Abstract
Ornithine decarboxylase (ODC) plays an indispensable role in the process of polyamine biosynthesis. Polyamines are a pivotal part of living cells and have diverse roles in the regulation of cell proliferation and apoptosis, aging and reproduction. However, to date, there have been no reports about ODC regulating follicular development in goose ovaries. Here, we constructed ODC siRNA and overexpression plasmids and transfected them into goose primary granulosa cells (GCs) to elucidate the effects of ODC interference and overexpression on the polyamine metabolism, hormone levels, cell apoptosis and proliferation of granulosa cells. After interfering with ODC in GCs, the mRNA and protein levels of ODC and the content of putrescine were greatly decreased (P < 0.05). When ODC was overexpressed, ODC mRNA and protein levels and putrescine content were greatly increased (P < 0.05). The polyamine-metabolizing enzyme genes ornithine decarboxylase antizyme 1 (OAZ1) and spermidine / spermine-N1-acetyltransferase (SSAT) were significantly increased, and spermidine synthase (SPDS) was significantly decreased when ODC was downregulated (P < 0.05). OAZ1, SPDS and SSAT were significantly increased when ODC was upregulated (P < 0.05). In addition, after interference with ODC, progesterone (P4) levels in the culture medium of GCs increased greatly (P < 0.05), while the overexpression of ODC caused the P4 level to decrease significantly (P < 0.05). After ODC downregulation, granulosa cell activity was significantly reduced, the apoptosis rate was significantly increased, and the BCL-2 / BAX ratio was downregulated (P < 0.05). Under ODC overexpression, the activity of GCs was notably increased, the apoptosis rate was significantly reduced, and the BCL-2 / BAX protein ratio was upregulated (P < 0.05). Our study successfully induced ODC interference and overexpression in goose ovarian GCs, and ODC regulated mainly putrescine content in GCs with a slight influence on spermidine and spermine. Moreover, ODC participated in the adjustment of P4 levels in the culture medium of GCs, promoted granulosa cell proliferation and inhibited granulosa cell apoptosis.
Key words: ornithine decarboxylase, polyamine, granulosa cells, proliferation, apoptosis
INTRODUCTION
The egg laying performance of poultry largely depends on the development of the follicles. The granulosa cells (GCs) in ovarian follicles participate in the regulation of follicular development through the synthesis of hormones. Polyamines mainly include putrescine, spermidine and spermine, which are indispensable components of living cells. They have diverse roles in the adjustment of gene expression, RNA translation, cell proliferation and apoptosis, as well as in the regulation of animal gametogenesis, embryo implantation, development and other reproductive functions (Kang et al., 2017a; Sobe et al., 2017; Elmetwally et al., 2018; Lane et al., 2018).
Ornithine decarboxylase (ODC) is a critical rate-limiting enzyme in polyamine biosynthesis that can catalyze the decarboxylation of ornithine to putrescine in cells and plays a pivotal role in polyamine metabolism (Pegg, 2006). Armstrong (1986) found that ODC activity in chicken ovarian granulosa layers increased with an increase in follicles. Moreover, after blocking the synthesis of ovarian ODC with the irreversible ODC inhibitor α-difluoromethylornithine (DFMO), ovarian and follicular production and luteinization were inhibited. Studies have found that ODC activity is regulated by ornithine decarboxylase antizyme (OAZ), which regulates the growth and development of animal follicles (Ivanov et al., 2000). After overexpression of OAZ1 in goose GCs, the concentrations of spermidine and putrescine in cells decreased, the level of spermine increased, and the expression of luteinizing hormone receptor (LHR), follicle-stimulating hormone receptor (FSHR) and estrogen receptor (ER) genes increased significantly. Research has demonstrated that polyamine deletion can inhibit the apoptosis of intestinal epithelial cells in rats by reducing the activity of CASPASE 3 and 9 and the transfer of BAX to mitochondria, thereby reducing cytochrome c efflux (Yuan et al., 2002). It was found that in many mammals (rats and pigs), ODC activity briefly increases before ovulation (Liu et al., 2016). Older mice showed decreased ovarian ODC and putrescine levels (Yong et al., 2015). Putrescine supplementation in drinking water before obtaining oocytes and supplementing putrescine in oocyte maturation medium in vitro can reduce oocyte aneuploidy in aged mice (Koehler et al., 2006). It is noteworthy that supplementation of putrescine in ovulating mice can significantly improve embryo quality, increase the number of blastocyst cells, reduce early embryonic death and increase the number of live births (Yong et al., 2015).
Inhibition of ODC activity not only sharply reduces polyamine content but also affects cell proliferation and apoptosis. Studies have found that ODC can directly act on ER and androgen receptor (AR) to regulate human breast cancer cell proliferation and apoptosis (Zhu et al., 2012). Lee et al. (2011) showed that during the proliferation stage, the expression of ODC in C2C12 cells was upregulated. After 48 and 72 h of DFMO treatment, the expression of ODC in C2C12 cells decreased by 40 and 66%, respectively, and cell proliferation decreased. The overexpression of the ODC gene promotes the proliferation of C2C12 cells, which indicated that ODC has an important regulatory effect on cell proliferation.
However, there is currently no information available on the influence of ODC on polyamine metabolism, reproductive hormone receptors, cell apoptosis and proliferation-related gene expression in poultry GCs. Focusing on these points, this work used goose primary GCs as the research object to clarify the influence of ODC on polyamine metabolism, reproductive hormone concentration, reproductive hormone receptor gene expression, apoptosis and proliferation in GCs through the interference and overexpression of the ODC gene.
MATERIALS AND METHODS
Animals and Ethics Statement
The regulations on the protection and use of Sichuan white geese were approved by the Animal Ethics Committee of the College of Animal Science and Technology of Sichuan Agricultural University. In this experiment, 40 Sichuan white geese weighing 3.5 ± 0.5 kg at the peak of laying eggs (35–40 wks old) were selected randomly from the poultry breeding farm of Sichuan Agricultural University and euthanized by cervical dislocation.
Cell Culture and Treatment
According to the methods of Kang et al. (2017b), hierarchical follicles (F1-F6) were rapidly collected from the ovaries of the geese, and primary granulosa cells were isolated. GCs were cultured in DMEM/F12 (Thermo Fisher Scientific, Shanghai, China) in a 37°C, 5% CO2 incubator.
Construction of ODC Interference and Overexpression Plasmids and Cell Transfection
To interfere with ODC expression, three ODC siRNA sequences (si-ODC-331, si-ODC-511, and si-ODC-894) and a negative control (NC) were designed and synthesized (Table 1). siRNAs were transfected when the granulosa cell density reached 70%. si-ODC-331, si-ODC-511, si-ODC-894 and NC were diluted with Opti-MEM (Thermo Fisher Scientific). Then, Lipofectamine 3000 (Thermo Fisher Scientific) was mixed with the plasmid and added to the GCs. The cells were cultured in an incubator at 37°C and 5% CO2 for 24, 48, and 72 h.
Table 1.
List of four siRNA sequences.
| Name | Sequences (5′ – 3′) | |
|---|---|---|
| si-ODC-331 | F: | GCAAAUCCCUGCAAACAAATT |
| R: | UUUGUUUGCAGGGAUUUGCTT | |
| si-ODC-511 | F: | GGAGCUACACUUAAGACAATT |
| R: | UUGUCUUAAGUGUAGCUCCTT | |
| si-ODC-894 | F: | GGAGCAAACAGGUUCUGAUTT |
| R: | AUCAGAACCUGUUUGCUCCTT | |
| NC | F: | UUCUCCGAACGUGUCACGUTT |
| R: | AGCUGACACGUUCGGAGAATT | |
The BglII and KpnI restriction endonucleases (New England Biolabs) for the pEGFP-N1 plasmid (Sangon Biotech, Shanghai, China) were a double enzyme, plastic recycling pEGFP-N1 skeleton DNA sequence. The primer sequences (F: 5′-CGCAAATGGGCGGTAGGCGTG-3′ and R: 5′-CGTCGCCGTCCAGCTCGACCAG-3′) were verified through bidirectional sequencing. All coding sequence (CDS) regions of ODC were correctly inserted into the pEGFP-N1 vector, indicating that the pEGFP-N1-ODC overexpression vector was successfully constructed. The transfection complex was prepared, and the compound was incubated at 25°C for 15 min and transfected into GCs.
qRT-PCR Was Used to Detect the Expression of Genes Associated With Polyamine Metabolism, Cell Proliferation and Apoptosis
Total RNA was extracted from GCs using TRIzol (Thermo Fisher Scientific). The cDNA templates were obtained from total RNA samples using a reverse transcription kit (Takara, Beijing, China). qRT-PCR detection was performed using a SYBR Green qPCR kit (Takara). The reaction system was: 95°C for 5 min, 95°C for 30 s, 55°C for 30 s, 72°C for 30 s, 39 cycles. The primer sequences are shown in Table 2. Gapdh was used as the internal standard, and 3 parallel replicates were performed for each sample. The 2−△△Ct method was used to calculate the Ct value based on the qRT-PCR.
Table 2.
List of primer sequences used for qRT-PCR.
| Genes | Primer sequences (5′ – 3′) | Product length (bp) | Annealing temperature (°C) | |
|---|---|---|---|---|
| ODC | F: | TGTATCTGCTTGACATTGGTGGTG | 146 | 60 |
| R: | CAGGAAGATACTATGTCGCATCAGC | |||
| OAZ1 | F: | ACTTCAGGAACCCTCGCATCAACT | 141 | 65 |
| R: | GCTGCCCTCATCTTTCTAATACGG | |||
| OAZ2 | F: | AAGCCTCATGTTGTCCACTTC | 142 | 63 |
| R: | GTGCTGATAACCCTTCTTTGC | |||
| SAMDC | F: | GCTTGACCCAGTAGTTATGGACCA | 180 | 55 |
| R: | TGAATAGTCCAGTAAGTTCCATCCG | |||
| AZIN1 | F: | GCTCTTACTGCACATTGCCACA | 180 | 58 |
| R: | TGAATGTACGTTTGCAGTTCCTTG | |||
| SPDS | F: | TCTGCTGCCAAGGTGAGTGC | 111 | 55 |
| R: | TAGGGATGGTGCAATAGGCGTA | |||
| SPMS | F: | GTGCTGATCCTTGGAGGTGGT | 110 | 58 |
| R: | TTACACCCGTCGATCACCATT | |||
| SSAT1 | F: | CACCCTTTCTACCACTGTCTG | 173 | 58 |
| R: | CCAATGCCAAGTCCTCTGT | |||
| APAO | F: | GAGTTTGAGCAACCCTTCTGG | 141 | 58 |
| R: | TGGCTGGAGGACCACAAA | |||
| SMO | F: | CTACCCACGGTGCTGTGCTTT | 124 | 59 |
| R: | GAATCGGGAGTTGGTGGTGTT | |||
| LHR | F: | GTAACACTGGAATAAGGGAAT | 191 | 54 |
| R: | GAAGGCTTGACTGTGGATA | |||
| ER | F: | ACCCAAACAGACCATTCAACGAA | 187 | 61 |
| R: | CGCCAGACTAAGCCAATCATCAG | |||
| FSHR | F: | TCCTGTGCTAACCCTTTCCTCTA | 207 | 59 |
| R: | AACCAGTGAATAAATAGTCCCATC | |||
| PR | F: | CCAGGATTTCGGAATTTAC | 187 | 55 |
| R: | GACACAGTGAATAGAACGATG | |||
| AR | F: | AGGAGTTTGGGTGGCTTCAGA | 201 | 55.7 |
| R: | GCTGGTAAAACCGCCTAGAGC | |||
| CCND1 | F: | TGTTTACGAGCCTGCCAAGAA | 109 | 55 |
| R: | CTGCTTCGTCCTCTACAGTCTTTG | |||
| PCNA | F: | AGAAATGAATGAGCCAGTCCAGC | 178 | 55 |
| R: | TTCAATCTTTGGAGCCAGGTAGT | |||
| SMAD1 | F: | AAGGGCTGCCGCATGTAATT | 146 | 55 |
| R: | CCGCTTGTAGTGGTAAGGATTGA | |||
| BAX | F: | GAAGCATTTACAGTTGCCATTACAG | 162 | 55 |
| R: | CCACAAGCAAGCAAAGAGCC | |||
| BCL-2 | F: | GATGCCTTCGTGGAGTTGTATG | 98 | 60 |
| R: | GCTCCCACCAGAACCAAAC | |||
| CASPASE 3 | F: | CTGGTATTGAGGCAGACAGTGG | 158 | 60 |
| R: | CAGCACCCTACACAGAGACTGAA | |||
| CASPASE 8 | F: | GGTGTCGCAGTTCAGGTA | 127 | 57 |
| R: | CATTGTAGTTTCAGGGCTT | |||
| CASPASE 9 | F: | TTCCAGGCTCTGTCGGGTAA | 150 | 64 |
| R: | GTCCAGCGTTTCCACATACCA | |||
Western Blotting Was Used to Detect ODC Expression, Cell Proliferation and Apoptosis-Related Proteins
GCs were cultured according to the above methods. After washing with PBS (Solarbio, Beijing, China) precooled at 4°C, RIPA lysate (Beyotime, Shanghai, China) containing protein inhibitor was added. The supernatant was analyzed using a BCA detection kit (Beyotime). Proteins were transferred to nitrocellulose membranes by 10% SDS-PAGE (Beyotime). Then, the cells were sealed for 2 h and incubated at 4°C for 13 h with primary anti-ODC (1: 1,000) (Abcam, Shanghai, China). The cell proliferation- and apoptosis-related protein antibodies and dilution ratios were as follows: anti-PARP (1: 1,000, Beyotime), anti-β-actin (1: 2,000, TransGen Biotech, Beijing, China), anti-Cyclin D1 (1: 1,000), anti-Bcl-2 (1: 1,000) and anti-Bax (1: 1,000) (Abcam). The goat anti-rabbit IgG labeled with 1: 2,000 diluted horseradish peroxidase was incubated at room temperature for 1 h and then washed with TBST (Beyotime). Finally, enhanced chemiluminescence reagent (Beyotime) was used for development on a gel imaging system instrument. Image Lab (Bio-Rad, Shanghai, China) was used to analyze the optical density levels and to calculate the relative protein content.
High-Performance Liquid Chromatography Was Used to Detect Polyamines
The polyamine content in the goose GCs was determined by high-performance liquid chromatography according to the methods of Kang et al. (2017b). The general steps were as follows. First, polyamine standard curves were prepared. Goose GCs were ultrasonically lysed, and 1 mL of 5% perchloric acid and 10 μL of 1,6-hexanediamine (Sigma, Shanghai, China) standard working solution were added. Then, the samples were sonicated for 10 min and centrifuged, and the supernatant was collected. Then, the samples were extracted again with 1 mL of 5% perchloric acid, the supernatant was removed, an equal volume of 2.5 mol/L NaOH and 7 μL of benzoyl chloride was added, and the samples were derivatized at 40°C for 1 h. The samples were adjusted to a neutral pH with 6 mol/L HCl, and a HyperSep C18 extraction column (Thermo Fisher Scientific) was used to extract and separate the derivative products. The extraction column was washed with 15 mL of ultrapure water and 15 mL of 15% (v/v) chromatographic grade aqueous methanol solution to purify the derivative products. Methanol was added to the extraction column for elution. The methanol: ultrapure water ratio was 62: 38 (v/v), and the column temperature of the Hypurity C18 chromatographic separation column was 40°C. The results were compared with the standard curve of polyamine.
Hormone (E2 and P4) Detection in the Culture Medium
According to the instructions of the goose estradiol (E2) ELISA kits (Qisong Biological Technology, Beijing, China) and progesterone (P4) ELISA kits (Qisong Biological Technology), the cell culture medium was collected and centrifuged at 4,000 r/min for 15 min. Then, horseradish peroxidase detection antibody was added to the sample wells and standard product wells, which were incubated at 37°C for 1 h, filled with washing buffer for 1 min and washed 5 times. Substrates A and B were added to each well, and the OD value was detected. The concentration of each sample was calculated according to the curve equation. The assay sensitivity was as follows: the minimum detection level of E2 was 1.0 pg/mL, and the maximum detection level was 480 pg/mL. The minimum detection level of P4 was 0.1 ng/mL and the maximum detection level was 8 ng/mL. The inter- and intra-assay coefficients of variability ≤7%. It showed that the experiment had good repeatability.
Analysis of Cell Proliferation in Transfected Granulosa Cells by MTT Assay
GCs were cultured in 96-well plates (n = 6), and the control and test groups were transfected as described above. Forty-eight or twenty-four hours after transfection of the interference and overexpression plasmids, 0.5 mg/mL MTT reagent (Beyotime) was added, and the cells were incubated for 4 h before discarding the DMEM. One hundred fifty μL/well DMSO (Beyotime) was added. A microplate reader was used to detect the OD value at 490 nm, and zero adjustment holes were set up (DMEM, MTT and DMSO). Calculated cell activity = (OD treatment – OD adjustment) / (OD control – OD adjustment).
Flow Cytometry Was Used to Detect the Apoptosis of Transfected GCs
According to the instructions of the Apoptosis Detection Kit (BD Biosciences, Shanghai, China), after the GCs were transfected, the cell culture medium was removed from the centrifuge tube. The cells were digested with trypsin after washing with 4°C precooled PBS (Solarbio). The cell culture medium collected before was added to stop digestion. After centrifugation, the cells were washed with precooled PBS once. The cells were resuspended in 1 × binding buffer, and the suspension was filtered through a strainer to adjust the cell concentration to 1 × 106/mL. Annexin V-FITC and PI staining solution was added to the cell suspension, mixed with 1 × binding buffer, and analyzed with flow cytometry. Analysis was performed by using the flow cytometry software CytExpert 2.0.
Statistical Analysis
The MEANS process in SPSS software (SPSS Inc., USA) was used for statistical analysis. ANOVA was used to compare multiple groups, Duncan's multiple comparisons test was used to identify significant relationships. GraphPad Prism 6 was applied for graphing, and the results were shown as the mean ± SEM. P < 0.05 * represents a significant difference, and P < 0.01 ** represents an extremely significant difference.
RESULTS
Effects of ODC Interference on the Levels of ODC mRNA and Protein in the Ovarian Granulosa Cells of Geese
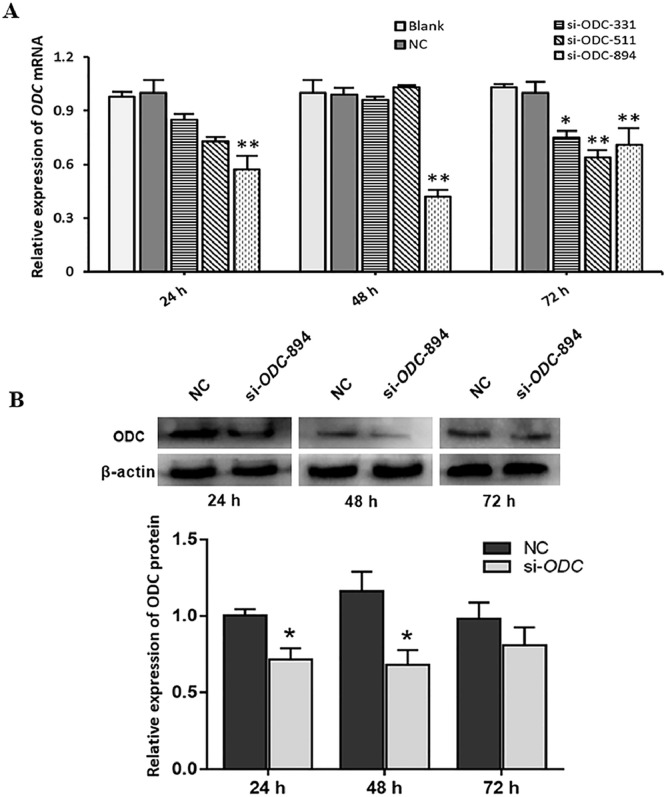
si-ODC-331, si-ODC-511 and si-ODC-894 were transfected into GCs for 24, 48, and 72 h, respectively. The interference efficiency of ODC was the highest when transfected with si-ODC-894 plasmid for 48 h, which was much lower than that of the Blank and NC groups (P < 0.01) (Figure 1A). Then, western blot analysis was utilized to further verify the level of ODC protein after the transfection of si-ODC-894 at different times. Figure 1B shows that the protein expression decreased significantly when ODC was interfered with for 48 h (P < 0.05), which was aligned with the results acquired by qRT-PCR. Therefore, si-ODC-894 transfected for 48 h was selected as the subsequent experimental condition.
Figure 1.
Levels of ODC mRNA and protein in GCs after transfection with ODC siRNA. (A) Expression of ODC mRNA after transfection of three si-ODC plasmids at different times, (B) Expression of ODC protein in GCs at different times. * P < 0.05, ** P < 0.01.
Effects of ODC Overexpression on the Levels of ODC mRNA and Protein in Goose Ovarian Granulosa Cells
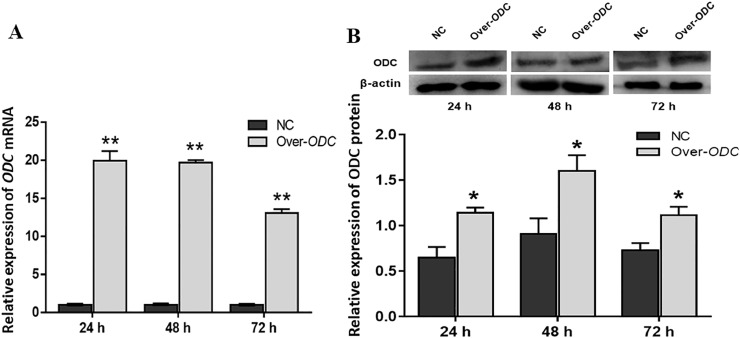
As shown in Figure 2A, when the ODC overexpression plasmid was transfected into GCs for 24, 48, and 72 h, ODC expression was considerably upregulated by 19.9 times, 19.7 times, and 13.1 times, respectively (P < 0.01). Figure 2B shows that after 24 h of overexpression, the protein level of ODC was significantly upregulated (P < 0.05), which was consistent with the results obtained by qRT-PCR. Thus, the transfection of GCs with the ODC overexpression plasmid for 24 h was selected as the subsequent experimental condition.
Figure 2.
Expression levels of ODC mRNA and protein in GCs after transfection with the ODC plasmid. (A) Expression of ODC mRNA after transfection of the overexpression plasmid at different times, (B) Expression of ODC protein in GCs. * P < 0.05, ** P < 0.01.
Effects of ODC on Polyamine Contents and Polyamine Metabolism-Related Gene Expression in GCs
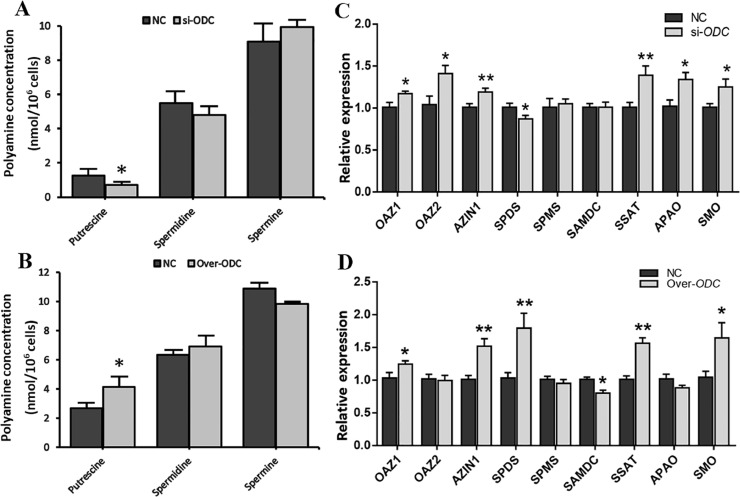
Upon ODC interference, the putrescine content in GCs was significantly decreased (P < 0.05) (Figure 3A). When ODC was overexpressed, the putrescine content in GCs was greatly increased (P < 0.05) (Figure 3B). Both the concentrations of spermidine and spermine were not significantly different under ODC interference or overexpression conditions (P > 0.05).
Figure 3.
Effect of ODC on polyamine content (A, B) and the expression of polyamine metabolism-related genes in GCs (C, D). (A, C) ODC interference, (B, D) ODC overexpression. * P < 0.05, ** P < 0.01.
Then, we studied the effect of ODC interference and overexpression on the gene expression of polyamine-metabolizing enzymes in GCs. The change in the expression of SPMS (spermine synthase) and SAMDC was not significant after interference with ODC (P > 0.05), while OAZ1, OAZ2, APAO, and spermine oxidase (SMO) gene expression was significantly upregulated (P < 0.05), antizyme inhibitor 1 (AZIN1) and SSAT expression was extremely significantly upregulated (P < 0.01), SPDS expression was remarkably decreased (P < 0.05) (Figure 3C). OAZ2, SPMS and APAO (acetyl-polyamine oxidase) expression was not significantly changed when ODC was overexpressed in GCs (P > 0.05), the expression of OAZ1 and SMO was markedly increased (P < 0.05), SPDS, AZIN1 and SSAT gene level was greatly significantly upregulated (P < 0.01), while SAMDC (s-adenosylmethionine decarboxylase) expression was greatly reduced (P < 0.05) (Figure 3D).
Effect of ODC on the Gene Expression of Reproductive Hormone Receptors in GCs and P4 and E2 Levels in Culture Medium
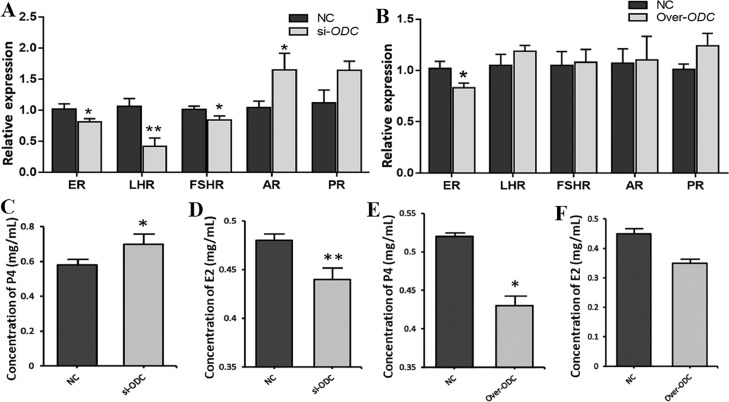
After interfering with ODC, the expression of ER and FSHR genes in GCs was greatly decreased (P < 0.05), while AR gene was markedly increased (P < 0.05), LHR gene was extremely significantly downregulated (P < 0.01) (Figure 4A). The P4 level in the culture medium was remarkably increased (P < 0.05) (Figure 4C), and the E2 content was tremendously decreased (P < 0.01) (Figure 4D). As shown in Figure 4B, when ODC was overexpressed, the expression of the ER gene in GCs was markedly downregulated (P < 0.05). LHR, FSHR, AR, and PR were not remarkably impacted (P > 0.05). The P4 level was significantly decreased (P < 0.05) (Figure 4E), while the E2 level did not markedly change (P > 0.05) (Figure 4F).
Figure 4.
Effect of ODC on the expression of reproductive hormone receptor genes (A, B) in GCs and on the levels of P4 (C, E) and E2 (D, F) in culture medium. (A, C, D) ODC interference, (B, E, F) ODC overexpression. * P < 0.05, ** P < 0.01.
The Viability of GCs Was Analyzed After ODC Interference and Overexpression
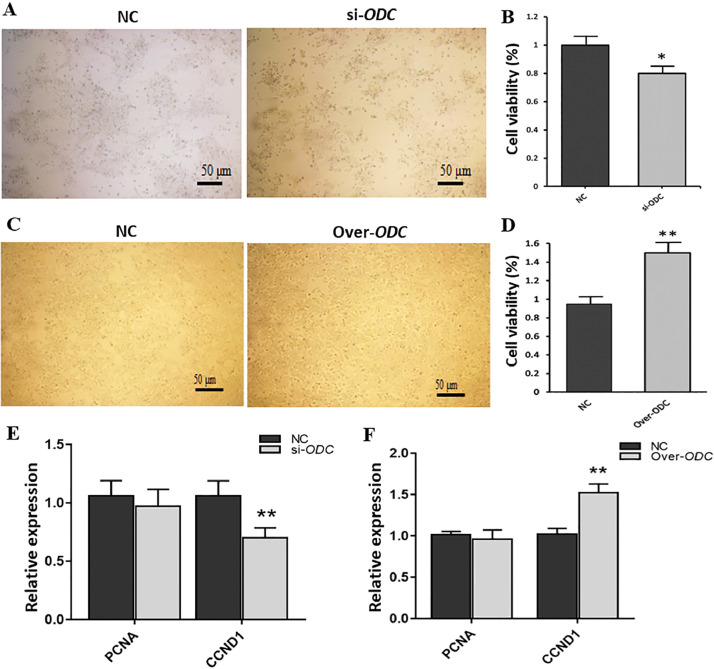
After ODC expression interference in goose ovarian GCs, the number of decreased and dead GCs was observed under the microscope and compared with that of the NC group (Figure 5A). MTT results showed that granulosa cell activity was remarkably reduced (P < 0.05) (Figure 5B). As shown in Figure 5C, after ODC was overexpressed, the number of GCs was significantly increased, and the cell morphology was fuller than that of the NC group. MTT results indicated that the activity of GCs was greatly increased after ODC overexpression (P < 0.01) (Figure 5D). Then, we explored the effects of ODC on proliferation-related proteins. Figure 5E and 5F show that the protein level of CCND1 decreased significantly when ODC was downregulated (P < 0.01), and the protein level of CCND1 was considerably increased when ODC was upregulated (P < 0.01).
Figure 5.
Effects of ODC on the morphology (A, C) and viability of goose GCs (B, D) and (E, F) cell proliferation-related genes. (A, B, E) ODC interference, (C, D, F) ODC overexpression. * P < 0.05, ** P < 0.01.
Effect of ODC Interference and Overexpression on the Apoptosis of GCs
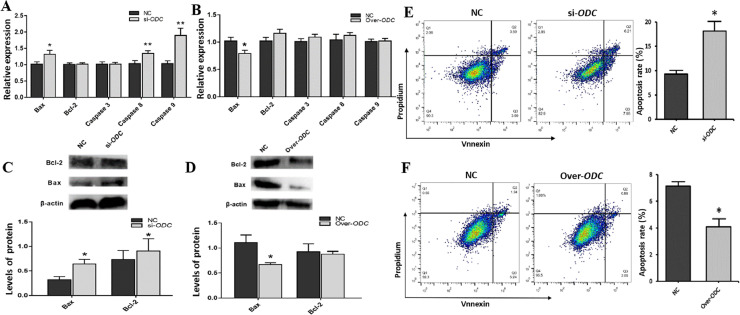
qRT-PCR was applied to detect the expression of cell apoptosis-related genes after ODC interference and overexpression. As shown in Figure 6A, under ODC expression interference, the relative expression of BAX mRNA was greatly upregulated (P < 0.05), the relative expression of CASPASE 8 and CASPASE 9 mRNA was markedly significantly upregulated (P < 0.01). When ODC was overexpressed (Figure 6B), the expression of the proapoptotic gene BAX was remarkably reduced (P < 0.05), and that of BCL-2, CASPASE 3, CASPASE 8 and CASPASE 9 was not significantly changed (P > 0.05).
Figure 6.
Effect of ODC on the apoptosis of GCs. (A, B) The expression of cell apoptosis-related genes, (C, D) and proteins in GCs, and (E, F) analysis of the cell apoptosis rate by flow cytometry. (A, C, E) ODC interference, (B, D, F) ODC overexpression. * P < 0.05, ** P < 0.01
Western blotting was applied to assess the influence of ODC interference and overexpression on the protein levels of apoptosis-related genes in GCs. BAX and BCL-2 increased significantly after ODC interference (P < 0.05) (Figure 6C). BAX was markedly reduced after ODC overexpression (P < 0.05), whereas BCL-2 showed no notable change (P > 0.05) (Figure 6D).
Furthermore, flow cytometry was utilized to test the influence of ODC interference and overexpression on the apoptosis of GCs. The apoptosis rate of GCs was greatly upregulated after interfering with ODC (P < 0.05) (Figure 6E), and the apoptosis rate of GCs was significantly downregulated when ODC was overexpressed (P < 0.05) (Figure 6F).
DISCUSSION
ODC is the pivotal rate-limiting enzyme for polyamine biosynthesis, and its activity can directly regulate the content of polyamines in cells. Studies have shown that changes in ODC expression and biological activity directly affect the contents of polyamine, which plays a crucial role in cell apoptosis, proliferation, and animal reproduction (Arisan et al., 2012; Wei et al., 2013). In this study, when GCs were transfected with the si-ODC-894 plasmid for 48 h, the mRNA and protein expression levels of ODC were remarkably reduced (P < 0.05). When the overexpressed ODC plasmid was transfected for 24 h, the mRNA and protein expression levels of ODC were considerably increased (P < 0.05), indicating that ODC interference and overexpression in GCs were successfully achieved.
Studies have reported that after ODC overexpression, the ODC activity in HEK293 cells increased by 100 times, and the content of putrescine increased by 10 times, but the concentrations of spermidine and spermine were only slightly reduced (Wilson et al., 2005). This experiment found that the content of putrescine in GCs increased significantly after the overexpression of ODC. In previous studies that transfected the ODC overexpression vector into H9c2 cells, ODC activity was significantly increased, and putrescine and spermidine levels were also significantly increased (Govoni et al., 2010). In this work, interfering with the expression of ODC reduced the content of putrescine in GCs, while the content of spermidine and spermine did not change significantly. Furthermore, putrescine content was significantly reduced after interfering with ODC expression in MCF7 breast cancer cells (Gupta et al., 2016). This experiment indicated that ODC expression changes mainly affected the putrescine content in GCs and had a small influence on the concentration of spermidine and spermine.
Previous studies found that the level of OAZ1 mRNA in the ovaries of laying geese was markedly higher than that in the ovaries of prelaying geese. This result indicated that the high expression of OAZ1 disrupted polyamine homeostasis by suppressing ODC activity and inhibited follicular development (Bo et al., 2017). At present, there is no direct evidence that ODC levels can affect OAZ1 gene expression, but ODC and OAZ1 are synergistically expressed in GCs, suggesting that enhanced ODC expression may cause corresponding changes in OAZ1 expression. Yue et al. (2008) showed that the overexpression of ODC can promote the expression of OAZ1 in endometrial cells, which corresponded with the results of this research. SSAT is regarded as the rate-limiting enzyme of polyamine catabolism. It has been confirmed in many cell lines that polyamine can promote the expression of SSAT (Fogel et al., 1996). This study found that putrescine content was reduced and SPDS gene expression was inhibited after interfering with ODC. Zhu et al. (2012) interfered with ODC gene expression in MCF7 and T47D cells and found that ODC protein and mRNA levels were remarkably downregulated while the genes expression of SMO and SSAT was upregulated, and the protein expression level of SMO was increased. This finding was consistent with the results of our experiment. After interfering with the ODC gene in goose GCs, in addition to upregulating the expression of the SMO and SSAT genes, the expression of the APAO gene also increased significantly. In this work, OAZ1 and OAZ2 expression increased greatly after ODC expression interference, and the expression of the SPMS and AZIN genes also increased significantly. SAMDC was considered to be the most important polyamine synthetase after ODC (Thomas et al., 2003). Studies have found that both ODC knockout and SAMDC knockout are lethal in mouse embryos (Pendeville et al., 2001; Nishimura et al., 2002). In this experiment, the interference or overexpression of ODC affected the gene expression of polyamine metabolism enzyme in GCs. Accordingly, we speculated that the protein and mRNA levels of ODC were significantly changed, resulting in changes in the content of putrescine in cells and in the stimulation of GCs, which affected the changes in the gene expression of polyamine metabolism enzymes.
ODC activity in mammalian ovaries was reported to transiently increase as oocytes mature in vivo, and oocyte maturation and development are regulated by GCs. In the early pregnancy of hamsters and rats, treatment with progesterone receptor antagonists can result in decreased ODC activity and eventually lead to miscarriage, which indicates that ODC activity in the ovary is closely related to hormones (Galliani et al., 1986; Jian et al., 2001). This study found that after interfering with ODC, the E2 level was remarkably reduced, and ER expression was greatly decreased. After the overexpression of the ODC gene, the E2 level in the medium did not change significantly, and ER expression decreased significantly. It is speculated that the expression of E2 and ER was affected not only by ODC but also by the content of polyamines in GCs. However, the detailed mechanism remains to be elucidated in the future. Poultry ovaries cannot form a corpus luteum after ovulation, poultry P4 is mainly produced by follicular GCs (Yang et al., 2005), and poultry follicles also have the ability to secrete P4 after ovulation (Estienne et al., 2020). Studies have shown that low levels of P4 can mediate an increase in low-frequency LH pulses, prolonging the duration of the dominant follicles and thus affecting the maturation and quality of oocytes (Savio et al., 1993), while high levels of P4 can inhibit the synthesis of luteinizing hormone and ultimately cause atresia of the dominant follicles (Gu and Zhao, 2015). This study showed that upon ODC interference, the level of P4 hormone in the culture medium of granulosa cells was remarkably increased. Moreover, studies have confirmed that PR genes play a critical role in regulating the development and reproduction of animal reproductive systems (Fahlén et al., 2018; Singhal et al., 2018). Bastida et al. (2002) found that after specifically using DFMO to inhibit ODC in the ovary before ovulation in mice, the level of progesterone in the ovary decreased, and then the level of progesterone in the serum of interestrus decreased. The above research showed that the ODC gene can regulate animal reproductive function by mediating progesterone production and its receptor gene expression.
Previous research found that ODC is an indispensable factor in yeast, and the loss of the ODC gene in yeast results in growth stagnation (Schwartz et al., 1995). This experiment demonstrated that the expression of BAX, CASPASE 8 and CASPASE 9 was considerably increased under ODC interference, and cell activity was significantly decreased, suggesting that ODC gene expression interference may upregulate BAX, CASPASE 8 and CASPASE 9 at the transcriptional level and promote cell apoptosis. He et al. (2017) found that interfering with ODC gene expression in esophageal phosphorus cells reduced the levels of PCNA and CCNB1, thereby inhibiting the proliferation of cells, significantly increasing the level of activated CASPASE 3 protein, and promoting the apoptosis of esophageal phosphorus cells. In HL-60 cell lines, the overexpression of ODC can inhibit the apoptosis induced by toxic carotene and maintain BCL-2 expression (Wei et al., 2010). CCND1 is the core component of cell cycle regulation and can promote the cell proliferation cycle. The results of this experiment showed that CCND1 expression was significantly decreased after interfering with ODC gene expression, and the expression levels of CCND1 were significantly increased after transfection with the ODC overexpression vector, which indicated that the upregulation of ODC promoted the differentiation and proliferation of GCs. When ODC was downregulated, granulosa cell activity was significantly reduced, downregulating the BCL-2 / BAX protein ratio (P < 0.05); when ODC was overexpressed, the activity of GCs was increased greatly, and the BCL-2 / BAX protein ratio was upregulated (P < 0.05). Overexpression of ODC can block the apoptosis induced by dibenzoylmethane in HL-60 cell lines, mainly by blocking the activation of CASPASE 9, reducing BAX expression, and upregulating the BCL-2 / BAX ratio (Wu et al., 2011). Studies have shown that the BAX protein cannot stimulate the release of cytochrome C. However, the oligomers formed by the transfer of BAX from the cytoplasm to the mitochondrial membrane form multimers with BCL-2 to enhance mitochondrial permeability, contributing to the release of cytochrome C and activating CASPASE 9. The enzymolysis cascade of the Caspase protease family is activated, which eventually leads to cell apoptosis. Overexpression of ODC may affect the cell cycle, and ODC activity increases in many cell types during the G1 phase (Kaczmarek et al., 1987; Fredlund and Oredsson, 2010). Inhibition of ODC activity by polyamine inhibitors or analogs produces different effects at different stages of the cell cycle (Pohjanpelto et al., 2010; Laitinen et al., 2015). Upregulation of ODC in the skin of transgenic mice can stimulate cell proliferation and enhance the activity of CCNE / CDK2- and CCNA / CDK2-associated kinases (Fredlund and Oredsson, 2010). Moreover, the flow cytometry results of this work illustrated that the apoptosis rate of GCs was markedly increased after interfering with ODC (P < 0.05), and when ODC was overexpressed, the apoptosis rate was significantly reduced (P < 0.05). The above results suggested that ODC accelerated the proliferation of goose ovarian GCs and suppressed cell apoptosis.
In conclusion, our study successfully achieved ODC interference and overexpression in goose ovarian GCs, and ODC mainly regulated the putrescine content in GCs and had little influence on spermidine and spermine. Furthermore, ODC participated in the adjustment of P4 levels in GCs, promoted GC proliferation and inhibited cell apoptosis.
ACKNOWLEDGMENTS
Funding was provided by the National Natural Science Foundation of China (31872358 and 31702116) and the Scientific Research Fund of Sichuan Provincial Education Department (18ZB0469)
DISCLOSURES
The authors declare no financial or personal conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101226.
Appendix. Supplementary materials
REFERENCES
- Arisan E.D., Obakan P., Coker A., Narcin P. Inhibition of ornithine decarboxylase alters the roscovitine-induced mitochondrial-mediated apoptosis in MCF-7 breast cancer cells. Mol. Med. Rep. 2012;5:1323–1329. doi: 10.3892/mmr.2012.786. [DOI] [PubMed] [Google Scholar]
- Armstrong D.G. Changes in ornithine decarboxylase activity in ovarian follicles of the laying hen (Gallus domesticus) during the ovulatory cycle. J. Endocrinol. 1986;110:211–216. doi: 10.1677/joe.0.1100211. [DOI] [PubMed] [Google Scholar]
- Bastida C.M., Tejada F., Cremades A., Rafael P. The preovulatory rise of ovarian ornithine decarboxylase is required for progesterone secretion by the corpus luteum. Biochem. Bioph. Res. Co. 2002;293:106–111. doi: 10.1016/S0006-291X(02)00191-2. [DOI] [PubMed] [Google Scholar]
- Bo K., Dongmei J., Hui H., Rong M., Zhixin Y., Ziyu C. Characterization of OAZ1 and its potential functions in goose follicular development. Electron. J. Biotechn. 2017;26:1–6. [Google Scholar]
- Elmetwally M.A., Lenis Y., Tang W., Fuller W.B. Effects of catecholamines on secretion of interferon tau and expression of genes for synthesis of polyamines and apoptosis by ovine trophectoderm. Biol. Reprod. 2018;99:611–628. doi: 10.1093/biolre/ioy085. [DOI] [PubMed] [Google Scholar]
- Estienne A., Brossaud A., Reverchon M. Adipokines expression and effects in oocyte maturation, fertilization and early embryo development: lessons from mammals and birds. Int J. Mol. Sci. 2020;21:3581. doi: 10.3390/ijms21103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlén M., Zhang H., Löfgren L., Britt M., Von S.E. Expression of progesterone and androgen receptors in the breast of premenopausal women, considering menstrual phase. Anticancer. Res. 2018;38:1499. doi: 10.21873/anticanres.12377. [DOI] [PubMed] [Google Scholar]
- Fogel P.M., Vujcic S., Brown P.J., Mari K.H., Carl W.P. Effects of polyamines, polyamine analogs, and inhibitors of protein synthesis on spermidine-spermine N1-acetyltransferase gene expression. Biochemistry. 1996;35:14436–14444. doi: 10.1021/bi9612273. [DOI] [PubMed] [Google Scholar]
- Fredlund J.O., Oredsson S.M. Impairment of DNA replication within one cell cycle after seeding of cells in the presence of a polyamine-biosynthesis inhibitor. Eur. J. Biochem. 2010;237:539–544. doi: 10.1111/j.1432-1033.1996.0539p.x. [DOI] [PubMed] [Google Scholar]
- Galliani G., Luzzani F., Colombo G., Conz A., Mistrello L., Barone D. On the mode of action of a new contragestational agent (DL 111-IT) Contraception. 1986;33:263–283. doi: 10.1016/0010-7824(86)90019-3. [DOI] [PubMed] [Google Scholar]
- Govoni M., Bonavita F., Shantz L.M., Carlo G., Emanuele G. Overexpression of ornithine decarboxylase increases myogenic potential of H9c2 rat myoblasts. Amino Acids. 2010;38:541–547. doi: 10.1007/s00726-009-0415-8. [DOI] [PubMed] [Google Scholar]
- Gu J.M., Zhao J.Z. The effect of progesterone on the development of endometrium, egg cells and embryos. Inter. J. Reprod. Heal. /Famy. Plann. 2015;34:239–242. [Google Scholar]
- Gupta E.D., Pachauri M., Ghosh P.C., Rajam M.V. Targeting polyamine biosynthetic pathway through RNAi causes the abrogation of MCF 7 breast cancer cell line. Tumor. Biol. 2016;37:1159–1171. doi: 10.1007/s13277-015-3912-2. [DOI] [PubMed] [Google Scholar]
- He W., Roh E., Yao K., Kang D.L., Xing M. Targeting ornithine decarboxylase (ODC) inhibits esophageal squamous cell carcinoma progression. N. P. J. Precis. Oncol. 2017;1:13. doi: 10.1038/s41698-017-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.P., Rohrwasser A., Terreros D.A., Raymond F.G., John F.A. Discovery of a spermatogenesis stage-specific ornithine decarboxylase antizyme: antizyme 3. P. Natl. Acad. Sci. USA. 2000;97:4808–4813. doi: 10.1073/pnas.070055897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian Z.S., Hui J.Z., Qiao J.H., Li M.W., Rui Y.F. Effects of DL111-IT or combined with RU486 on uterine polyamines biosynthesis in rats during early gestation. Contraception. 2001;63:283–287. doi: 10.1016/s0010-7824(01)00197-4. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L., Calabretta B., Ferrari S., Jon K.D.R. Cell-cycle-dependent expression of human ornithine decarboxylase. J. Cell. Physiol. 1987;132:545. doi: 10.1002/jcp.1041320318. [DOI] [PubMed] [Google Scholar]
- Kang B., Jiang D., Ma R., Hui H., Zhi X.Y., Zi Y.C. OAZ1 knockdown enhances viability and inhibits ER and LHR transcriptions of granulosa cells in geese. Plos One. 2017;12 doi: 10.1371/journal.pone.0175016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B., Jiang D.M., He H., Rong M., Zi Y.C., Zhi X.Y. Effect of Oaz1 overexpression on goose ovarian granulosa cells. Amino Acids. 2017;49:1–10. doi: 10.1007/s00726-017-2411-8. [DOI] [PubMed] [Google Scholar]
- Koehler K.E., Schrump S.E., Cherry J.P., Terry J.H., Patri4cia A.H. Near-human aneuploidy levels in female mice with homeologous chromosomes. Curr. Biol. 2006;16:579–580. doi: 10.1016/j.cub.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Laitinen J., Stenius K., Eloranta T.O., Erkki H. Polyamines may regulate S-phase progression but not the dynamic changes of chromatin during the cell cycle. J. Cell. Biochem. 2015;68:200–212. doi: 10.1002/(sici)1097-4644(19980201)68:2<200::aid-jcb7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lane D.J.R., Bae D.H., Siafakas A.R., Yohan S.R., Lina A., Patric J.J. Coupling of the polyamine and iron metabolism pathways in the regulation of proliferation: Mechanistic links to alterations in key polyamine biosynthetic and catabolic enzymes. BBA-Mol. Basis. Dis. 2018;1864:2793–2813. doi: 10.1016/j.bbadis.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N.K., Skinner J.P., Zajac J.D., Helen E.M. Ornithine decarboxylase is upregulated by the androgen receptor in skeletal muscle and regulates myoblast proliferation. Am. J. Physiol. Endocrinol. Metab. 2011;301:172–179. doi: 10.1152/ajpendo.00094.2011. [DOI] [PubMed] [Google Scholar]
- Liu X.J. Targeting oocyte maturation to improve fertility in older women. Cell Tissue Res. 2016;363:57–68. doi: 10.1007/s00441-015-2264-y. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Nakatsu F., Kashiwagi K., Hiroshi O., Saito T., Kazuei I. Essential role of S-adenosylmethionine decarboxylase in mouse embryonic development. Genes Cells. 2002;7:41–47. doi: 10.1046/j.1356-9597.2001.00494.x. [DOI] [PubMed] [Google Scholar]
- Pegg A.E. Regulation of ornithine decarboxylase. J. Biol. Chem. 2006;281:14529–14532. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- Pendeville H., Carpino N., Marine J.C., Yutaka T., Marc M., Joseph M. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol. Cell. Biol. 2001;21:6549. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjanpelto P., Nordling S., Knuutila S. Flow cytometric analysis of the cell cycle in polyamine-depleted cells. Cytometry. 2010;16:331–338. doi: 10.1002/cyto.990160407. [DOI] [PubMed] [Google Scholar]
- Savio J.D., Thatcher W.W., Morris G.R., Mattiacci M.R. Effects of induction of low plasma progesterone concentrations with a progesterone-releasing intravaginal device on follicular turnover and fertility in cattle. J. Reprod. Fertil. 1993;98:77–84. doi: 10.1530/jrf.0.0980077. [DOI] [PubMed] [Google Scholar]
- Schwartz B., Hittelman A., Daneshvar L., Hirak S.B. A new model for disruption of the ornithine decarboxylase gene, SPE1, in Saccharomyces cerevisiae exhibits growth arrest and genetic instability at the MAT locus. Biochem. J. 1995;312:83. doi: 10.1042/bj3120083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal H., Greene M.E., Zarnke A.L., Muriel L., Rose A.A. Progesterone receptor isoforms, agonists and antagonists differentially reprogram estrogen signaling. Oncotarget. 2018;9:4282–4300. doi: 10.18632/oncotarget.21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobe R.C., Bond W.G., Wotanis C.K., Josiah P.Z., Marybeth A.B., Nicolas L.F. Spermine inhibits Vibrio cholerae biofilm formation through the NspS-MbaA polyamine signaling system. J. Biol. Chem. 2017;292:17025–17036. doi: 10.1074/jbc.M117.801068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Thomas T.J. Polyamine metabolism and cancer. J. Cell. Mol. Med. 2003;7:113–126. doi: 10.1111/j.1582-4934.2003.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Chun X.X., Guo S.H., You G.C., Jia X.X., Xian X.L. Downregulation of tumstatin expression by overexpression of ornithinedecarboxylase. Oncol. Rep. 2013;30:2042–2048. doi: 10.3892/or.2013.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C.H., Pei C.H., Ya F.L., Chih L.L. Overexpression of ornithine decarboxylase suppresses thapsigargin-induced apoptosis. Mol. Cells. 2010;30:311–318. doi: 10.1007/s10059-010-0120-1. [DOI] [PubMed] [Google Scholar]
- Wilson S.M., Iii L.H., Pastorian K.E., Craig V.B. A stable, inducible, dose-responsive ODC overexpression system in human cell lines. BBA Gene Struct. Expr. 2005;1732:103–110. doi: 10.1016/j.bbaexp.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Wu C.L., Liao Y.F., Hung Y.C., Hsiu L.K., Hung H.C. Ornithine decarboxylase prevents dibenzoylmethaneâinduced apoptosis through repressing reactive oxygen species generation. J. Biochem. Mol. Toxic. 2011;25:312–319. doi: 10.1002/jbt.20391. [DOI] [PubMed] [Google Scholar]
- Yang S.H., Xu S.W., Li J.L. The effect of reproductive hormones on the apoptosis of poultry follicle cells. Jilin Ani. Harv. Veteri. 2005;10:12–17. [Google Scholar]
- Yong T., Dandan L., Guolong M., Hong M.W., Liu X.J. Peri-ovulatory putrescine supplementation reduces embryo resorption in older mice. Hum. Reprod. 2015;30:1867–1875. doi: 10.1093/humrep/dev130. [DOI] [PubMed] [Google Scholar]
- Yuan Q., Ray R.M., Johnson L.R. Polyamine depletion prevents camptothecin-induced apoptosis by inhibiting the release of cytochrome c. Am J Physiol. Cell Physiol. 2002;282:1290–1297. doi: 10.1152/ajpcell.00351.2001. [DOI] [PubMed] [Google Scholar]
- Yue C.Z., Yu J.C., Yong S.Y., Ji L.L., Ren W.S., Xing H.M. Polyamines are essential in embryo implantation: expression and function of polyamine-related genes in mouse uterus during peri-implantation period. Endocrinology. 2008;149:2325–2332. doi: 10.1210/en.2007-1420. [DOI] [PubMed] [Google Scholar]
- Zhu Q., Jin L., Casero R.A., Nancy E.D., Yi H. Role of ornithine decarboxylase in regulation of estrogen receptor alpha expression and growth in human breast cancer cells. Breast. Cancer. Res. Tr. 2012;136:57–66. doi: 10.1007/s10549-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.