Abstract
Snakebite envenoming is a set of intoxication diseases that disproportionately affect people of poor socioeconomic backgrounds in tropical countries. As it is highly dependent on the environment its burden is expected to shift spatially with global anthropogenic environmental (climate, land use) and demographic change. The mechanisms underlying the changes to snakebite epidemiology are related to factors of snakes and humans. The distribution and abundance of snakes are expected to change with global warming via their thermal tolerance, while rainfall may affect the timing of key activities like feeding and reproduction. Human population growth is the primary cause of land-use change, which may impact snakes at smaller spatial scales than climate via habitat and biodiversity loss (e.g. prey availability). Human populations, on the other hand, could experience novel patterns and morbidity of snakebite envenoming, both as a result of snake responses to environmental change and due to the development of agricultural adaptations to climate change, socioeconomic and cultural changes, development and availability of better antivenoms, personal protective equipment, and mechanization of agriculture that mediate risk of encounters with snakes and their outcomes. The likely global effects of environmental and demographic change are thus context-dependent and could encompass both increasing and or snakebite burden (incidence, number of cases or morbidity), exposing new populations to snakes in temperate areas due to “tropicalization”, or by land use change-induced snake biodiversity loss, respectively. Tackling global change requires drastic measures to ensure large-scale ecosystem functionality. However, as ecosystems represent the main source of venomous snakes their conservation should be accompanied by comprehensive public health campaigns. The challenges associated with the joint efforts of biodiversity conservation and public health professionals should be considered in the global sustainability agenda in a wider context that applies to neglected tropical and zoonotic and emerging diseases.
Keywords: Snakebite, Global change, Sustainability agenda, Snake ecology, Climate change, Land use change, Envenoming, Venomous snakes, Poverty, Agriculture
Graphical abstract

Highlights
-
•
Distribution and abundance of snakes are expected to be affected by climate change.
-
•
Land-use change may also impact snakes but at smaller spatial scales than climate.
-
•
Human populations could experience novel patterns and morbidity of snakebite.
-
•
Reducing snakebite should be accompanied by actions that protect snake diversity.
1. Introduction
Snakebite envenoming, like other neglected tropical diseases (NTDs) is mediated by biodiversity and the environment, and its epidemiology is, therefore, susceptible to shift spatially in unexpected ways with global anthropogenic environmental and social change (climate, land use, demographic and socio-economic, hereafter global change; Booth, 2018). At its core, snakebite is the interaction of two species, hence large scale environmental factors that affect the frequency and outcome of human-snake interactions will change the spatial variability of its incidence and morbidity (burden). For instance, climate change could increase or decrease the size of snake populations, affecting the chances of encounter (Yañez-Arenas et al., 2016, 2014). In fact, of the global change axes, climate and land use change are widespread threats to medically-relevant snakes (Böhm et al., 2013), in a process widely known as the biodiversity loss crisis (Krauss et al., 2010; Rosales, 2008). As human populations and their economies are expected to keep growing (Jones and O'Neill, 2016; Tatem, 2017), biodiversity loss will continue unabated unless drastic measures are in place (Bradshaw et al., 2021, Dirzo et al., 2014, Zipkin et al., 2020). Those measures aim at preserving all life forms, although preserving snakes, unfortunately, could also represent an obstacle in the battle for human health, especially for biodiversity-borne diseases like snakebite envenoming (Dunn, 2010; Garchitorena et al., 2017; Morand and Lajaunie, 2021, p. 202).
While the transmission dynamics of snakebite are relatively simple, in comparison to zoonotic infectious diseases, the biology of snakebite risk geographic patterns can be complex. For instance, snakebite envenoming actually comprises a diverse set of diseases caused by snake venom composition: snakes in the Elapidae family (e. g. cobras, kraits and coral snakes) have predominantly neurotoxic venoms, while snakes in the Viperidae family (e. g. true vipers and pit vipers such as rattlesnakes) have predominantly cytotoxic, proteolytic and haemolytic venoms (Tasoulis and Isbister, 2017). In addition to a diverse range of envenoming diseases, the geography of snakebite risk depends on different levels of ecological organization that may be impacted by the various global change dimensions, including: 1) climate change affects snakes and humans at large scales via physiological resistance (deMenocal, 2011, Wu, 2016) or by regulating reproduction and feeding (Sengupta et al., 1994); 2) land use change, while widespread, interacts with climate change and potentially enhances it at small scales (Krauss et al., 2010; Newbold et al., 2015); and 3) human population and economic growth are the driving forces of climate and land use change and also represent the main sources of individual snakebite risk factors. As global change progresses, it will reconfigure snakebite epidemiology via these dynamically interconnected processes with outcomes that may seem contradictory across space and time. Using our current knowledge of snake biology, ecology, and snakebites, we can untwine some of connections between them to draw the potential pathways for snakebite epidemiology and explain, to a certain degree, how environmentally similar processes may lead to divergent outcomes.
We identify three aspects critical for understanding global change impacts on snakebite epidemiology. First is the distributional ecology of snake species, which refers to the spatial scales at which different biotic and abiotic factors (prey species, climate, topography, vegetation; Soberon and Nakamura, 2009) shape the geographical patterns of abundance and temporal patterns of activity of medically-relevant snake species (Yañez-Arenas et al., 2014). Second is the eco-epidemiology of snakebite which refers to the role that societal, demographic, and economic drivers of land cover have on the heterogeneity of human-snake interactions (Parkin et al., 2020), as depicted in Fig. 1. And third is snakebite envenoming risk management in the context of the global sustainability agenda, which includes potential conflicts between health and nature conservation policies.
Fig. 1.
Impacts of global change begin with global climate, which incfluences the type of ecosystems (e. g. temperate, tropical, desert). Ecosystems in turn influence the number and type of snake species present. For instance, arboreal venomous snakes are mostly present in tropical ecosystems. Then, at the landscape scale, changes in vegetation structure caused by humans determine which of the local species may interact with humans depending on the type of environments created by humans (number 1 and 2 in the figure), which also depend on socio-economic and cultural characteristics. For instance, mechanization of agriculture reduces risk of bites to workers (number 1).
Global change may impact snakebite epidemiology in multiple ways, such as altering annual cycles of incidence. However, in this review, we primarily describe the relationship between snake distributional ecology, snakebite eco-epidemiology, and global change mitigation in order to understand the nature of global change impacts on the geography of snakebite epidemiology.
2. Distributional ecology of venomous snakes
The distribution of a species is an expression of many factors that interact dynamically (Gaston, 2003, Pearson and Dawson, 2003) and are defined by its ecology and evolutionary history (Brown, 1995). In a very general but practical view, these factors can be summarized in three classes: abiotic conditions, accessibility (available areas to colonize), and interactions with other organisms (Soberon and Peterson, 2005). The strength with which these factors influence the distribution of a species depends on their natural history and the spatial scale of analysis (Gaston, 2003, Pearson and Dawson, 2003). Concerning venomous snakes, at coarse spatial scales, climate is the main factor limiting their geographic ranges as it is related to their physiological adaptations to withstand temperature and humidity regimes (Kearney et al., 2018). At finer spatial scales, climate may be related to a wide variety of biological processes such as the timing of reproduction and food availability (Chaves et al., 2015, Sengupta et al., 1994).
Since snakes are ectotherm organisms, low temperatures tend to decrease their population densities. Except for the European adder (Vipera berus) which occurs inside the Arctic Circle, no other species inhabits the polar regions of the world, above 4000 m. a.s.l. (Gloydius himalayanus) (Warrell, 2003) or below 43° south (Bothrops ammodytoides) (Carrasco et al., 2010). These characteristics of snake biodiversity result in high snakebite envenoming incidence in the warm tropics (Yañez-Arenas et al., 2014), again as a result of higher venomous snake abundance (Luiselli et al., 2020). Also, snakebite envenoming incidence is higher in the tropics because high climatic suitability allows snake populations to grow faster and achieve higher population densities (Holt, 2020; Osorio-Olvera et al., 2019).
The clear link between snakes and climate indicates that climate change will affect their geographical distributions and patterns of abundance. However, the width of snakes' thermal tolerance can also determine the extent of climate change impacts on them (Saupe et al., 2015; Soberón and Arroyo-Peña, 2017). The geographical distributions of snakes with narrow climatic tolerance might be expected to shrink earlier and to a greater extent than species with large continuous distributions and broad climatic tolerance (Soberón and Arroyo-Peña, 2017). The most medically-relevant snakes, such as the Indian cobra (Naja naja), the common krait (Bungarus caeruleus), Russel's viper (Daboia russelli) and the saw scaled viper (Echis carinatus) in South Asia; the black mamba (Dendroaspis polylepis), the carpet viper (Echis ocellatus), and gaboon viper (Bitis gabonica) in Sub-Saharan Africa; the terciopelo (Bothrops asper) in tropical America, and the eastern copperhead (Agkistrodon contortrix) in North America have large geographical distributions, covering large and small cities with millions of people. The relationship between distributional size and human population at risk means that anticipating global change impacts for these species is more important from a mitigation and prevention perspective. However, as mentioned above organisms with large distributions may respond more erratically in relation to our predictive capacity than species with narrower distributions.
Despite medical importance and implications for global change, information on the above species’ populations in relation to environmental conditions is still scarce. Naturally, our capacity to anticipate the impacts of climate change on them is limited to analyses of the occupied climatic conditions historically which will differ greatly from climate change predictions. For instance, the maximum temperature of the warmest month and the minimum temperature of the coldest month associated with the localities where the presence of the eastern copperhead (A. contortrix) has been reported are considerably lower than what they are predicted to be in some future scenarios. The lower panel of Fig. 2 shows a 2-dimensional plot of historical values of these two variables (blue circles) at each presence record of A. contortrix, compared with their respective values in a pessimistic future scenario, based on the human development pathway assumed to drive greenhouse gas concentration (ssp585 with global circulation model CANESM5, full details in Fick and Hijmans, 2017). Data pertaining to predicted climate in the 2081–2100 period in the locations where A. contortrix has been observed is represented with red circles in Fig. 2.
Fig. 2.
Crosses show the geographic locations where Agkistrodon contortrix has been registered, whereas circles in the bottom panel scatter plot show the climatic space associated with the geographic records of the eastern copperhead. Present climate in blue and future climate (2081–2100) in red. Photo credit: Carlos Yañez-Arenas. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In contrast with climate, the effect of land cover on snake distributions and abundance is less well understood than that of climate. On one hand, land cover, unlike climate which is a global system, is more variable over small spatial scales and can immediately reflect the impacts of human activities (Krauss et al., 2010). On the other, many snake species seem to be less sensitive to habitat-related anthropogenic disturbances in comparison to other species (Suazo-Ortuño et al., 2008), probably because snakes have biological traits that make them less prone to extinction, such as larger clutches at larger body sizes compared to mammals because snake offspring size does not increase in proportion to size at reproductive maturity (Reed, 2003). The snake species most affected by human activities and land use change have relatively narrow geographical distributions, feed on vertebrates (as opposed to molluscs or arthropods), and are aquatic (Luiselli et al., 2020; Todd et al., 2017). Also, there is little evidence that the negative public perception of venomous snakes is a widespread extinction risk factor (Todd et al., 2017), even though active persecution can in fact decrease snake populations (Means, 2009). In light of snakes’ probable resistance to land use change, the general scarcity of information is a critical information void which should be filled by systematic snake surveys in relation to land use change.
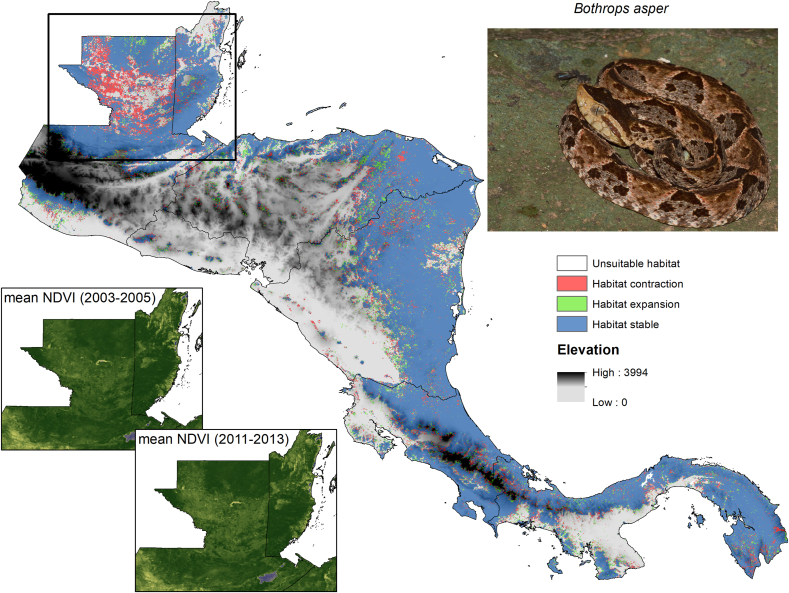
To illustrate land use change effects on snakes, we modeled the influence of land cover change on suitable habitat for the terciopelo (Bothrops asper), the most significant venomous snake in Central America (Bravo-Vega et al., 2019), during the period 2003–2004 to 2011–2013 in Central America. We used monthly MODIS (Moderate Resolution Imaging Spectroradiometer; https://modis.gsfc.nasa.gov/) data to characterize, through the NDVI (Normalized Difference Vegetation Index), the greenness of each pixel, and assess potential changes in vegetation cover. From the monthly data, we computed the average, maximum, minimum, and standard deviation of the NDVI as environmental predictors in each three-year period. The presence records used to model habitat suitability with the Maxent algorithm (Philips et al., 2006) were obtained from the Global Biodiversity Information Facility (https://www.gbif.org/). We calibrated models in the period 2003–2005 using the Maxent bootstrapping functionality (10 replicates), with 80% of presence records used as training data (20% as test data), Cloglog output, and default features and regularization multiplier. The models were then transferred to the second time period (2011–2013), and the median of the ten replicates on each time was used as the final model. Finally, we generated binary habitat suitability maps using a five percentile thresholding criteria, with which we highlight the areas where habitat is being predicted to be lost (red areas), gained (green) or remain stable (blue) for B. asper between both time periods (Fig. 3).
Fig. 3.
Habitat suitability change during 2001–2013 across the range of the terciopelo (Bothrops asper). Photo credit: Brian Gratwicke. The regional zoom to Guatemala shows the area where the habitat of the species was most altered during the analyzed period (2001–2013), where more intense green denotes areas with more dense vegetation. Habitat contraction (red) indicates areas that lost the vegetation preferred by B. asper, while expansion (green) indicates areas where low-density vegetation was replaced by higher density vegetation and became more suitable. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Both cases, A. contortrix and B. asper, case studies are fairly basic and simple but separately represent clear examples of two of the environmental change phenomena that influence the distribution and demographics of medically important snakes. As mentioned above, the factors that shape the geographic ranges and demographics of any snake interact dynamically across different times and geographical scales. Therefore, the effects of climate and land use change should be estimated together at scales that represent the various biological aspects of snakes (physiological tolerance, demographic processes and interactions with other organisms) to draw an integral picture of snake distribution and abundance. In relation to snakebite, we infer that for all snake species, changes in response to deforestation occur at smaller scales (e. g. landscape) than for climate change because the latter, by definition, is a dynamic global system in which changes occur simultaneously over vast geographical extensions. Hence, in response to land use change, snakebite epidemiology may change in response to probability of encountering local snake species, while climate change may drive broad scale changes to ensembles of venomous snakes, thereby altering the nature of the locally known envenoming diseases.
2.1. Example box 1: snake distributional changes driven by environmental change
Empirical evidence of climate and land-use change effects on venomous snake populations is scarce, yet current evidence is revealing.
2.1.1. Climate change
The New Mexico ridge-nosed rattlesnake (Crotalus willardi obscurus) has a distribution restricted to three mountain peaks in New Mexico, where temperature restricts its mobility between mountain peaks. Recent warming has been pushing the remaining individuals towards higher altitudes as they seek to track their preferred lower temperatures. At higher altitudes, however, wildfires have become more frequent and increased snake mortality, leaving no alternative areas to inhabit (Davis et al., 2015). Furthermore, warmer temperatures (and habitat fragmentation) in the lowlands make between-mountain dispersal even more difficult, reinforcing the isolation between populations and making each more vulnerable to local extirpation (Davis et al., 2015).
In a similar case, the timber rattlesnake (Crotalus horridus) has seen regional declines caused by climate change (together with habitat fragmentation). Here, changing rainfall patterns increase susceptibility to water-borne fungal infections that increase snake mortality (Clark et al., 2011).
2.1.2. Land use change
Changes in snake habitats have been observed to reconfigure venomous snake assemblages in some regions, by facilitating species invasions and replacement of biting snake species. For instance, deforestation of the Atlantic rainforest allowed the establishment of Crotalus durissus populations in the state of Valenca, Brazil, after being washed by floodwaters during the 1950–60s (Bastos et al., 2005). Once established, C. durissus became a successful competitor of Bothrops jararaca, which is associated with more densely vegetated areas (Sazima, 1992). The local expansion of C. durissus was noticed in hospital admissions with higher numbers of bites. Likewise, the spitting cobra (Naja nigricollis) is gradually replacing the black forest cobra (Naja melanoleuca) following deforestation in Nigeria (Luiselli, 2001).
2.1.3. Summary
The above cases illustrate some of the environmental change effects on snakes that alter snakebite epidemiology: 1) climate change makes snake populations physiologically unable to cope with higher temperatures and decline (C. willardi; Davis et al., 2015); 2) climate change interacts with land use change to decrease connectivity resulting in genetic bottlenecks and facilitate detrimental biological interactions (e.g., water-borne infectious diseases in C. horridus; Clark et al., 2011); 3) land use change can create hospitable conditions for species that might not be able to adapt in the absence of those anthropogenic habitat perturbations (Naja nigricollis in Nigeria (Bastos et al., 2005; Luiselli, 2001); and further create habitat corridors that allow dispersal of distant snake species (C. durissus invasion in Brazil; Sazima, 1992).
At a global scale, these examples indicate that the nature of global change impacts depend deeply on climate-land use change interactions. If habitat continuity allows dispersal, global warming will drive declines in tropical areas and colonisations in subtropical and temperate regions. Where land use or habitat block species dispersal, we might witness large declines potentially accompanied by decreasing snakebite risk (e. g. Yañez-Arenas et al., 2016); or where land use change is accompanied by human population growth, there may be momentary increases in number of cases until snake numbers plummet with increasing urbanisation. Ultimate changes in snakebite envenoming cases will depend on the size of human populations exposed to the new or disappearing snake assemblages.
3. Eco-epidemiology of snakebites
3.1. Climate and land use change
Anthropogenic impacts on the environment are having a profound toll on vertebrate biodiversity (Dirzo et al., 2014). Of the 3879 snake species known (Uetz et al., 2021), 12% are currently considered endangered, but the number is likely to change because there is insufficient data for nearly 25% of the species (Böhm et al., 2013). This phenomenon is an underappreciated issue in snakebite epidemiology, as loss of snake species or reductions in their local abundance in the long term seem likely to decrease snakebite risk, unless snake species adapt well to urbanisation or human disturbances (e. g. C. durissus and N. nigricollis examples). In general, we still need to determine how key medically-relevant species respond to biodiversity loss. Recent studies suggest that snakes collapse following loss of amphibians, one of their common vertebrate prey (Zipkin et al., 2020), which coincides with the identified snake extinction risk factors (Todd et al., 2017). Also, snakebite envenoming burden is higher in biodiversity hotspots, which are known to harbor larger snake populations (Luiselli et al., 2020). However, some species might manage to persist and thrive in biologically impoverished environments, such as agricultural lands probably due to prey abundance (Suazo-Ortuño et al., 2008).
In the short term, the impacts of environmental change on snakebite envenoming burden are also unclear. Generally, snakebites are higher in warmer regions, but the effect of rainfall is context-dependent: snakebites in California significantly increase in response to precipitation levels with an 18-month delay, and significantly decrease with droughts in the following six months (Phillips et al., 2019); while in Costa Rica, snakebites decrease 11 months after precipitation (Chaves et al., 2015). Such relationships with precipitation are likely driven by demographic and activity patterns of snakes in response to precipitation levels (Chaves et al., 2015), and precipitation is also known to influence the composition of local venomous snake ensembles. Furthermore, flooding events are widely considered a primary driver of snakebite risk as they wash snakes towards lowlands which usually have higher population density (Ochoa et al., 2020). On the other hand, the positive effect of temperature on snakebites may be related to overall diversity (Luiselli et al., 2020). It is therefore possible that global warming is changing seasonal patterns of bites according to local snake species’ biology (Chaves et al., 2015) and other human-related factors such as agricultural practices (see below).
Lastly, land use change is an immediate reflection of human activities, which is correlated with occupational and socioeconomic characteristics of local residents (Ganzeboom et al., 1992), and often depends on upstream demands of certain goods. Socioeconomic status and occupation have long been recognized as important risk factors for snakebite (Fig. 1; Harrison et al., 2009). Land conversion from forest to agriculture is representative of a growing population of agricultural workers who are the most frequent snakebite victims (Figueroa-Mise et al., 2019; Pierini et al., 1996). For this reason, snakebites tend to be concentrated in peri-urban and rural settings (Gutiérrez et al., 2010; Minghui et al., 2019). Likewise, urban parks are frequent sources of snakebites, as these provide refugia for animal species in human-dominated landscapes (Parkin et al., 2020; Perry et al., 2020; Soga and Gaston, 2020). Although the effect of land use change on snakebite is highly variable between regions, it can be explained by the composition of snake assemblages and their local population sizes (Oliveira et al., 2020). In summary, land clearing and its associated demographic and occupational factors provide the ideal settings for human-snake encounters (Soga and Gaston, 2020) and a biological basis to explain the role of land use change on snakebite risk patterns. The effect of land use change on snakebite envenoming incidence and morbidity will thus depend on changes in the human population at risk and the response of resident snakes to anthropogenic disturbances.
3.2. Human population growth and socio-economy
Ecologically, snakebites occur following an interaction between two different species. Any process or factor that affects the frequency and outcome of human-snake contacts will change its epidemiology. While climate, human population growth, and land use change directly affect the capacity of snake populations to grow, achieve high population densities, disperse to nearby habitats (see above) and sustain viable populations, socio-economic and demographic changes among humans can modify their susceptibility to snakebites (Harrison et al., 2009), thereby offsetting the effect of snake population size on snakebites. As ongoing socio-economic and cultural changes are inherent to the global dynamics of environmental change, we must strive to understand the factors that make certain occupations, socio-economic strata and genders more or less susceptible to snakebites.
Snakebite envenoming risk is related to economic status and occupation (Figueroa-Mise et al., 2019; Harrison et al., 2009). Agricultural workers living in low and middle income tropical areas, are at the highest risk of snakebites (Ediriweera et al., 2016; Figueroa-Mise et al., 2019), and the factors that make agricultural workers more susceptible than other occupations to snakebites differ among regions. For instance, barefoot rice paddy farming by the male is common in India and Sri Lanka (Hellung-Schønning et al., 2019) and is a major risk factor for Russel's viper bites because this species is attracted to agricultural lands (Kularatne, 2002; Silva et al., 2014). Whereas among Brazilian farmers, who are also at high risk of snakebites (Figueroa-Mise et al., 2019), there is wider use of footwear, and most snakebites are caused by difficult-to-spot jararaca snakes (Bothrops atrox juveniles) hence bites occur in the upper limbs (Oliveira et al., 2020; Pierini et al., 1996).
Poverty and inequality increase snakebite envenoming risk by other means such as lack of accessibility to safe housing, bedding, rodent control, and by being geographically closer to the forest-agricultural frontier (Bawaskar and Bawaskar, 2019; Kularatne, 2002). First, safe housing impedes indoors access to snakes, and the use of raised beds prevents snakebites while asleep (Bawaskar and Bawaskar, 2019; Kularatne, 2002); rodent control decreases prey availability for snakes (Pandey et al., 2020) and closeness to the agricultural-forest frontier puts people in proximity to larger and more diverse snake populations (Oliveira et al., 2020; Pierini et al., 1996; Silva et al., 2020).
Gender-specific risks are also known to occur and result in different envenoming diseases. For instance, women in Sri Lanka are traditionally in charge of house garden maintenance, where species of the hump-nosed viper species complex (Hypnale hypnale, H. zara, H. Nepa (Maduwage et al., 2009); occur in leaf litter. Naturally most Hypnale spp. bite victims in Sri Lanka are women and the most frequent site is the hand or upper limb (Ariaratnam et al., 2008). The mentioned risk factors illustrate that socio-economic and cultural changes could differ among nations and ecological settings (Fig. 1), both as a result of the nature of local risk factors, from snake species’ biologies, and from the environmental changes that take place.
In addition to socio-economic changes affecting snakebites, agriculture is predicted to require certain adaptations to climate change which may both increase or decrease risk levels to workers (Bennett and McMichael, 2010). The predicted and recommended changes from an agricultural perspective could have deep health implications in general (Bennett and McMichael, 2010; Wilcox et al., 2019) and for snakebite envenoming burden in particular. Two notable examples of high snakebite envenoming risk agricultural occupations are rubber tapping and rice paddy farming (Chanhome et al., 1998; Chippaux, 1998). Since both farming types depend on rainfall seasonal patterns and its levels (Naylor et al., 2007; Rao et al., 1998; Wassmann et al., 2009), farmers are likely to adapt to novel climatic conditions, modifying the structure of plantations and paddies (e.g. Barbier et al., 2009; Mesike et al., 2015) thereby affecting snake abundance. Also, changing rainfall patterns may require different planting and/or harvesting dates and changing the frequency of rubber tapping (Esham and Garforth, 2013, Kudaligama et al., 2012, Matthews et al., 1997, Mesike et al., 2015), also affecting the probability of encountering snakes among seasons (Chaves et al., 2015). Predicting the effect of these processes on snakebite is a great challenge, although there are novel computational approaches to examine changes in snakebites as a socio-ecological system (Goldstein et al., 2021).
Ongoing socioeconomic changes are altering the burden and epidemiology of NTDs (Hotez, 2017) and in some regions probably snakebites (e.g. Soga and Gaston, 2020). Further epidemiological changes will depend on the interaction between snake species’ biology, agricultural practices, accessibility to basic goods, services, and facilities. Some of the changes necessary to reduce snakebite envenoming burden could occur with the natural progression of socioeconomic and cultural changes (housing, bedding, mechanization of agriculture, gender equality). However, availability of safe, and effective antivenoms, trained medical staff and knowledge on the best snakebite envenoming management practices should still be actively pursued in areas at risk as a frontline reactive approach to snakebite (Bawaskar and Bawaskar, 2019; Longbottom et al., 2018).
3.2.1. Example box 2: environmental change impacts on snakebite
Empirical evidence of global change impacts on snakebite are even scarcer than it is for snakes, owing to the scale of the process and the fact that snakebites remain neglected among NTDs. Thus, current evidence stems from a handful of regional studies that did not specifically aim to characterize global change impacts on snakebite.
3.2.1.1. Impacts of human population density on snakes and snakebite
Some of the most comprehensive attempts to characterize the geography of snakebite incidence indicate that human population density tends to reduce incidence (Ediriweera et al., 2016, Molesworth et al., 2003). While there is still insufficient information as to how widespread this phenomenon is, it might be related to the reduction of snake abundance in population centers because human activities have been observed to significantly reduce abundance of venomous snakes (Parkin et al., 2020).
3.2.1.2. Impacts of land use change on snakebite
These may be context-dependent, thus highly variable, difficult to understand and examples are scarce. One of the few existing records, however, suggests that unplanned land use change can increase risk of mortality after a snakebite, as access to remote locations with poor quality roads is problematic for ambulances and there is low antivenom availability in such places (Fan and Monteiro, 2018, Hansson et al., 2013). Remotely populated areas lacking emergency services for snakebite are one of the many global challenges (Longbottom et al., 2018).
3.2.1.3. Impacts of climate change on snakebite
We have described above how climate change, particularly via temperature, may impact snake populations, thereby resulting in potential changes to snakebite epidemiology. However, short term flooding events, such as monsoons, have been observed to lead to large numbers of snakebites by putting humans and snakes in proximity. The most likely mechanisms could be related to snakes being dragged by floodwaters from their natural home ranges or distributions (as has been the case of C. durissus in brazil; Bastos et al., 2005), to areas with higher human populations, and then seeking shelter from deep water in higher grounds along with people (Ochoa et al., 2020). As climate change is altering the frequency, severity and location of flooding events, snakebite burden is expected to keep changing with flooding events. In the long term the effect of flooding events will also depend on how such flooding events will impact snake populations.
3.2.1.4. Summary
The scarcity of examples of environmental change impacts on snakebite shows that snakebite eco-epidemiology has a long way to go, especially with regards to characterizing snakebite statistics in relation to ecological determinants of risk patterns, such as those described for flooding events (Ochoa et al., 2020). As shown previously along this review untangling the latter is possible although, doing so at a global scale is an enormous but necessary challenge.
3.3. Conflicts between global change mitigation and snakebite
The sustainable development goals (SDGs) are an urgent call to curb and reverse the negative impacts of global environmental and socio-economic change. The SDGs include specific goals around reducing NTDs (Bangert et al., 2017). Among the pillars of the SDGs, nature conservation stands out as a potential source of ongoing conflict for managing snakebites. While snake conservation is important to maintain resilient ecosystems and livelihoods (Downing et al., 2012; Ritchie et al., 2012), safeguarding venomous snakes from environmental change will naturally preserve snakebites as an ecosystem disservice (Dunn, 2010; Morand and Lajaunie, 2021; Soga and Gaston, 2020). Therefore preventing snakebites (alongside other NTDs and emerging infections) remains a significant challenge for sustainable development (Bangert et al., 2017).
In the context of the SDGs, a prevention-centered agenda for snakebites is more likely to render satisfactory outcomes for both conflicting parties (conservation vs public health). Prevention in the first place will improve prosperity by decreasing life years of disability (Hotez et al., 2016; 2014; Warrell, 2010) and ease nature conservation by reducing snake-human conflicts and the persecution of venomous snakes (Fita et al., 2010; Means, 2009; Pandey et al., 2016), reduce the burden on public health services and improve safety standards in food production (Warrell, 2010). In order to prevent conflicts among SDGs snakebite prevention should encompass the different levels of ecological organization summarized above, ranging from strategic management of the forest-agricultural frontier and urban sprawl (Perry et al., 2020), education, adequate collection of snakebite statistics to monitor their response to environmental changes and conservation policies, to developing and optimizing the delivery of effective antivenoms and personal protective equipment (Gutiérrez et al., 2010; Longbottom et al., 2018).
There are numerous opportunities for interventions with the joint effort of traditionally disparate governance agencies (environment, public health, economy, education, and scientific development) as disjoint efforts are likely to result in conflicts that preclude any transformational outcomes (Trisos, 2019; Wong and Heijden, 2019). As we have shown, meeting the goals set by the global snakebite action plan (Minghui et al., 2019) should be in line with mitigation of global environmental change and with the SDGs to take advantage of the potential synergies for NTDs (Bangert et al., 2017).
4. Summary
The three dimensions of global environmental change considered here (climate, land use, and socioeconomic) can modify the epidemiology of snakebites on both of its snake and human dimensions. Snake geographic distributions and abundance can change across large spatial scales in response to climate change and alter the extent of regions at risk, while land use change may modify species’ ensembles at smaller scales. With respect to humans, land use change may depend on both climate and socioeconomic changes which will dictate the nature of snakebite risks depending on local snake species, predominant occupations, and accessibility to basic goods and services. Managing snakebite envenoming risks in response to environmental changes requires joint efforts between nature conservation and public health authorities in order to secure satisfactory outcomes for societies.
Credit author statement
Design of the review: Gerardo Martín; Carlos Yáñez-Arenas. Writing of the first version of the review: Gerardo Martín; Carlos Yáñez-Arenas. Revision and editing of the first version: Gerardo Martín; Carlos Yáñez-Arenas; Rodrigo Rangel-Camacho; Kris A. Murray, Eyal Goldstein; Takuya Iwamura, Xavier Chiappa-Carrara. Final revision of the manuscript: Gerardo Martín; Carlos Yáñez-Arenas; Rodrigo Rangel-Camacho; Kris A. Murray, Eyal Goldstein; Takuya Iwamura, Xavier Chiappa-Carrara.
Bullet points.
-
•
Distributional areas and demographics of venomous snakes are being, and will continue to be affected by global environmental change.
-
•
Climate change effects on venomous snakes are gradual and may occur at coarse spatial scales (e.g. distributional ranges).
-
•
Human population growth is the main determinant of land-use change, which impacts snake’ populations at smaller landscape scales than climate change.
-
•
Global environmental change could lead to novel patterns of incidence and morbidity of snakebites, both as a result of snake responses to environmental change and socioeconomic and demographic changes among humans.
-
•
Developing strategies to mitigate global change requires drastic measures that guarantee ecosystem functionality which include protecting venomous snakes. Thus, to prevent conflicts, global change mitigation should encompass overarching public health campaigns.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This article was written under the Postdoctoral scholarship by the Dirección General del Personal Académico (POSDOC-DGAPA-UNAM) granted to GM, and supervised by XCC.
Handling Editor: Dr. Raymond Norton
References
- Ariaratnam C.A., Thuraisingam V., Kularatne S.A.M., Sheriff M.H.R., Theakston R.D.G., de Silva A., Warrell D.A. Frequent and potentially fatal envenoming by hump-nosed pit vipers (Hypnale hypnale and H. nepa) in Sri Lanka: lack of effective antivenom. Trans. R. Soc. Trop. Med. Hyg. 2008;102:1120–1126. doi: 10.1016/j.trstmh.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Bangert M., Molyneux D.H., Lindsay S.W., Fitzpatrick C., Engels D. The cross-cutting contribution of the end of neglected tropical diseases to the sustainable development goals. Infect. Dis. Poverty. 2017;6:73. doi: 10.1186/s40249-017-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier B., Yacouba H., Karambiri H., Zoromé M., Somé B. Human vulnerability to climate variability in the sahel: farmers' adaptation strategies in northern Burkina Faso. Environ. Manag. 2009;43:790–803. doi: 10.1007/s00267-008-9237-9. [DOI] [PubMed] [Google Scholar]
- Bastos E.G. de M., Araújo A.F.B., de Silva H.R. Records of the rattlesnakes Crotalus durissus terrificus (Laurenti) (Serpentes, Viperidae) in the State of Rio de Janeiro, Brazil: a possible case of invasion facilitated by deforestation. Rev. Bras. Zool. 2005;22:812–815. doi: 10.1590/S0101-81752005000300047. [DOI] [Google Scholar]
- Bawaskar H.S., Bawaskar P.H. Snake bite: prevention and management in rural Indian settings. Lancet Glob. Health. 2019;7:e1178. doi: 10.1016/S2214-109X(19)30275-X. [DOI] [PubMed] [Google Scholar]
- Bennett C.M., McMichael A.J. Non-heat related impacts of climate change on working populations. Glob. Health Action. 2010;3:5640. doi: 10.3402/gha.v3i0.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm M., Collen B., Baillie J.E.M., Bowles P., Chanson J., Cox N., Hammerson G., Hoffmann M., Livingstone S.R., Ram M., Rhodin A.G.J., Stuart S.N., van Dijk P.P., Young B.E., Afuang L.E., Aghasyan A., García A., Aguilar C., Ajtic R., Akarsu F., Alencar L.R.V., Allison A., Ananjeva N., Anderson S., Andrén C., Ariano-Sánchez D., Arredondo J.C., Auliya M., Austin C.C., Avci A., Baker P.J., Barreto-Lima A.F., Barrio-Amorós C.L., Basu D., Bates M.F., Batistella A., Bauer A., Bennett D., Böhme W., Broadley D., Brown R., Burgess J., Captain A., Carreira S., Castañeda M., del R., Castro F., Catenazzi A., Cedeño-Vázquez J.R., Chapple D.G., Cheylan M., Cisneros-Heredia D.F., Cogalniceanu D., Cogger H., Corti C., Costa G.C., Couper P.J., Courtney T., Crnobrnja-Isailovic J., Crochet P.-A., Crother B., Cruz F., Daltry J.C., Daniels R.J.R., Das I., de Silva A., Diesmos A.C., Dirksen L., Doan T.M., Dodd C.K., Doody J.S., Dorcas M.E., Duarte de Barros Filho J., Egan V.T., El Mouden E.H., Embert D., Espinoza R.E., Fallabrino A., Feng X., Feng Z.-J., Fitzgerald L., Flores-Villela O., França F.G.R., Frost D., Gadsden H., Gamble T., Ganesh S.R., Garcia M.A., García-Pérez J.E., Gatus J., Gaulke M., Geniez P., Georges A., Gerlach J., Goldberg S., Gonzalez J.-C.T., Gower D.J., Grant T., Greenbaum E., Grieco C., Guo P., Hamilton A.M., Hare K., Hedges S.B., Heideman N., Hilton-Taylor C., Hitchmough R., Hollingsworth B., Hutchinson M., Ineich I., Iverson J., Jaksic F.M., Jenkins R., Joger U., Jose R., Kaska Y., Kaya U., Keogh J.S., Köhler G., Kuchling G., Kumlutaş Y., Kwet A., La Marca E., Lamar W., Lane A., Lardner B., Latta C., Latta G., Lau M., Lavin P., Lawson D., LeBreton M., Lehr E., Limpus D., Lipczynski N., Lobo A.S., López-Luna M.A., Luiselli L., Lukoschek V., Lundberg M., Lymberakis P., Macey R., Magnusson W.E., Mahler D.L., Malhotra A., Mariaux J., Maritz B., Marques O.A.V., Márquez R., Martins M., Masterson G., Mateo J.A., Mathew R., Mathews N., Mayer G., McCranie J.R., Measey G.J., Mendoza-Quijano F., Menegon M., Métrailler S., Milton D.A., Montgomery C., Morato S.A.A., Mott T., Muñoz-Alonso A., Murphy J., Nguyen T.Q., Nilson G., Nogueira C., Núñez H., Orlov N., Ota H., Ottenwalder J., Papenfuss T., Pasachnik S., Passos P., Pauwels O.S.G., Pérez-Buitrago N., Pérez-Mellado V., Pianka E.R., Pleguezuelos J., Pollock C., Ponce-Campos P., Powell R., Pupin F., Quintero Díaz G.E., Radder R., Ramer J., Rasmussen A.R., Raxworthy C., Reynolds R., Richman N., Rico E.L., Riservato E., Rivas G., da Rocha P.L.B., Rödel M.-O., Rodríguez Schettino L., Roosenburg W.M., Ross J.P., Sadek R., Sanders K., Santos-Barrera G., Schleich H.H., Schmidt B.R., Schmitz A., Sharifi M., Shea G., Shi H.-T., Shine R., Sindaco R., Slimani T., Somaweera R., Spawls S., Stafford P., Stuebing R., Sweet S., Sy E., Temple H.J., Tognelli M.F., Tolley K., Tolson P.J., Tuniyev B., Tuniyev S., Üzüm N., van Buurt G., Van Sluys M., Velasco A., Vences M., Veselý M., Vinke S., Vinke T., Vogel G., Vogrin M., Vogt R.C., Wearn O.R., Werner Y.L., Whiting M.J., Wiewandt T., Wilkinson J., Wilson B., Wren S., Zamin T., Zhou K., Zug G. The conservation status of the world's reptiles. Biol. Conserv. 2013;157:372–385. doi: 10.1016/j.biocon.2012.07.015. [DOI] [Google Scholar]
- Booth M. Advances in Parasitology. Elsevier; 2018. Climate change and the neglected tropical diseases; pp. 39–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C.J.A., Ehrlich P.R., Beattie A., Ceballos G., Crist E., Diamond J., Dirzo R., Ehrlich A.H., Harte J., Harte M.E., Pyke G., Raven P.H., Ripple W.J., Saltré F., Turnbull C., Wackernagel M., Blumstein D.T. Underestimating the Challenges of Avoiding a Ghastly Future. Front. Conserv. Sci. 2021;1:615419. doi: 10.3389/fcosc.2020.615419. [DOI] [Google Scholar]
- Bravo-Vega C.A., Cordovez J.M., Renjifo-Ibáñez C., Santos-Vega M., Sasa M. Estimating snakebite incidence from mathematical models: A test in Costa Rica. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.H. Macroecology. The University of Chigao Press; Chicago. United States: 1995. [Google Scholar]
- Carrasco P., Scrocchi G., Leynaud G. Redescription of the southernmost snake species, Bothrops ammodytoides (Serpentes: Viperidae: Crotalinae) Amphib Reptilia. 2010;31:323–338. doi: 10.1163/156853810791769491. [DOI] [Google Scholar]
- Chanhome L., Cox M.J., Wilde H., Jintakoon P., Chaiyabutr N., Sitprija V. Venomous snakebite in Thailand I: medically important snakes. Mil. Med. 1998;163:310–317. doi: 10.1093/milmed/163.5.310. [DOI] [PubMed] [Google Scholar]
- Chaves L.F., Chuang T.-W., Sasa M., Gutiérrez J.M. Snakebites are associated with poverty, weather fluctuations, and El Niño. Sci. Adv. 2015;1 doi: 10.1126/sciadv.1500249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux J.-P. Snake-bites: appraisal of the global situation. Bull. World Health Organ. 1998;76:515–524. [PMC free article] [PubMed] [Google Scholar]
- Clark R.W., Marchand M.N., Clifford B.J., Stechert R., Stephens S. Decline of an isolated timber rattlesnake (Crotalus horridus) population: interactions between climate change, disease, and loss of genetic diversity. Biol. Conserv. 2011;144:886–891. doi: 10.1016/j.biocon.2010.12.001. [DOI] [Google Scholar]
- Davis M.A., Douglas M.R., Webb C.T., Collyer M.L., Holycross A.T., Painter C.W., Kamees L.K., Douglas M.E. Nowhere to go but up: impacts of climate change on demographics of a short-range endemic (Crotalus willardi obscurus) in the sky-islands of southwestern North America. PloS One. 2015;10 doi: 10.1371/journal.pone.0131067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deMenocal P.B. Climate and Human Evolution. Science. 2011;331:540–542. doi: 10.1126/science.1190683. [DOI] [PubMed] [Google Scholar]
- Dirzo R., Young H.S., Galetti M., Ceballos G., Isaac N.J.B., Collen B. Defaunation in the anthropocene. Science. 2014;345:401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- Downing A.S., van Nes E.H., Mooij W.M., Scheffer M. The resilience and resistance of an ecosystem to a collapse of diversity. PloS One. 2012;7 doi: 10.1371/journal.pone.0046135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn R.R. Global mapping of ecosystem disservices: the unspoken reality that nature sometimes kills us: ecosystem disservices. Biotropica. 2010;42:555–557. doi: 10.1111/j.1744-7429.2010.00698.x. [DOI] [Google Scholar]
- Ediriweera D.S., Kasturiratne A., Pathmeswaran A., Gunawardena N.K., Wijayawickrama B.A., Jayamanne S.F., Isbister G.K., Dawson A., Giorgi E., Diggle P.J., Lalloo D.G., de Silva H.J. Mapping the risk of snakebite in Sri Lanka - a national survey with geospatial analysis. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esham M, Garforth C. Climate change and agricultural adaptation in Sri Lanka: a review. Clim. Dev. 2013;5(1):66–76. [Google Scholar]
- Fan H.W., Monteiro W.M. History and perspectives on how to ensure antivenom accessibility in the most remote areas in Brazil. Toxicon. 2018;151:15–23. doi: 10.1016/j.toxicon.2018.06.070. [DOI] [PubMed] [Google Scholar]
- Figueroa-Mise Y., Lira-da-Silva R.M., Carvalho F.M. Fatal snakebite envenoming and agricultural work in Brazil: a case–control study. Am. J. Trop. Med. Hyg. 2019;100:150–154. doi: 10.4269/ajtmh.18-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick S.E., Hijmans R.J. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37:12. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- Fita D.S., Costa Neto E.M., Schiavetti A. “Offensive” snakes: cultural beliefs and practices related to snakebites in a Brazilian rural settlement. J. Ethnobiol. Ethnomed. 2010;6:13. doi: 10.1186/1746-4269-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzeboom H.B.G., De Graaf P.M., Treiman D.J. A standard international socio-economic index of occupational status. Soc. Sci. Res. 1992;21:1–56. doi: 10.1016/0049-089X(92)90017-B. [DOI] [Google Scholar]
- Garchitorena A., Sokolow S.H., Roche B., Ngonghala C.N., Jocque M., Lund A., Barry M., Mordecai E.A., Daily G.C., Jones J.H., Andrews J.R., Bendavid E., Luby S.P., LaBeaud A.D., Seetah K., Guégan J.F., Bonds M.H., De Leo G.A. Disease ecology, health and the environment: a framework to account for ecological and socio-economic drivers in the control of neglected tropical diseases. Philos. Trans. R. Soc. B Biol. Sci. 2017;372:20160128. doi: 10.1098/rstb.2016.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston K.J. The Structure and Dynamics of Geographic Ranges, Firrst. Oxford University Press; Oxford, New York: 2003. [Google Scholar]
- Goldstein E., Erinjery J.J., Martin G., Kasturiratne A., Ediriweera D.S., de Silva H.J., Diggle P., Lalloo D.G., Murray K.A., Iwamura T. Integrating human behavior and snake ecology with agent-based models to predict snakebite in high risk landscapes. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Williams D., Fan H.W., Warrell D.A. Snakebite envenoming from a global perspective: towards an integrated approach. Toxicon. 2010;56:1223–1235. doi: 10.1016/j.toxicon.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Hansson E., Sasa M., Mattisson K., Robles A., Gutiérrez J.M. Using geographical information systems to identify populations in need of improved accessibility to antivenom treatment for snakebite envenoming in Costa Rica. PLoS Neglected Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R.A., Hargreaves A., Wagstaff S.C., Faragher B., Lalloo D.G. Snake envenoming: a disease of poverty. PLoS Neglected Trop. Dis. 2009;3:e569. doi: 10.1371/journal.pntd.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellung-Schønning M., Phelps M.D., Warnasekara J., Agampodi S.B., Furu P. A case–control study of environmental and occupational risks of leptospirosis in Sri Lanka. EcoHealth. 2019;16:534–543. doi: 10.1007/s10393-019-01448-w. [DOI] [PubMed] [Google Scholar]
- Holt R.D. Reflections on niches and numbers. Ecography. 2020;43:387–390. doi: 10.1111/ecog.04828. [DOI] [Google Scholar]
- Hotez P.J. Ten failings in global neglected tropical diseases control. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Alvarado M., Basáñez M.-G., Bolliger I., Bourne R., Boussinesq M., Brooker S.J., Brown A.S., Buckle G., Budke C.M., Carabin H., Coffeng L.E., Fèvre E.M., Fürst T., Halasa Y.A., Jasrasaria R., Johns N.E., Keiser J., King C.H., Lozano R., Murdoch M.E., O'Hanlon S., Pion S.D.S., Pullan R.L., Ramaiah K.D., Roberts T., Shepard D.S., Smith J.L., Stolk W.A., Undurraga E.A., Utzinger J., Wang M., Murray C.J.L., Naghavi M. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Neglected Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Damania A., Naghavi M. Blue marble health and the global burden of disease study 2013. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., O'Neill B.C. Spatially explicit global population scenarios consistent with the Shared Socioeconomic Pathways. Environ. Res. Lett. 2016;11 doi: 10.1088/1748-9326/11/8/084003. [DOI] [Google Scholar]
- Kearney M.R., Munns S.L., Moore D., Malishev M., Bull C.M. Field tests of a general ectotherm niche model show how water can limit lizard activity and distribution. Ecol. Monogr. 2018;88:672–693. doi: 10.1002/ecm.1326. [DOI] [Google Scholar]
- Krauss J., Bommarco R., Guardiola M., Heikkinen R.K., Helm A., Kuussaari M., Lindborg R., Öckinger E., Pärtel M., Pino J., Pöyry J., Raatikainen K.M., Sang A., Stefanescu C., Teder T., Zobel M., Steffan-Dewenter I. Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels: immediate and time-delayed biodiversity loss. Ecol. Lett. 2010;13:597–605. doi: 10.1111/j.1461-0248.2010.01457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudaligama K.V.V.S., Rodrigo V.H.L., Fernando K.M.E.P., Yapa P.A.J. Effect of low frequency harvesting in Hevea brasiliensis on major raw rubber properties. J. Rubber Res. Inst. Sri Lanka. 2012;92:1. doi: 10.4038/jrrisl.v92i0.1856. [DOI] [Google Scholar]
- Kularatne S.A.M. Common krait (Bungarus caeruleus) bite in Anuradhapura, Sri Lanka: a prospective clinical study, 1996-98. Postgrad. Med. 2002;78:276–280. doi: 10.1136/pmj.78.919.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longbottom J., Shearer F.M., Devine M., Alcoba G., Chappuis F., Weiss D.J., Ray S.E., Ray N., Warrell D.A., Ruiz de Castañeda R., Williams D.J., Hay S.I., Pigott D.M. Vulnerability to snakebite envenoming: a global mapping of hotspots. Lancet. 2018;392:673–684. doi: 10.1016/S0140-6736(18)31224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiselli L. The ghost of a recent invasion in the reduced feeding rates of spitting cobras during the dry season in a rainforest region of tropical Africa? Acta Oecol. 2001;22:311–314. doi: 10.1016/S1146-609X(01)01113-4. [DOI] [Google Scholar]
- Luiselli L., Sale L., Akani G.C., Amori G. Venomous snake abundance within snake species' assemblages worldwide. Diversity. 2020;12:69. doi: 10.3390/d12020069. [DOI] [Google Scholar]
- Maduwage K., Silva A., Manamendra-Arachchi K., Pethiyagoda R. A taxonomic revision of the South Asian hump-nosed pit vipers (Squamata: Viperidae: Hypnale) Zootaxa. 2009;2232:1–28. doi: 10.11646/zootaxa.2232.1.1. [DOI] [Google Scholar]
- Matthews R.B., Kropff M.J., Horie T., Bachelet D. Simulating the impact of climate change on rice production in Asia and evaluating options for adaptation. Agric. Syst. 1997;54:399–425. doi: 10.1016/S0308-521X(95)00060-I. [DOI] [Google Scholar]
- Means B.D. Effects of rattlesnake roundups of the eastern diamondback rattlesnake (Crotalus adamanteus) Herpetol. Conserv. Biol. 2009;4:132–141. [Google Scholar]
- Mesike CS, Ugwa IK, Esekhade TU. Adaptation to climate change among rubber farmers in delta state, Nigeria. Climate Change. 2015;1(2):98–104. [Google Scholar]
- Minghui R., Malecela M.N., Cooke E., Abela-Ridder B. WHO's Snakebite Envenoming Strategy for prevention and control. Lancet Glob. Health. 2019;7:e837–e838. doi: 10.1016/S2214-109X(19)30225-6. [DOI] [PubMed] [Google Scholar]
- Molesworth A.M., Harrison R., David R., Theakston G., Lalloo D.G. Geographic information system mapping of snakebite incidence in northern Ghana and Nigeria using environmental indicators: a preliminary study. Trans. R. Soc. Trop. Med. Hyg. 2003;97:188–192. doi: 10.1016/S0035-9203(03)90115-5. [DOI] [PubMed] [Google Scholar]
- Morand S., Lajaunie C. Outbreaks of vector-borne and zoonotic diseases are associated with changes in forest cover and oil pals expansion at global scale. Front. Vet. Sci. 2021 doi: 10.3389/fvets.2021.661063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor R.L., Battisti D.S., Vimont D.J., Falcon W.P., Burke M.B. Assessing risks of climate variability and climate change for Indonesian rice agriculture. Proc. Natl. Acad. Sci. Unit. States Am. 2007;104:7752–7757. doi: 10.1073/pnas.0701825104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold T., Hudson L.N., Hill S.L.L., Contu S., Lysenko I., Senior R.A., Börger L., Bennett D.J., Choimes A., Collen B., Day J., De Palma A., Díaz S., Echeverria-Londoño S., Edgar M.J., Feldman A., Garon M., Harrison M.L.K., Alhusseini T., Ingram D.J., Itescu Y., Kattge J., Kemp V., Kirkpatrick L., Kleyer M., Correia D.L.P., Martin C.D., Meiri S., Novosolov M., Pan Y., Phillips H.R.P., Purves D.W., Robinson A., Simpson J., Tuck S.L., Weiher E., White H.J., Ewers R.M., Mace G.M., Scharlemann J.P.W., Purvis A. Global effects of land use on local terrestrial biodiversity. Nature. 2015;520:45–50. doi: 10.1038/nature14324. [DOI] [PubMed] [Google Scholar]
- Ochoa C., Bolon I., Durso A.M., Ruiz-de-Castañeda R., Alcoba G., Babo-Martins S., Chappuis F., Nicolas R. Assessing the increase of snakebite incidence in relationship to flooding events. J. Env. Pub. Health. 2020 doi: 10.1155/2020/6135149. [DOI] [Google Scholar]
- Oliveira L.P. de, Moreira J.G., do V., Sachett J., de A.G., Monteiro W.M., Meneguetti D.U., de O., Bernarde P.S. Snakebites in rio branco and surrounding region, acre, western Brazilian amazon. Rev. Soc. Bras. Med. Trop. 2020;53 doi: 10.1590/0037-8682-0214-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio‐Olvera L., Soberón J., Falconi M. On population abundance and niche structure. Ecography. 2019;42:1415–1425. doi: 10.1111/ecog.04442. [DOI] [Google Scholar]
- Pandey D.P., Bhattarai P., Piya R.C. Food spectrum of common kraits (Bungarus caeruleus): an implication for snakebite prevention and snake conservation. J. Herpetol. 2020;54:87. doi: 10.1670/18-054. [DOI] [Google Scholar]
- Parkin T., Jolly C.J., Laive A., Takach B. Snakes on an urban plain: temporal patterns of snake activity and human–snake conflict in Darwin, Australia. Austral Ecol. aec. 2020 doi: 10.1111/aec.12990. 12990. [DOI] [Google Scholar]
- Pearson R.G., Dawson T.P. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol. Biogeogr. 2003;12:361–371. doi: 10.1046/j.1466-822X.2003.00042.x. [DOI] [Google Scholar]
- Perry G., Lacy M., Das I. Snakes, snakebites, and humans. In: Angelici F.M., Rossi L., editors. Problematic Wildlife II. Springer International Publishing; Cham: 2020. pp. 561–580. [DOI] [Google Scholar]
- Phillips C., Lipman G.S., Gugelmann H., Doering K., Lung D. Snakebites and climate change in California, 1997–2017. Clin. Toxicol. 2019;57:168–174. doi: 10.1080/15563650.2018.1508690. [DOI] [PubMed] [Google Scholar]
- Pierini S.V., Warrell D.A., De Paulo A., Theakston R.D.G. High incidence of bites and stings by snakes and other animals among rubber tappers and amazonian indians of the Juruá Valley, Acre State, Brazil. Toxicon. 1996;34:225–236. doi: 10.1016/0041-0101(95)00125-5. [DOI] [PubMed] [Google Scholar]
- Rao P.S., Saraswathyamma C.K., Sethuraj M.R. 1998. Studies on the relationship between yield and meteorological parameters of para rubber tree žHeÕea brasiliensis/11. [Google Scholar]
- Reed R.N. Interspecific patterns of species richness, geographic range size, and body size among New World venomous snakes. Ecography. 2003;26:107–117. doi: 10.1034/j.1600-0587.2003.03388.x. [DOI] [Google Scholar]
- Ritchie E.G., Elmhagen B., Glen A.S., Letnic M., Ludwig G., McDonald R.A. Ecosystem restoration with teeth: what role for predators? Trends Ecol. Evol. 2012;27:265–271. doi: 10.1016/j.tree.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Rosales J. Economic growth, climate change, biodiversity loss: distributive justice for the global North and south. Conserv. Biol. 2008;22:1409–1417. doi: 10.1111/j.1523-1739.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- Saupe E.E., Qiao H., Hendricks J.R., Portell R.W., Hunter S.J., Soberón J., Lieberman B.S. Niche breadth and geographic range size as determinants of species survival on geological time scales: determinants of species survival. Global Ecol. Biogeogr. 2015;24:1159–1169. doi: 10.1111/geb.12333. [DOI] [Google Scholar]
- Sazima I. Natural history of the jararaca pitviper, Bothrops jararaca, in southeastern Brazil. Biol. Pitvipers. 1992:199–2015. [Google Scholar]
- Silva A., Marikar F., Murugananthan A., Agampodi S. Awareness and perceptions on prevention, first aid and treatment of snakebites among Sri Lankan farmers: a knowledge practice mismatch? J. Occup. Med. Toxicol. 2014;9:20. doi: 10.1186/1745-6673-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S.R., Tare T.G., Sutar N.K., Renapurkar D.M. Ecology and distribution of Echis carinatus snakes in Deogad Taluka and other areas of Maharashtra State, India. J. Wilderness Med. 1994;5:282–286. doi: 10.1580/0953-9859-5.3.282. [DOI] [Google Scholar]
- Silva J.L. da, Fonseca W.L. da, Mota da Silva A., Amaral G.L.G. do, Ortega G.P., Oliveira A. de S., Correa R.R., Oliveira I., Monteiro W.M., Bernarde P.S. Venomous snakes and people in a floodplain forest in the Western Brazilian Amazon: potential risks for snakebites. Toxicon. 2020;187:232–244. doi: 10.1016/j.toxicon.2020.09.007. [DOI] [PubMed] [Google Scholar]
- Soberón J., Arroyo-Peña B. Are fundamental niches larger than the realized? Testing a 50-year-old prediction by Hutchinson. PloS One. 2017;12 doi: 10.1371/journal.pone.0175138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon J., Nakamura M. Niches and distributional areas: concepts, methods, and assumptions. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:19644–19650. doi: 10.1073/pnas.0901637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon J., Peterson A.T. Interpretation of models of fundamental ecological niches and species' distributional areas. Biodivers. Inf. 2005;2 doi: 10.17161/bi.v2i0.4. [DOI] [Google Scholar]
- Soga M., Gaston K.J. The ecology of human–nature interactions. Proc. R. Soc. B Biol. Sci. 2020;287:20191882. doi: 10.1098/rspb.2019.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suazo-Ortuño I., Alvarado-Díaz J., Martínez-Ramos M. Effects of conversion of dry tropical forest to agricultural mosaic on herpetofaunal assemblages: tropical dry forest disturbance and herpetofauna. Conserv. Biol. 2008;22:362–374. doi: 10.1111/j.1523-1739.2008.00883.x. [DOI] [PubMed] [Google Scholar]
- Tasoulis T., Isbister G. A Review and Database of Snake Venom Proteomes. Toxins. 2017;9:290. doi: 10.3390/toxins9090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem A.J. WorldPop, open data for spatial demography. Sci. Data. 2017;4:170004. doi: 10.1038/sdata.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd B.D., Nowakowski A.J., Rose J.P., Price S.J. Species traits explaining sensitivity of snakes to human land use estimated from citizen science data. Biol. Conserv. 2017;206:31–36. doi: 10.1016/j.biocon.2016.12.013. [DOI] [Google Scholar]
- Trisos C.H. vol. 2. 2019. p. 3. (Mosquito Net Fishing Exemplifies Conflict Among Sustainable Development Goals). [Google Scholar]
- Uetz P., Hallermann J., Hošek J. 2021. The Reptile Database.http://www.reptile-database.org/ [Google Scholar]
- Warrell D.A. Snake bite. Lancet. 2010;375:77–88. doi: 10.1016/S0140-6736(09)61754-2. [DOI] [PubMed] [Google Scholar]
- Wassmann R., Jagadish S.V.K., Sumfleth K., Pathak H., Howell G., Ismail A., Serraj R., Redona E., Singh R.K., Heuer S. Advances in Agronomy. Elsevier; 2009. Chapter 3 regional vulnerability of climate change impacts on asian rice production and scope for adaptation; pp. 91–133. [DOI] [Google Scholar]
- Wilcox B.A., Echaubard P., de Garine-Wichatitsky M., Ramirez B. Vector-borne disease and climate change adaptation in African dryland social-ecological systems. Infect. Dis. Poverty. 2019;8:36. doi: 10.1186/s40249-019-0539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R., Heijden J. Avoidance of conflicts and trade‐offs: a challenge for the policy integration of the United Nations Sustainable Development Goals. Sustain. Dev. 2019;27:838–845. doi: 10.1002/sd.1944. [DOI] [Google Scholar]
- Wu J. Detecting and Attributing the Effects of Climate Change on the Distributions of Snake Species Over the Past 50 Years. Environ. Manag. 2016;57:207–219. doi: 10.1007/s00267-015-0600-3. [DOI] [PubMed] [Google Scholar]
- Yañez-Arenas C., Peterson A.T., Mokondoko P., Rojas-Soto O., Martínez-Meyer E. The use of ecological niche modeling to infer potential risk areas of snakebite in the Mexican state of veracruz. PloS One. 2014;9 doi: 10.1371/journal.pone.0100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yañez-Arenas C., Townsend Peterson A., Rodríguez-Medina K., Barve N. Mapping current and future potential snakebite risk in the new world. Climatic Change. 2016;134:697–711. doi: 10.1007/s10584-015-1544-6. [DOI] [Google Scholar]
- Zipkin E.F., DiRenzo G.V., Ray J.M., Rossman S., Lips K.R. Tropical snake diversity collapses after widespread amphibian loss. Science. 2020;367:814–816. doi: 10.1126/science.aay5733. [DOI] [PubMed] [Google Scholar]