vessels. Eggs are produced, passed in the host's feces, and hatch in water and infect snails of the family Lymnaeidae in this case. Schistosomes reproduce asexually in the snail and release or “shed” many cercariae into water. Cercariae then penetrate the skin of their definitive host and migrate via the lungs to the liver and develop into adult worms.
The only snail species thus far found to be naturally infected with H. americana [12] is the small amphibious lymnaeid Galba cubensis [13]. It ranges from Florida to Texas with populations known from Oklahoma, Georgia and the Carolinas. Not surprisingly, the historical range of H. americana thus appears to correspond with the presence of this snail. Populations of G. cubensis also occur in Mexico, South America and the West Indies [14,15]. The cosmopolitan aquatic lymnaeid Pseudosuccinea columella [16] is sometimes experimentally susceptible [7] but has not been found naturally infected.
Upon exposure to H. americana, dogs experience dermatitis (penetrating cercariae), bloody diarrhea, weight loss, anorexia, vomiting, lethargy, polyuria and polydipsia, and collapse as clinical signs. Granulomatous responses provoked by H. americana eggs occur in the liver, intestine, pancreas, and lungs. Similar pathology occurs in raccoons [17]. A distinctive feature of H. americana pathology in dogs is hypercalcemia, probably a consequence of granulomatous disease [18] which can lead to renal failure [19,20]. In horses, H. americana infection can result in granuloma formation in the lungs, possibly leading to congestive heart failure [21]. Dogs infected with H. americana can be treated with praziquantel or fenbendazole. Two consistent messages from veterinarians are that H. americana is underdiagnosed and an increasing problem, especially in dogs and horses [22].
Below we provide evidence for the westward expansion of H. americana, implicate for the first time a widespread new snail vector Galba humilis in natural transmission, provide further details regarding variation within H. americana and highlight aspects of the biology of the parasite and its hosts that may both favor spread and complicate control in the future.
2. Materials and methods
2.1. Collection and screening of field snails
Snails were collected from Mulberry Grove Pond, Moab (Table 1) with handled kitchen strainers swept through aquatic vegetation near the shoreline, or at or above the waterline using forceps. Snails were washed, isolated in individual wells of 24-well plates at mid-day, and left for 2–3 h before screening to identify individual snails shedding cercariae. Snails were re-screened in the early evening (6-8 pm) and the following morning. Snails not shedding cercariae were maintained in the laboratory and re-examined at two-week intervals. H. americana cercariae were photographed, preserved in 100% ethanol, or used to infect mice.
Table 1.
Museum vouchers, locality information, and GenBank Accession numbers of parasites and snails collected as a part of this study. The Louisiana isolate of H. americana and several of the G. humilis (and other lymnaeid) isolates are part of the existing collections of the Parasite Division, Museum of Southwestern Biology. Detailed information for each specimen is on the Arctos Database (http://arctos.database.museum/SpecimenSearch.cfm).
| GenBank Accession numbers |
|||||
|---|---|---|---|---|---|
| Species | Museum Voucher | Locality | Source of Host or Parasite | cox1 | ITS |
| Heterobilharzia americana | MSB:Para:19286 | Louisiana | Adult worm from raccoon | MZ020157 | – |
| MSB:Para:18951 | Indiana | Adult worm from raccoon | – | – | |
| MSB:Para:31807 | Moab, Utah | Eggs from raccoon feces | MW425690 | MW425378 | |
| MSB:Para:31790 | Moab, Utah | Adults from experimental infection | – | – | |
| MSB:Para:31806 | Moab, Utah | Cercariae from field-derived snails | MW963185 | – | |
| MSB:Para:31995 | Austin, Texas | Eggs from dog feces | MW963186, MW963187 | – | |
| Galba humilis | MSB:Host:24243 | Moab, Utah | Field-derived snails | MW425684-MW425688 | MW427222-MW427227 |
| MSB:Host:24242 | Moab, Utah | Lab-reared snail | MW879390 | MW879712 | |
| MSB:Host:22266 | Lake Winnibigoshish, Minnesota | Field-derived snail | MW879391 | – | |
| MSB:Host:22819 | Tingley Beach, New Mexico | Field-derived snail | MW879392 | – | |
| MSB:Host:23338 | Eagles Nest, New Mexico | Field-derived snail | MW879394 | – | |
| MSB:Host:23347 | Eagles Nest, New Mexico | Field-derived snail | – | – | |
| MSB:Host:23371 | Valles Caldera, New Mexico | Field-derived snail | MW879393 | – | |
| Galba schirazensis | MSB:Host:23332 | Valle Escondito, New Mexico | Field-derived snail | MW879397 | – |
| Galba sp. | MSB:Host:20433 | Bosque del Apache, New Mexico | Field-derived snail | MW879396 | MW879710 |
| MSB:Host:20480 | Bosque del Apache, New Mexico | Field-derived snail | MW879395 | MW879711 | |
| Stagnicola elodes | MSB:Host:22097 | Angel Fire, New Mexico | Field-derived snail | MW879399 | |
| MSB:Host:24244 | Rio Cebolla, New Mexico | Field-derived snail | MW879398 | MW879714 | |
| MSB:Host:23703 | Pecos Baldy Lake, New Mexico | Field-derived snail | MW879400 | MW879713 | |
| MSB:Host:23153 | Serpent Lake, New Mexico | Field-derived snail | MW879401 | – | |
| MSB:Host:23349 | Black Lake, New Mexico | Field-derived snail | – | MW979408 | |
| Pseudosuccinea columella | MSB:Host:24245 | PetSmart, Albuquerque, New Mexico | Lab-reared snail | – | – |
| Procyon lotor (feces+eggs) | MSB:Host:24240/MSB:Para:31807 | Moab, Utah | Raccoon feces and miracidia | MW425353 | – |
| MSB:Host:24241/MSB:Para:31808 | Moab, Utah | Raccoon feces and miracidia | – | – | |
2.2. Establishment and maintenance of a laboratory colony of Galba humilis
Mud from Mulberry Grove Pond was placed in plastic dishes (28 cm diameter, 4 cm deep) and sloped to a high point in the center. Spring water was added, leaving a central area uncovered but moist. Snails (20–40 per dish) were fed crushed shrimp pellets and leaf lettuce every other day. Snails were maintained in an ambient light:dark cycle at 25 ± 2 °C. Water was changed regularly. Snails produced egg masses, and progeny were used in experimental infections.
2.3. Maintenance of aquatic lymnaeids
Lab-reared Pseudosuccinea columella (Albuquerque Pet Smart store), Stagnicola cf. elodes from Serpent Lake, New Mexico or from Rio Cebolla, Jemez National Recreation Area, New Mexico were maintained in aerated aquaria and fed on leaf lettuce, temperature 25 ± 2 °C.
2.4. Examination of fecal samples
At the start of the outbreak, fecal samples from dogs living in the vicinity of Mulberry Grove Pond were collected and sent to the College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, and an established diagnostic PCR assay based on amplification of a portion of the 18S ribosomal gene of H. americana [18] was used to determine presence of the parasite. Later, fresh canine, deer or raccoon fecal samples were collected from the ground near the pond; fresh raccoon scats were collected from raccoon latrines. Five grams of fecal material was dispersed in spring water in a 10 cm diameter culture dish, and particles were allowed to settle. The cloudy supernatant was decanted. This process was repeated twice more. The settled material in the dish was examined for the presence of H. americana eggs, or swimming miracidia were collected for infection of snails. Some eggs or miracidia were set aside for photography or preserved in 100% ethanol. Identity of the host of origin for positive fecal samples was confirmed as described in the technical appendix. A canine fecal sample from Austin, Texas positive for H. americana was later obtained and eggs isolated and stored in 100% ethanol for use in molecular analyses.
2.5. Exposure of snails to H. americana miracidia
Miracidia were placed in individual wells of 24-well plates, the number varying depending on the trial. A single uninfected lab-reared lymnaeid snail was added to each well and left for three hours. Snails were then maintained as described above. At four weeks post-exposure, and weekly thereafter, exposed snails were isolated and checked for shedding cercariae. Some exposed G. humilis and P. columella were collected for histological study at 2, 4, 8, or 40 days post-exposure (dpe).
2.6. Exposure of mice to H. americana cercariae
Cercariae were collected from shedding snails and pooled (numbers depending on the experiment) in beakers containing spring water to a depth of 0.5 cm. A Swiss Webster outbred mouse was placed into each beaker for one hour. Fecal samples from exposed mice were checked weekly starting at 49 dpe for H. americana eggs. After 70 dpe, mice were injected with heparin, euthanized using Nembutol [23] and perfused with 199 medium (Sigma-Aldrich, Wisconsin, USA) to collect adult worms. Swimming miracidia for snail exposures were pipetted from water containing homogenized livers of mice with patent infections. Worms were put in 100% ethanol for molecular study or were fixed in 10% buffered formalin for permanent whole mounts [24]. Animal use for this study was approved by the University of New Mexico Institutional Animal Care and Use Committee (IACUC #19-200,813-MC).
2.7. Molecular techniques
Because multiple approaches were taken, details of methods for extractions, primers, amplifications, sequencing approaches and phylogenetic analyses of the parasite and snail mitochondrial cox1 and snail ITS1 datasets are provided in Technical Appendix #1.
3. Results
3.1. Original outbreak and presumed transmission focus
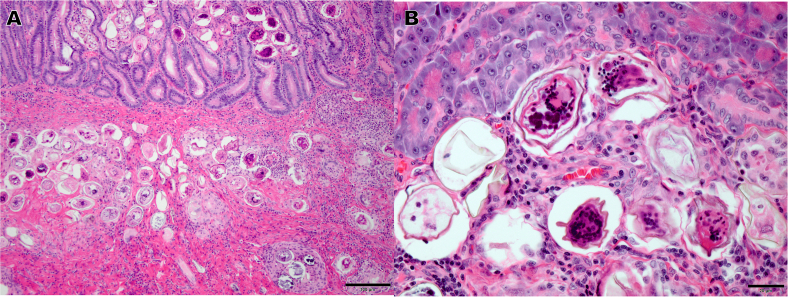
In fall, 2018, an 8-year-old dog presented with decreased appetite, lethargy, vomiting, diarrhea, polydipsia, and gradual weight loss. The symptoms had begun 3 weeks prior to the other dog in the household being euthanized for similar symptoms. Diagnostic bloodwork revealed azotemia, hypercalcemia, and hyperglobulinemia. One week later, the symptoms had progressed, and diagnostic bloodwork showed renal failure and elevated liver enzymes. The patient was euthanized one week later, and a necropsy performed. On gross examination, the spleen, liver, intestines, kidneys, and pancreas appeared unhealthy and enlarged. Biopsies were submitted for histopathology to Colorado State University. Histopathology revealed mineralization (calcification) of the kidneys. In addition, the intestines and pancreas were infiltrated with trematode eggs resulting in localized tissue inflammation (Fig. 1a,b). All histopathology findings were consistent with schistosomiasis.
Fig. 1.
(A) Histological section of intestine of euthanized dog showing large nests of H. americana eggs and granulomatous reactions to them, in both the mucosa and submucosa. Scale bar is 100 μm. (B) Histological section of pancreas of euthanized dog showing sections of H. americana eggs, some containing developed miracidia. Scale bar is 20 μm.
The two deceased dogs had lived adjacent to a small man-made irrigation pond (Mulberry Grove Pond) in east-central Moab (Fig. 2a). The pond receives water from nearby Mill Creek and has been filled since at least 2014. We subsequently submitted 12 fecal samples to the Texas A&M Gastrointestinal Laboratory from dogs living in the neighborhood of the pond or that were known to have swum in the pond. Ten of the 12 (83%) were diagnosed positive by PCR for H. americana. Some dogs were symptomatic, while others were clinically normal. All dogs were treated with praziquantel and fenbendazole. Symptoms resolved in all treated dogs, and subsequent fecal tests were negative.
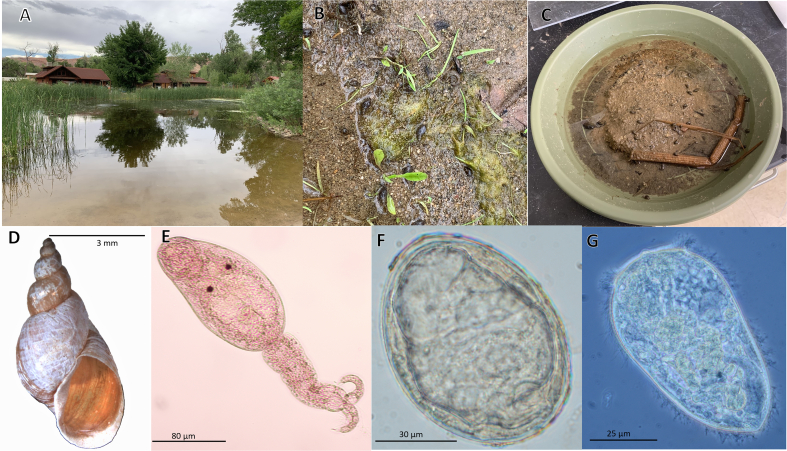
Fig. 2.
(A) Mulberry Grove Pond in residential area in east-central Moab. (B) Muddy bank of pond, showing several specimens of G. humilis. (C) Shallow, flat-bottomed dish containing mud from pond, in which lab-reared populations of G. humilis were grown. (D) Shell of G. humilis. The largest snails we observed were approximately 11 mm in shell length. (E) Cercaria of H. americana. Note the presence of eyespots, an unusual feature for mammalian schistosomes. Scale bar =80 μm. (F) Egg of H. americana. Note lack of a spine, and the miracidium within. Scale bar =30 μm. (G) Miracidium of H. americana. Scale bar = 50 μm.
3.2. Parasitological examinations at mulberry grove pond
The pond was visited on multiple occasions to collect either snails or mammalian fecal samples. The pond harbored populations of the aquatic snails Physa acuta, Planorbella trivolvis, and Gyraulus parvus. At the waterline and on the banks of the pond, a population of amphibious snails was found, provisionally identified as Galba humilis (Figs. 2b-d, 3) by conchological means [14]. Collections of G. humilis made in April 2019 (123 individuals), and July 2019 (25 individuals) were negative for schistosome infections, as were individuals of each of the three aquatic snail species present.
Fig. 3.
Representatives of Galba humilis. Galba humilis from North America H = shell height: 1- Tioga, New York, H = 12.6 mm; 2- Emmet, Michigan, H = 11.0 mm; 3- Beaver Dam, New Mexico, H = 10.3 mm; 4- Campus Lake, Louisiana, H = 10.1 mm; 5- Bizard Island, Montreal, Canada, H = 9.7 mm; 6- Augusta, Virginia, H = 9.7 mm; 7- Philadelphia, Pennsylvania, H = 7.5 mm; 8- Mulberry Grove Pond, Utah, H = 9.6 mm; 9- Mulberry Gove Pond, Utah, H = 5.8 mm; 10- Anderson, Tennessee, H = 6.9 mm; 11- San Antonio, Texas, H = 6.0 mm; 12- Randolph, North Carolina, H = 5.9 mm; 13- Hamilton, Ohio, H = 5.8 mm; 14- Kershaw, South Carolina, H = 5.6 mm.
In June 2020, 272 G. humilis were collected, three (1.1%) of which shed cercariae (Fig. 2e) consistent with those of H. americana [12]. None of the strictly aquatic snail species examined at this time shed schistosome cercariae. Positive G. humilis snails kept on an ambient light regime shed cercariae between 6 and 7:30 pm.
Fecal samples from a dog (1), deer (2), and raccoons (9) were collected near the pond. Two of the raccoon scats (2/9, 22.2%) were positive for H. americana, one recovered in December 2019, and one in November 2020. The first positive scat was confirmed to be of raccoon origin based on 16S mtDNA sequence; the second has yet to yield amplifiable DNA. (Table 1). Eggs (Fig. 2f) typical of H. americana were found, and miracidia (Fig. 2g) hatched from them. Our examinations indicated that transmission of H. americana had persisted for at least two years in Moab. Specimens of snails and worms were vouchered at the Museum of Southwestern Biology, Division of Parasites (Table 1).
3.3. Experimental infections of snails and mice
Miracidia from the December 2019 raccoon scat were used to expose 26 lab-reared Pseudosuccinea columella (10 miracidia per snail), but none became infected. Miracidia from the November 2020 raccoon scat were used to expose 40 lab-reared G. humilis (4-8 mm shell length), 4 Stagnicola elodes (>10 mm shell length) from Serpent Lake, and 7 S. elodes (5- > 10 mm shell length) from Rio Cebolla, all to 5–15 miracidia/snail. Only G. humilis became infected and shed cercariae (31 of 40 snails, 77.5%).
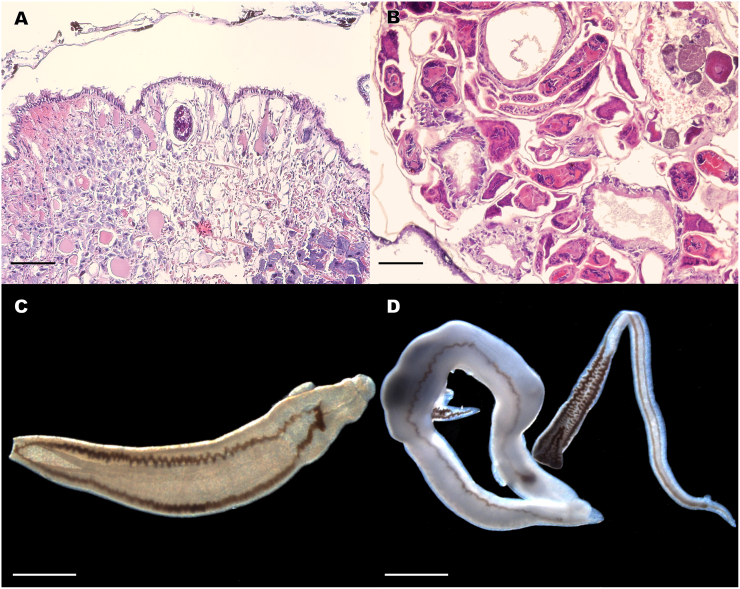
Another 23 lab-reared G. humilis were later exposed to 1–5 miracidia per snail, and 7 P. columella (5-12 mm shell length) were batch-exposed to an undetermined number of miracidia. Only 9 of the G. humilis survived, 3 of which were positive (33.3%). Once again, none of the P. columella were positive. Histological sections of G. humilis exposed to H. americana revealed developing schistosome sporocysts (Fig. 4a,b). Infected G. humilis were again observed to release cercariae in the early evening hours. Shedding G. humilis had shells up to 11 mm long, and 5 of the heavier shedders released an average of 810 (range 740–860) cercariae/snail/shed.
Fig. 4.
(A) Histological section of G. humilis exposed to H. americana miracidia, 8 days post exposure. Note presence of mother sporocyst developing in the head foot of the snail. Scale bar = 60 μm. (B) Histological section of G. humilis exposed to H. americana miracidia, 28 days post-exposure. Note presence of daughter sporocysts harboring developing cercariae in the digestive gland of the snail. Scale bar = 80 μm. (C) Live, unstained adult male of H. americana from experimental infection (101 days post-infection), dorsal view, with straight body conformation, testes (white spheres) visible at posterior end. (D) Live, unstained worms, adult male (left) harboring one female within its gynecophoric canal (note bulge containing folded female worm with dark gut and her protruding posterior end) and to the male's right, another female just released from its gynecophoric canal, from infected mouse, 77 days post-infection). Scale bar for adult worms = 500 μm.
First using cercariae pooled from two naturally infected G. humilis, we exposed two mice to infection. At 101 dpe, they were euthanized and perfused and both found to contain only male H. americana (Fig. 4c). Using cercariae obtained from experimentally infected G. humilis, one mouse was exposed to 100 cercariae pooled from 20 snails and another to 150 cercariae pooled from 15 snails. At 77 dpe, adult schistosomes consistent with H. americana males and females were perfused from the portal vein or dissected from the mesenteric veins of both mice. The male photographed initially harbored two females in its gynecophoric canal (Fig. 4d). Numerous miracidia were obtained from the liver and used to infect G. humilis snails for subsequent life cycle maintenance.
3.4. Molecular confirmation of identifications of Galba humilis and Heterobilharzia americana
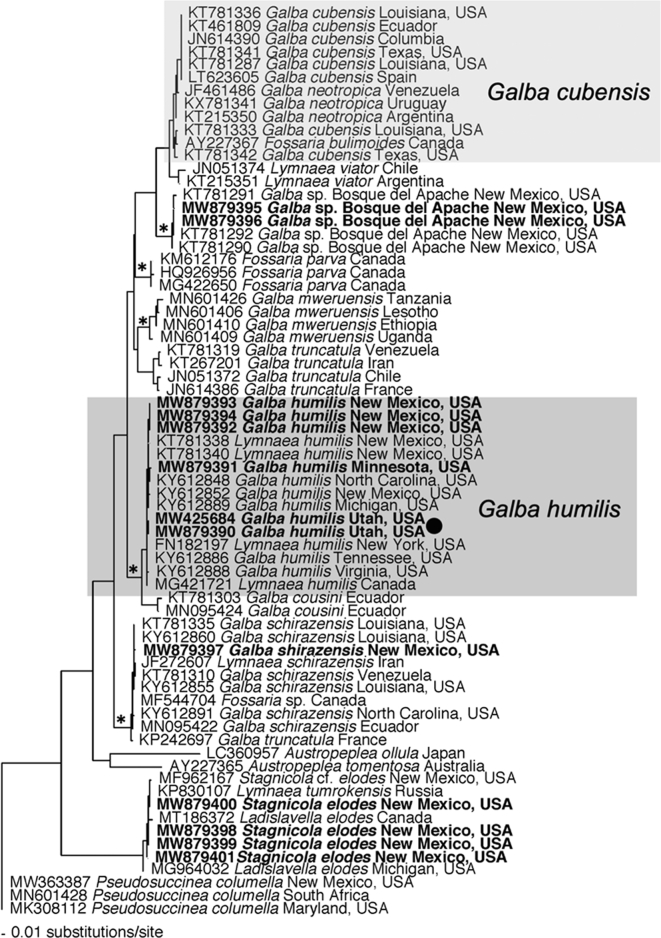
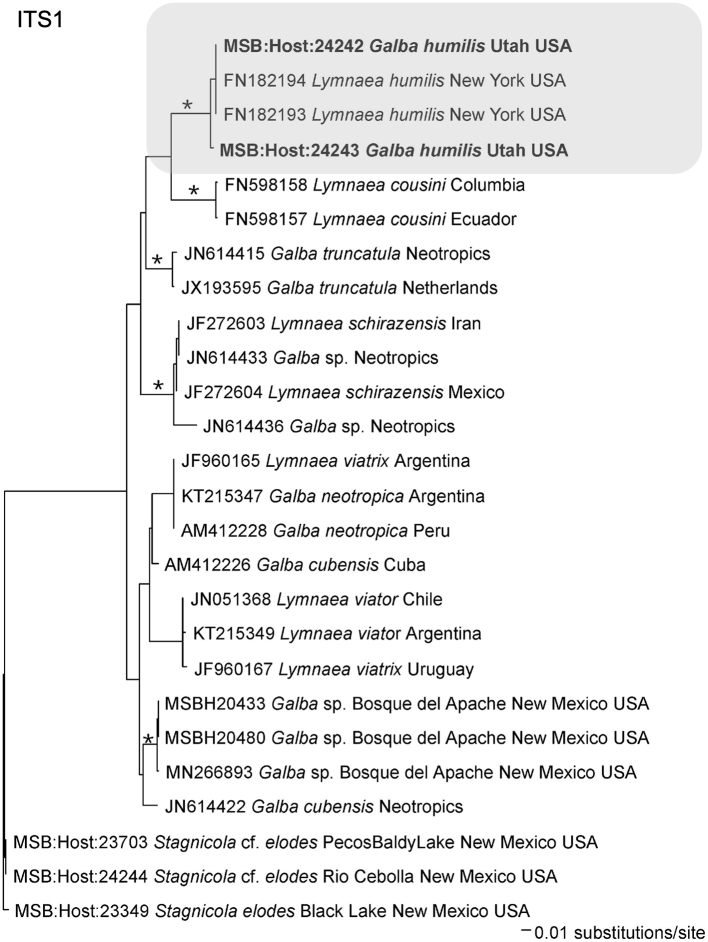
Snails provisionally identified as Galba humilis were confirmed as such based on comparisons with cox1 sequences from several relevant lymnaeid taxa [13] and from our own collections (Table 1). This comparison groups the Moab specimens with 13 other samples identified as G. humilis from different parts of North America (Fig. 3, Fig. 5). Supplementary Fig. 1 is of an ITS1 tree, which includes ITS1 sequences from G. humilis from this study.
Fig. 5.
Phylogenetic tree for relevant snails based on Bayesian analysis of cox1 with nodal support indicated on the branches by posterior probabilities as asterisk for >0.98. The clade for G. humilis is outlined in a dark gray box, light gray box are G. cubensis, the other species found infected in nature. The samples from this study examined for Heterobilharzia americana infections and/or used in experimental exposures are in bold. The relevant sample from Utah is noted with black circle. Additional taxa examined in New Mexico are from the MSB Parasite Division. Taxa are preceded with their corresponding GenBank accession number and followed by their collection locality (U.S. state or country). See Table 1 for accession numbers and MSB numbers.
The following are the supplementary data related to this article.Supplementary Fig. 1.
Phylogenetic tree for Galba humilis based on Bayesian analysis of ITS1 with nodal support indicated on the branches by posterior probabilities as asterisk for >0.98. The clade for G. humilis is outlined in a light gray box. See Table 1 for accession numbers and MSB numbers. Samples in bold are part of this study.
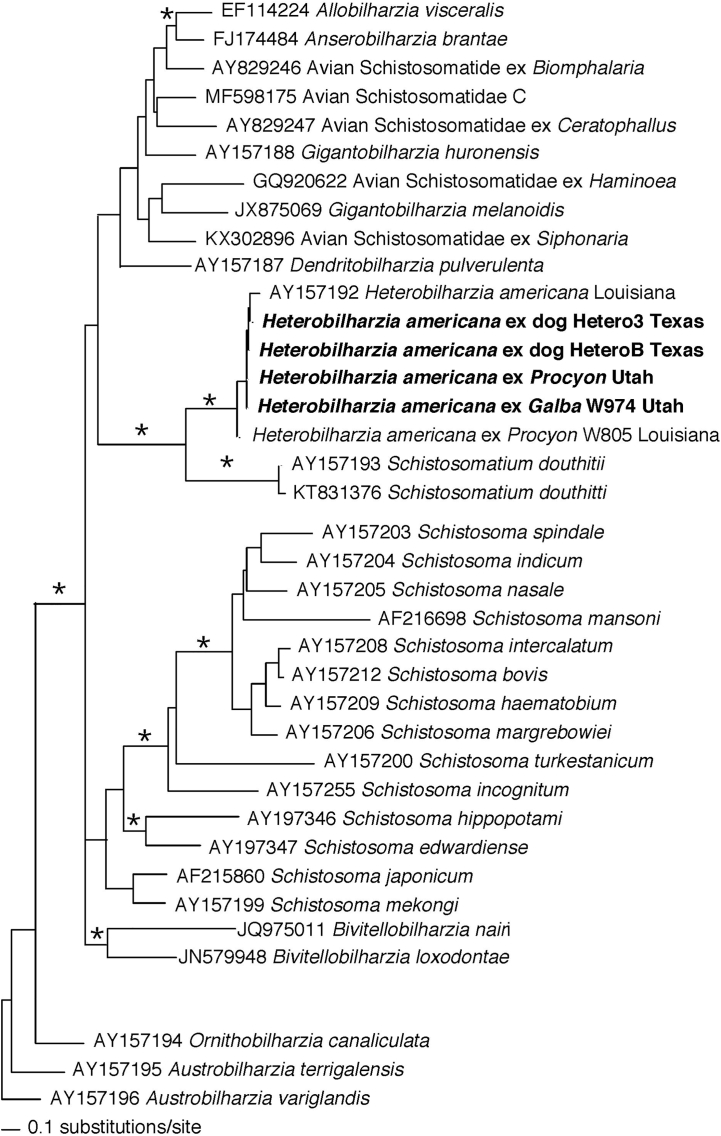
For H. americana from Utah, cox1 sequences derived from eggs and miracidia obtained from the 2019 raccoon scat sample and cercariae from experimentally-infected G. humilis in 2020 showed uncorrected p-distances of 0% with H. americana from Texas, 4% with H. americana from Louisiana, and ~ 20% with Schistosomatium douthitti, the monospecific sister genus of North American mammalian schistosome (Fig. 6).
Fig. 6.
Phylogenetic tree for relevant schistosomes, including Heterobilharzia americana, based on Bayesian analysis of cox1 with nodal support indicated on the branches by posterior probabilities as asterisk for >0.98. The samples from this study are in bold and outlined in a gray box. Taxa names are preceded by their corresponding GenBank accession number and followed by their collection locality (U.S. state). See Table 1 for accession numbers and MSB numbers.
3.5. Phylogenetic considerations for Galba humilis and Heterobilharzia americana
Galba humilis from Moab groups with high support with other representatives of this species from widely separated parts of North America (Fig. 3, Fig. 5). It also groups in a clade separate from that of the other two known host species of H. americana, G. cubensis, and P. columella.
The close relationships between H. americana and S. douthitti is confirmed (Fig. 6). The results also revealed isolates of H. americana from Utah and Texas to be more similar to each other than to an isolate from Louisiana.
4. Discussion
The appearance of H. americana in Utah marks the most westerly report for this endemic North American schistosome. Coupled with the Indiana report [11], this parasite has either had a wider distribution than heretofore appreciated or is currently expanding its range. Northward range expansion of G. cubensis could play a role in range expansion of the schistosome, and this snail has been reported as far north as Oklahoma, but the infected snails recovered in Utah were not G. cubensis, but G. humilis. It also seems unlikely that the semi-tropical species G. cubensis could have been involved in transmission of H. americana in Indiana [11].
Malek [[24], [25]] noted that G. humilis from Michigan was marginally susceptible (0.7–1.6% positive) to experimental infection with H. americana. Insofar as the Moab isolate of G. humilis was 77.5% positive following experimental exposure, and we noted, as have others [26], that P. columella was not compatible, it seems likely that the spread of H. americana has been facilitated by acquiring increased compatibility with G. humilis. G. humilis occurs widely in Utah and North America. It also serves as an intermediate host for Fasciola hepatica [27].
Galba humilis is one of at least 40 related subspecies and species of “fossarine” lymnaeids, all relatively small (<15 mm in shell height), living at or above the waterline but at times submersed as well [14,28]. Many of these taxa will eventually be synonymized [13]. Collectively their range covers much of North America, many Caribbean islands, and parts of Central and South America. Although their occurrence tends to be spotty, they can be locally very abundant [28]. Susceptibility to H. americana of fossarine lymnaeids from most of these areas is unknown. Our phylogenetic analysis showing other species of Galba as close relatives to G. cubensis and G. humilis suggests the possibility they too could potentially be infected by H. americana.
The fact that cercariae are released from snails at night is likely an indication of the extent to which this parasite is adapted to raccoons which commonly forage in and around aquatic habitats at night [29]. Raccoons (see https://upload.wikimedia.org/wikipedia/commons/0/04/Raccoon-range.png for range map) were not common in Utah through the 20th century [30], but as with other locations throughout the American west, they have since become much more abundant, particularly in urban areas. Elimination of predators, expanding agriculture and urbanization, and climate-related increases in available food all favor the spread of raccoons [31]. A recent climate modeling analysis suggested raccoons will expand their range northward but may diminish in numbers in more tropical areas of their known range [32,33]. This may help to explain the apparent absence of H. americana from areas south of Texas given that a permissive snail host, G. cubensis, is known from Mexico, South America, and Cuba. Alternatively, this apparent absence may reflect a lack of survey information.
Recovery of H. americana from Utah and Indiana suggests it is not precluded from colder climates. Its closest relative, S. douthitti, occurs as far north as Alaska [34]. The establishment of the mostly tropical species Schistosoma haematobium in Corsica has prompted the suggestion that schistosomes are “pre-adapted” to colder climates [35].
Infected dogs from endemic areas may also be critical in introducing H. americana into new habitats (possibly including Moab). Parasites carried in dog feces may infect local snail populations, which in turn might permit spread of infection to local raccoons, which are probably better maintenance hosts. Construction of man-made habitats like Mulberry Grove Pond also favor H. americana by attracting dogs and raccoons to these new snail-supporting habitats [36].
Our sequencing results suggest there might be strain- or possibly species-level differences within H. americana, a supposition already discussed by others [37,38]. We found a Louisiana isolate from raccoons differed by as much as 4.1% in cox1 sequences from our Utah and Texas isolates, values approaching those often considered to be typical of species delineations [[39], [40], [41]]. Our results also suggest that H. americana from Moab may have originated from Texas. Further studies are needed to learn if the isolates from different states have diverged sufficiently to be considered different species.
5. Conclusions
If raccoons or dogs infected with H. americana colonize areas where susceptible amphibious lymnaeids like G. humilis are common, then the spread of H. americana in North America will likely continue. Any mammals using such habitats would be at risk, potentially suffering symptoms ranging from dermatitis to granulomatous disease as a consequence. The role of raccoons in the transmission of H. americana adds to the health concerns they already pose as hosts for rabies and the intestinal roundworm Baylisascaris procyonis [42]. Introductions of raccoons into Europe have already led to emergence of B. procyonis there and increases the likelihood that H. americana could also extend its range to Europe. Amphibious lymnaeids such as Galba truncatula are common in Eurasia and might prove to be susceptible to H. americana. Additional study of possible incorporation of new snail hosts and range shifts are clearly warranted for H. americana, an emerging schistosome capable of infecting a broad range of mammals with potentially severe pathological consequences. Furthermore, the difficulties involved in controlling raccoons, or snails colonizing muddy banks of an increasing numbers of man-made aquatic habitats, will prove to be challenging, just as are efforts to control human schistosomiasis.
Supplementary material
Funding
This work was supported by the core laboratory support provided by The National Institutes of Health [R37AI101438] and technical assistance at the University of New Mexico Molecular Biology Facility was supported by the National Institute of General Medical Sciences of the National Institutes of Health [P30GM110907]. The content for this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Competing Interest
None.
Acknowledgments
The authors wish to thank Patsy Wilmerding for her hospitality and Robin Loker for assisting with collections. We thank the Colorado State University Veterinary Histopathology Laboratory for the provision of histological sections and associated photographs of the necropsied dog, and the Gastrointestinal Diagnostic Unit of Texas A & M University for identifying 10 Moab dogs with fecal samples positive for Heterobilharzia americana. We also thank Ms. Karen Buehler from Tri-Core Laboratories in Albuquerque for her timely assistance in producing histological sections of snails.
References
- 1.Hosie M.J., Jasim S. The case for adopting a combined comparative medicine and one health approach to tackle emerging diseases. Vet. Rec. 2020;187:24–26. doi: 10.1136/vr.m2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laing G., Vigilato M.A.N., Cleaveland S., Thumbi S.M., Blumberg L., Salahuddin N., Abdela-Ridder B., Harrison W. One health for neglected tropical diseases. Trans. R. Soc. Trop. Med. Hyg. 2021;115:182–184. doi: 10.1093/trstmh/traa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P., Shi Z.L. SARS-CoV-2 spillover events. Science. 2021;371:120–122. doi: 10.1126/science.abf6097. [DOI] [PubMed] [Google Scholar]
- 4.Sonenshine D.E. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health. 2018;15 doi: 10.3390/ijerph15030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wasel S.M., Samuel W.M., Crichton V. Distribution and ecology of meningeal worm, Parelaphostrongylus tenuis (Nematoda), in northcentral North America. J. Wildl. Dis. 2003;39:338–346. doi: 10.7589/0090-3558-39.2.338. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez J.Y., Lewis B.C., Snowden K.F. Distribution and characterization of Heterobilharzia americana in dogs in Texas. Vet. Parasitol. 2014;203:35–42. doi: 10.1016/j.vetpar.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Malek E.A., Armstrong J.C. Infection with Heterobilharzia americana in primates. Am. J. Trop Med. Hyg. 1967;16:708–714. [PubMed] [Google Scholar]
- 8.McKown R.D., Veatch J.K., Fox L.B. New locality record for Heterobilharzia americana. J. Wildl. Dis. 1991;27:156–160. doi: 10.7589/0090-3558-27.1.156. [DOI] [PubMed] [Google Scholar]
- 9.Duncan K.T., Koons N.R., Litherland M.A., Little S.E., Nagamori Y. Prevalence of intestinal parasites in fecal samples and estimation of parasite contamination from dog parks in Central Oklahoma. Vet. Parasitol. Reg. Stud. Rep. 2020;19:100362. doi: 10.1016/j.vprsr.2019.100362. [DOI] [PubMed] [Google Scholar]
- 10.Johnson E.M. Canine schistosomiasis in North America: an underdiagnosed disease with an expanding distribution. Compend Contin Educ. Vet. 2010;32:E1–E4. [PubMed] [Google Scholar]
- 11.Rodriguez J.Y., Camp J.W., Lenz S.D., Kazacos K.R., Snowden K.F. Identification of Heterobilharzia americana infection in a dog residing in Indiana with no history of travel. J. Am. Vet. Med. Assoc. 2016;248:827–830. doi: 10.2460/javma.248.7.827. [DOI] [PubMed] [Google Scholar]
- 12.Lee H.F. Life history of Heterobilharzia americana Price 1929, a schistosome of the raccoon and other mammals in southeastern United States. J. Parasitol. 1962;48:728–739. [PubMed] [Google Scholar]
- 13.Alda P., Lounnas M., Vazquez A.A., Ayaqui R., Calvopina M., Celi-Erazo M., Dillon R.T., Jr., Gonzalez Ramirez L.C., Loker E.S., Muzzio-Aroca J., Narvaez A.O., Noya O., Pereira A.E., Robles L.M., Rodriguez-Hidalgo R., Uribe N., David P., Jarne P., Pointier J.P., Hurtrez-Bousses S. Systematics and geographical distribution of Galba species, a group of cryptic and worldwide freshwater snails. Mol. Phylogenet. Evol. 2021;157:107035. doi: 10.1016/j.ympev.2020.107035. [DOI] [PubMed] [Google Scholar]
- 14.Burch J. Vol. 1. 1982. North American Freshwater Snails: Identification Keys, Generic Synonymy, Supplemental Notes, Glossary, References, Index, Walkerana; pp. 216–364.https://www.fwgna.org/species/lymnaeidae/l_cubensis.html [Google Scholar]
- 15.Dillon A.M., Kohl M., Reeves W., Smith T., Stewart T., Watson B. 2013. Lymnaea (Fossaria) cubensis, The Freshwater Gastropods of North America. [Google Scholar]
- 16.Madsen H., Frandsen F. The spread of freshwater snails including those of medical and veterinary importance. Acta Trop. 1989;46:139–146. doi: 10.1016/0001-706X(89)90030-2. [DOI] [PubMed] [Google Scholar]
- 17.Bartsch R.C., Ward B.C. Visceral lesions in racoons naturally infected with Heterobilharzia americana. Vet. Pathol. 1976;13:241–249. doi: 10.1177/030098587601300401. [DOI] [PubMed] [Google Scholar]
- 18.Corapi W.V., Ajithdoss D.K., Snowden K.F., Spaulding K.A. Multi-organ involvement of Heterobilharzia americana infection in a dog presented for systemic mineralization. J. Vet. Diagn. Investig. 2011;23:826–831. doi: 10.1177/1040638711407894. [DOI] [PubMed] [Google Scholar]
- 19.Fabrick C., Bugbee A., Fosgate G. Clinical features and outcome of Heterobilharzia americana infection in dogs. J. Vet. Intern. Med. 2010;24:140–144. doi: 10.1111/j.1939-1676.2009.0429.x. [DOI] [PubMed] [Google Scholar]
- 20.Fradkin J.M., Braniecki A.M., Craig T.M., Ramiro-Ibanez F., Rogers K.S., Zoran D.L. Elevated parathyroid hormone-related protein and hypercalcemia in two dogs with schistosomiasis. J. Am. Anim. Hosp. Assoc. 2001;37:349–355. doi: 10.5326/15473317-37-4-349. [DOI] [PubMed] [Google Scholar]
- 21.Corapi W.V., Snowden K.F., Rodrigues A., Porter B.F., Buote M.A., Birch S.M., Jackson N.D., Eden K.B., Whitley D.B., Mansell J., Edwards J.F., Hardy J., Chaffin M.K. Natural Heterobilharzia americana infection in horses in Texas. Vet. Pathol. 2012;49:552–556. doi: 10.1177/0300985811432346. [DOI] [PubMed] [Google Scholar]
- 22.Corapi W.V., Eden K.B., Edwards J.F., Snowden K.F. Heterobilharzia americana infection and congestive heart failure in a llama (Lama glama) Vet. Pathol. 2015;52:562–565. doi: 10.1177/0300985814540541. [DOI] [PubMed] [Google Scholar]
- 23.Duvall R.H., DeWitt W.B. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am. J. Trop Med. Hyg. 1967;16:483–486. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]
- 24.Kruse M.H.P.G.O. The Harold W. Manter Laboratory, University of Nebraska Press., Technical Bulletin; 1982. The Collection and Preservation of Animal Parasites; p. 141. [DOI] [Google Scholar]
- 25.Malek E.A. Experimental infection of several lymnaeid snails with Heterobilharzia americana. J. Parasitol. 1967;53:700–702. doi: 10.2307/3276755. [DOI] [PubMed] [Google Scholar]
- 26.Goff W.L., Ronald N.C. Certain aspects of the biology and life cycle of Heterobilharzia americana in east Central Texas. Am. J. Vet. Res. 1981;42:1775–1777. [PubMed] [Google Scholar]
- 27.Briem R.M. Plant and Wildlife Sciences, BYU. 1971. A study of snail hosts for Fasciola hepatica in Utah Valley. Theses and Dissertations. [Google Scholar]
- 28.McCraw B.M. The ecology of the snail, Lymnaea humilis Say. Trans. Am. Microsc. Soc. 1959;78:101–121. doi: 10.2307/3223807. [DOI] [Google Scholar]
- 29.Combes C., Fournier A., Moné H., Théron A. Behaviours in trematode cercariae that enhance parasite transmission: patterns and processes. Parasitology. 2017;109:S3–S13. doi: 10.1017/S0031182000085048. [DOI] [PubMed] [Google Scholar]
- 30.Kamler J.F., Ballard Warren B., Helliker Brent R., Stiver San. Range expansions of raccoons in western Utah and central Nevada. West N Am. Nat. 2021:17. 63 pp. [Google Scholar]
- 31.Larivière S. Range expansion of raccoons in the Canadian prairies: review of hypotheses. Wildl. Soc. Bull. 2004;32:955–963. doi: 10.2193/0091-7648(2004)032[0955:REORIT]2.0.CO;2. [DOI] [Google Scholar]
- 32.Louppe V., Leroy B., Herrel A., Veron G. Current and future climatic regions favourable for a globally introduced wild carnivore, the raccoon Procyon lotor. Sci. Rep. 2019;9:9174. doi: 10.1038/s41598-019-45713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roug A., Clancy C.S., Detterich C., Van Wettere A.J. Cerebral larva migrans caused by Baylisascaris spp. in a free-ranging North American porcupine (Erethizon dorsatum) J. Wildl. Dis. 2016;52:763–765. doi: 10.7589/2015-11-316. [DOI] [PubMed] [Google Scholar]
- 34.Malek E.A. Geographical distribution, hosts, and biology of Schistosomatium douthitti (Cort, 1914) Price, 1931. Can. J. Zool. 1977;55:661–671. doi: 10.1139/z77-087. [DOI] [PubMed] [Google Scholar]
- 35.Mulero S., Rey O., Arancibia N., Mas-Coma S., Boissier J. Persistent establishment of a tropical disease in Europe: the preadaptation of schistosomes to overwinter. Parasit. Vectors. 2019;12:379. doi: 10.1186/s13071-019-3635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carolus H., Muzarabani K.C., Hammoud C., Schols R., Volckaert F.A.M., Barson M., Huyse T. A cascade of biological invasions and parasite spillback in man-made Lake Kariba. Sci. Total Environ. 2019;659:1283–1292. doi: 10.1016/j.scitotenv.2018.12.307. [DOI] [PubMed] [Google Scholar]
- 37.Short R.B., Teehan W.H., Liberatos J.D. Chromosomes of Heterobilharzia americana (Digenea: Schistosomatidae), with ZWA sex determination, from Louisiana. J. Parasitol. 1987;73:941–946. [PubMed] [Google Scholar]
- 38.Malek E.A., Short R.B., Teehan W.H., Jama A. Differential susceptibility of snail hosts to Heterobilharzia americana from Texas and Louisiana. J. Parasitol. 1987;73:872–873. [PubMed] [Google Scholar]
- 39.Vilas R., Criscione C.D., Blouin M.S. A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology. 2005;131:839–846. doi: 10.1017/S0031182005008437. [DOI] [PubMed] [Google Scholar]
- 40.Brant S.V., Loker E.S. Schistosomes in the Southwest United States and their potential for causing cercarial dermatitis or ‘swimmer’s itch. J. Helminthol. 2009;83:191–198. doi: 10.1017/S0022149X09308020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ebbs E.T., Loker E.S., Davis N.E., Flores V., Veleizan A., Brant S.V. Schistosomes with wings: how host phylogeny and ecology shape the global distribution of Trichobilharzia querquedulae (Schistosomatidae) Int. J. Parasitol. 2016;46:669–677. doi: 10.1016/j.ijpara.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Graeff-Teixeira C., Morassutti A.L., Kazacos K.R. Update on baylisascariasis, a highly pathogenic zoonotic infection. Clin. Microbiol. Rev. 2016;29:375–399. doi: 10.1128/CMR.00044-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material