Abstract
Purpose
To determine whether Mel4-coated antimicrobial contact lenses (MACLs) can reduce the incidence of corneal infiltrative events (CIEs) during extended wear.
Methods
A prospective, randomized, double-masked, single-center, contralateral, extended contact lens wear clinical trial was conducted with 176 subjects. Each participant was randomly assigned to wear a MACL in one eye and an uncoated control contact lens in the contralateral eye or an extended-wear biweekly disposable modality for 3 months. The main outcome measures were the incidence of CIEs per 100 eye-months, identification of the microbial types colonizing the contact lenses or eyes at the time of the CIEs, and their susceptibility to Mel4.
Results
Nine participants (5.1%) experienced unilateral CIEs; six participants had contact lens acute red eye, and three participants had infiltrative keratitis. The incidence rate for CIEs (0.4 events per 100 participant months; 1.7%) in the Mel4-coated lenses (test) was 69% less than that of the control lenses (1.3 events per 100 participant months; 3.4%; P = 0.29). All Gram-negative bacteria isolated from lenses and lids of participants with CIEs (Citrobacter diversus, Acinetobacter haemolyticus, and Acinetobacter lwoffii) were susceptible to Mel4 peptide; minimum inhibitory concentrations ranged from 15.6 to 62.5 µg/mL. Reduction of adhesion of these bacteria by Mel4-coated lenses ranged from 2.1 to 2.2 log10 colony-forming units/lens.
Conclusions
MACLs had the capacity to reduce CIEs by at least 50% compared with uncoated control lenses during extended wear over 3 months; however, due to the relatively low rates of CIEs, the reduction was not statistically different compared with control lenses.
Translational Relevance
This study provides evidence that antimicrobial contact lenses have the potential to reduce the incidence of corneal infiltrative events during extended wear.
Keywords: antimicrobial peptide, Mel4 peptide, antimicrobial contact lenses, corneal infiltrative events, clinical trial
Introduction
Microbial contamination of contact lenses may lead to corneal infection or inflammatory events such as microbial keratitis (MK), contact lens acute red eye (CLARE) and contact lens peripheral ulcers (CLPUs).1–4 Pseudomonas aeruginosa is the most common microbe associated with MK during contact lens wear.5,6 CLARE is commonly caused by Gram-negative colonization of contact lenses,7–9 and CLPUs are commonly associated with Gram-positive contamination of contact lenses.9,10 Collectively, along with infiltrative keratitis (IK), which can also be associated with microbial contamination of lenses,1,7 these adverse events are known as microbially driven corneal infiltrative events (CIEs).
The incidence of MK per year for extended wear of contact lenses is approximately 20 per 10,000 wearers; for daily wear, it is approximately three per 10,000 wearers.11–15 The incidence of other bacterially driven CIEs is approximately 0.5 to 26.7 per 100 wearers during extended wear 16–19 and 0 to 25.5 per 100 eyes during daily wear.3,17,20,21 The use of silicone hydrogel lenses has been associated with a two-times greater risk of CIEs.18
Inhibition of microbial adhesion to contact lenses during wear is likely to reduce the incidence of CIEs. Several potential antimicrobial contact lenses have been produced, although most have not progressed to human clinical trials. Impregnation of silver into etafilcon A hydrogel contact lenses reduced microbial colonization in an in vitro study.22 Selenium (Se) covalently coated onto balafilcon A silicone hydrogel lenses reduced bacterial colonization in vitro while not adversely affecting the corneal health of rabbits in vivo.23 The overall clinical performance of the Se-coated lenses was comparable to that of the commercially available balafilcon A lens, and the efficacy of Se-coated lenses was maintained after 24 hours of extended wear.24 Fimbrolide covalently attached to lotrafilcon A (silicone hydrogel) contact lenses showed good antibacterial activity, and there were no significant differences in ocular responses to fimbrolide-coated lenses compared with uncoated control lenses in either a 1-month animal trial or an overnight human trial.25 The arginine- and lysine-rich, 29-amino-acid peptide melimine, when immobilized on contact lenses, reduced the severity and incidence of CLARE, CLPUs, and MK in animal models and was not cytotoxic to mammalian cells in vitro.26–28 Clinical responses to wearing melimine-coated hydrogel contact lenses did not differ from those to wearing uncoated control lenses in animal safety trials; in human trials, an increase in punctate fluorescein corneal staining after 1 day of wear has been reported.27,28 Due to the increase in punctate fluorescein corneal staining, a shorter derivative of melimine, referred to as Mel4, was developed. Mel4-coated silicone hydrogel lenses (lotrafilcon A, comfilcon A, and somofilcon A) showed a high level of antimicrobial inhibition in vitro and did not have any cytotoxic effects in vivo when rabbits wore them for 1 week.29 Furthermore, Mel4-coated hydrogel lenses showed no evidence of corneal staining in human phase I clinical trials.29–31
The current study was designed to determine whether Mel4-coated lenses can reduce the incidence of microbially driven CIEs during extended wear. This study hypothesized that etafilcon A lenses coated with Mel4 would be able to reduce the incidence of microbially driven CIEs due to extended wear.
Materials and Methods
Production of Mel4-Coated Contact Lenses
Etafilcon A contact lenses (Acuvue 2; Johnson & Johnson Vision Care, Jacksonville, FL) were used for this study. Mel4 peptide (amino acid sequence, KNKRKRRRRRRGGRRRR; American Peptide Company, Sunnyvale, CA) was synthesized by conventional solid-phase peptide synthesis with >95% purity. The procedure for covalently attaching Mel4 to contact lenses has been reported elsewhere.28,30–32 Briefly, washed contact lenses were placed in 0.1 mol/L sodium acetate buffer, pH 5.0, containing 2 mg/mL 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) with 0.3 mg/mL N-hydroxysuccinimide for 30 minutes at 28°C. After washing in phosphate-buffered saline (PBS) the lenses were resuspended in 3.1 mg/mL of Mel4 in PBS and incubated for 20 hours at 37°C. Subsequently, lenses were washed three times in sterile PBS and then resuspended in 2 mL of 10% w/v NaCl for 2 hours at 28°C. This was followed by soaking in PBS for 2 hours and autoclaving at 121°C for 20 minutes. The lenses were then stored in glass vials at room temperature. Control etafilcon A lenses were removed from their packs, washed, and autoclaved in glass vials, as above.
Quantification of Mel4 Peptide Attachment and Activity on Contact Lenses
Mel4-coated lenses for the clinical trial were produced in 31 different batches. Amino acid analysis was performed using high-performance liquid chromatography to quantify the presence of Mel4 peptide on the coated lenses. Two Mel4-coated lenses from each batch were assessed by amino acid analysis to confirm the presence and amount of peptide on the lens surface. Briefly, Milli-Q (MilliporeSigma, Billerica, MA) water was used to wash Mel4-coated and control uncoated lenses. The lenses then underwent 24 hours of gas-phase hydrolysis in 6-M HCl (Ajax Finechem, Taren Point, NSW, Australia) at 110°C. Amino acids were extracted into 20% acetonitrile with trifluoroacetic acid (Thermo Fisher Scientific, Waltham, MA) and analyzed using an AccQ-Tag Ultra chemistry kit (Waters Corp., Milford, MA). The sum of all of the amino acids derived from the lens was regarded as the total amount of Mel4 attached to the lens surface.
After the production of Mel4-coated lenses and prior to the lens-dispensing visit, two Mel4-coated and control lenses from each batch were assessed for adhesion of P. aeruginosa ATCC 27853 and Staphylococcus aureus L2260/15. The bacterial adhesion protocol has been reported in prior studies.30,33 Briefly, 1.0 × 106 colony-forming units (CFU)/mL of bacterial cell suspensions in 1× PBS were transferred to the wells of 24-well tissue culture plates containing control or Mel4-coated lenses. The plates were incubated for 18 hours with shaking (120 rpm) at 37°C. The lenses were then washed three times with 1× PBS and then stirred in a vortex mixer rapidly in 2 mL of fresh 1× PBS containing a small magnetic stirring bar for a minute. The resulting lens slurry was serially diluted (1/10), and 3 × 20 µL of each dilution was transferred to nutrient agar for recovery of cells. After incubation for 24 hours at 37°C, viable microorganisms were enumerated as CFU/mm2 of a lens.
Study Participants
All procedures were conducted according to the tenets of the Declaration of Helsinki and were approved by the Human Research Ethics Committee of the University of New South Wales (HC15436) and the Institutional Ethics Committee of Hyderabad Eye Research Foundation (LEC 05-15-057). Also, the clinical trial was registered with Clinical Trials Registry–India (CTRI Trial ID CTRI/2015/10/006327) following approval from the Central Drugs Standard Control Organization and Drug Controller General of India (DCGI 4-MD/CT-148/2015-DC). The inclusion criteria required participants to be older than 18 years and in good health, to not be taking any medications, and to have correctable vision to 6/12 or better in each eye. Also required were contact lens correction between –1.00 and –6.00 diopter (D) sphere and the subject to be either experienced or new to contact lens wear. The exclusion criteria were any pre-existing ocular irritation; injury or condition (including infection or disease) of the cornea, conjunctiva, or eyelids that would interfere with contact lens fitting and the safe wearing of contact lenses; any systemic disease; eye surgery; systemic or topical medication up to 12 weeks before or during the trial that could adversely affect ocular health; and being or planning on becoming pregnant. All participants gave prior informed consent, and all were residents of Hyderabad, Telangana, India. The study was conducted at the L V Prasad Eye Institute (LVPEI), Hyderabad, India.
The study sample size was calculated based on a previous study at the same site,34 which reported that the rate of CIEs within 3 months of extended contact lens wear was 6.7%. The sample size for this study was estimated for incidence rates over 3 months represented as events per 100 participant months. PS software was used to calculate sample sizes.35 A total of 415 participant-months was required to determine a difference in incidence rates of 6.7 versus 2.4% at a 5% level of significance with 80% power. A low correlation of 0.15 for the CIE outcome between matched test and control eyes was used in the calculations. The sample was adjusted for a 15% loss of data over 3 months. The resulting sample of 495 participant months was divided by 3 months to arrive at a minimum sample of 165 participants.
Clinical Trial Design
This Mel4-coated antimicrobial contact lens clinical trial was a phase II/III, prospective, randomized, double-masked, single-center, contralateral design with 3 months of extended contact lens wear. Participants were randomized (using a computer-generated random allocation table) to wear Mel4-coated antimicrobial contact lens in one eye and uncoated etafilcon A control lenses in the contralateral eye. All of the participants were instructed to replace the lenses every 2 weeks.
The study required seven visits: baseline (visit 1), lens dispensing (visit 2), one night of lens wear (visit 3), 2 weeks of lens wear (visit 4), 1 month of lens wear (visit 5), and 3 months of lens wear (visit 6), followed by a 1 month follow-up visit after study lens discontinuation (visit 7). The seventh follow-up visit to test for any delayed responses to the investigational product at the end of 3 months of extended wear did not assign contact lens wear, and the participants were free to wear their glasses, if desired. Experienced wearers were given study lenses at the baseline visit. For adaptation purposes, neophytes were required to wear commercially available etafilcon A (Acuvue 2) lenses for 1 week on a daily-wear schedule using Biotrue contact lens cleaning solution (Bausch & Lomb, Rochester, NY) for rinsing and disinfecting the lenses. Study lenses were then dispensed at the subsequent lens dispensing visit. To reduce the possibility of participants mixing right and left eye lenses, all right eye and left eye lens vials were affixed with green and white labels, respectively. The baseline visit included health and contact lens history, keratometry, subjective refraction, and visual acuity assessments. All measurements for the study were performed by optometrists who were masked as to the types of lenses worn in each eye. At each visit, ocular characteristics and subjective responses of each subject were assessed. Slit-lamp biomicroscopy was performed for anterior eye assessment and included bulbar, limbal and palpebral redness, palpebral roughness, and conjunctival and corneal staining using standard clinical techniques and standardized grading scales. All of the clinical grading was conducted using the Cornea and Contact Lens Research Unit grading scales36 (0 to 4 units) interpolated into 0.1 increments, except for corneal staining, which was graded in 1.0 steps for extent and depth and 0.5 steps for type. Concordance training for the optometrists was conducted before study commencement, and concordance was measured every 6 months during the study. All masked optometrists were allowed to examine study participants if they scored more than 70% concordance for each grading scale, and they were retrained if concordance dropped below this level.
If the participants needed to remove their lenses temporarily, they were given Biotrue contact lens care solution and a case only for temporary storage. All participants were issued with a “red eye kit” at the end of the lens dispensing visit which consisted of a single 5-mL unit dose of sterile saline (sodium chloride; Pfizer, Sydney, Australia) and a fresh lens case (Bausch & Lomb) with right (R) and left (L) clearly marked with colored stickers. In the case of unusual symptoms such as redness, pain, watering, photophobia, decrease in vision, or noticing a white spot on the eye, participants were instructed to report to the clinic at their earliest possible convenience, or, if unable to present to the clinic, they were instructed to wash their hands with soap and air dry their hands before lens removal. Further, they were asked to use the red eye kit and place an approximately 2.5-mL unit dose of sterile saline in each lens case well, aseptically placing the right and left eye contact lenses appropriately. They were also asked to present to the clinic as soon as possible along with their red eye kit. The primary outcome measure for this clinical trial was the incidence of microbially driven adverse events (corneal infiltrative events) in each eye of the participants.
Adverse Events
The clinical assessment and classification of CIEs were adopted from previous studies.37,38 MK was classified as a serious adverse event. Significant events included CLARE, CLPUs, or IK. Non-significant events included asymptomatic IK or asymptomatic infiltrates. During the presentation of any adverse event, clinical optometrists made a primary diagnosis.37 A clinical audit team consisting of optometrists, ophthalmologists, and basic scientists reviewed all of the cases to confirm or amend the diagnosis prior to data analysis. All participants who were classified as having a CIE were permanently discontinued from lens wear and the study, and they were regularly monitored until the condition resolved completely. In the event of adverse events, upper bulbar conjunctiva and lower lid swabs, as well as contact lenses that had been worn, where available, were aseptically collected for microbiological analysis. Aseptically collected contact lenses and swabs from the CIE participants were placed in 2 mL of sterile PBS and transported immediately to the microbiology laboratory for analysis. The methodology for processing collected contact lenses and swabs has been described elsewhere.39,40
Susceptibility of Microbes to Mel4 Peptide
Gram-negative bacteria are the most common bacteria type isolated from contact lenses. When CIEs have occurred in previous studies,1,7–9 the susceptibility to Mel4 of Gram-negative bacteria isolated from swabs or lenses at the time of a CIE was assessed by determining the minimum inhibitory concentration of Mel4 that reduced the growth of the microbes by 50% using a previously published method.30 The adhesion of these Gram-negative bacteria to Mel4 and control lenses was also examined as outlined above for P. aeruginosa 27853.
Statistical Analysis
The rate of CIEs was expressed as a rate per 100 participant-months. The differences in the incidence of CIEs between participants wearing the test lenses and those wearing the control lenses were compared using the McNemar test. The log-rank (Mantel–Cox) test was used to examine the survival analysis of participants who had CIEs. A Mann–Whitney test was used to assess differences in the numbers of bacteria adhering to the Mel4-coated and control lenses. For all tests, the level of statistical significance was set at P < 0.05.
Results
A total of 775 participants (neophytes and experienced contact lens wearers) were screened for suitability. Of these, 176 participants (128 neophytes and 48 experienced contact lens wearers) were enrolled and given study lenses between January 2015 to December 2017. The participants were 18 to 42 years old and had contact lens powers ranging from –1.00 to –6.00 D sphere. Baseline clinical characteristics of the subjects are shown in Supplementary Table S1. All participants achieved visual acuity of at least 6/12 or better with correction and were willing to comply with wearing the study contact lenses. Among the participants, 129 completed the 4-month study. Thirty-nine participants (22%) discontinued lens wear permanently prior to completion of the study due to adverse events, fever, epidemic viral conjunctivitis, chickenpox, relocation, or disinterest. Another eight participants (5%) were lost to follow-up during the study.
Analyses of Mel4-Coated Lenses Prior to Lens Wear
More than 1500 Mel4-coated lenses were produced for the clinical trial in 31 batches. The amino acid analyses revealed that the Mel4-coated contact lenses had 62.6 ± 26.4 µg of Mel4 peptide per lens, confirming that the lenses had been coated with Mel4 peptide. Unworn Mel4-coated lenses significantly reduced the adhesion of P. aeruginosa (2.42 ± 1.16 log10 CFU/lens reduction; P < 0.001) and S. aureus (1.82 ± 0.46 log10 CFU/lens reduction; P < 0.001) compared with control uncoated lenses. These results demonstrate that the participants in the trial were prescribed with active Mel4-coated contact lenses.
Incidence of Contact Lens-Induced Corneal Infiltrative Events
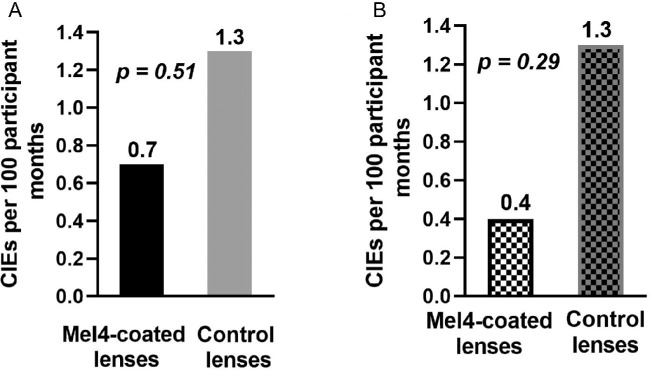
During the 3 months of extended lens wear, nine participants (5.1%; nine eyes of the nine participants) experienced CIEs. These nine participants included three participants (1.7%; three eyes) who wore the Mel4-coated lenses and six participants (3.4%; six eyes) who wore the control lenses. Six of the nine participants with CIEs had CLARE, and three participants had IK. All of these participants experienced unilateral CIEs. The clinical features of CIEs with Mel4-coated lens and control lens has shown in supplementary Figure S1. The incidence of CIEs based on 100 participant-months is presented in Figure 1A. The incidence rate for CIEs in the Mel4-coated lenses (0.7 events per 100 participant-months) was approximately half that of the control lenses (1.3 events per 100 participant-months). However, there were no significant differences between Mel4-coated lenses and control lenses (P = 0.51). One of the CIEs in the Mel4-coated lenses occurred after the participant had worn the lenses for 22 days, which is outside the maximum number of days allowed in the trial. When this case was removed for the analysis, there was a 69% reduction in the incidence of CIEs (Fig. 1B) for Mel4-coated lenses that had been worn (0.4 events per 100 participant-months) compared with control lenses that had been worn (1.3 events per 100 participant-months). However, this was also not statistically different (P = 0.29).
Figure 1.
CIE rates for the Mel4-coated lenses and control lenses: (A) all participants, and (B) after removing the participant who wore the lenses for 22 days (exceeding the maximum number of days allowed in the trial).
Survival analyses for CIEs (Fig. 2A) found no significant differences between Mel4 and control lenses (P = 0.32). Mel4-coated lens survival probability was 1.00 until 12 days and was reduced to 0.98 on the 47th day. For the control lens, the survival probability was 1.00 on day one but dropped to 0.96 by the 68th day. Removing the participant who wore the contact lenses longer than prescribed resulted in the Mel4-coated lenses having two events and the control lenses having six events (Fig. 2B), but this was not a statistically significant difference (P = 0.16). Figures 3A and 3B show the number of days the study lenses were worn until adverse events occurred and the number of days the study lenses were worn within the scheduled 14 days of lens wear until adverse events occurred. The median number of days until adverse events occurred was 11 days (range, 2–14) for control lenses and 13 days (range, 13–22; 13 days only if the non-compliant lens wearer was removed) for Mel4-coated lenses.
Figure 2.
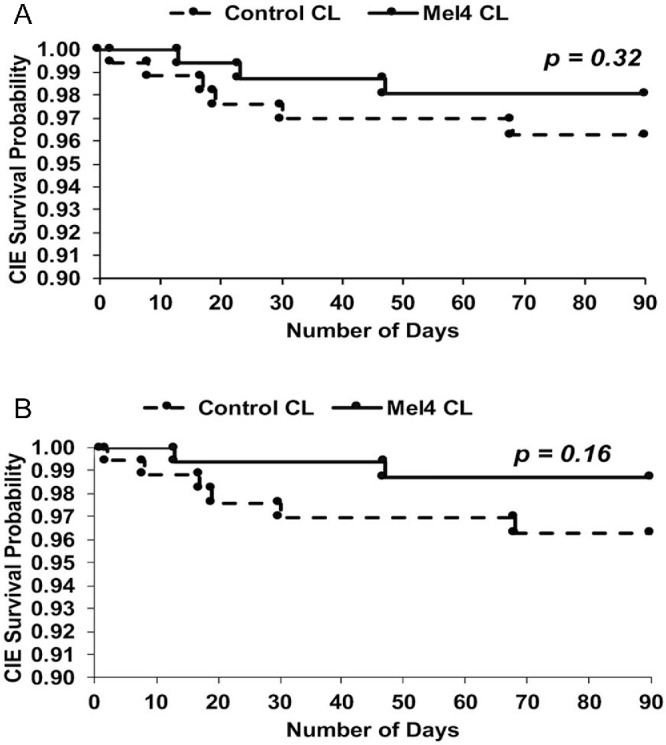
CIE survival analysis for Mel4-coated lenses and control lenses: (A) all participants, and (B) after removing the participant who wore the lenses for 22 days (exceeding the maximum number of days allowed in the trial).
Figure 3.
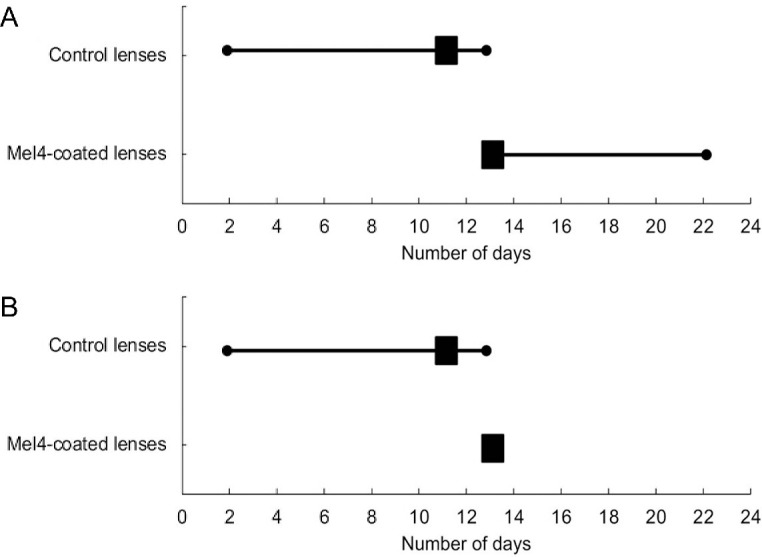
Number of days the study lenses were worn until adverse events occurred for (A) all participants and (B) those within the scheduled 14 days of lens wear. The box represents the median number of days, and the lines represent the total number of days.
Microbial Growth From Contact Lenses and Affected Eyes for CIE Participants
Of the nine participants’ contact lens samples, eight lenses were available for culture. The results of the microbiological analysis of the microbes grown from the contact lenses of the affected eyes for the participants with CIEs are presented in Table 1. Of these, four samples (two Mel4-coated lenses and two control lenses) did not show any growth of microbes. Citrobacter diversus (a Gram-negative bacteria) was cultured from one Mel4-coated lens (of subject 15). Acinetobacter haemolyticus (a Gram-negative bacteria) and Staphylococcus hominis (a Gram-positive bacteria) were cultured from control lenses collected from two participants.
Table 1.
Microbiological Analysis of the Microbes Grown From Contact Lenses and Eyes of Participants Experiencing CIEs
| Location | |||||
|---|---|---|---|---|---|
| Subject ID | Diagnosis | Contact Lens | Contact Lens (CFU/lens) | Upper Bulbar Conjunctiva (CFU/mL) | Lower Lid Swab (CFU/mL) |
| 15 | CLARE | Mel4-coated lens | Citrobacter diversus (30) | No growth | No growth |
| 117 | CLARE | No growth | No growth | No growth | |
| 103a,b | IK | No growth | Staphylococcus gallinarum (2) | No growth | |
| 37 | CLARE | Control lens | Acinetobacter haemolyticus (1050) | Staphylococcus epidermidis (1) | Not available |
| 68b | CLARE | No growth | Staphylococcus epidermidis (5) | Staphylococcus epidermidis (260) Acinetobacter lwoffii (3) | |
| 90 | CLARE | No growth | No growth | Staphylococcus hominis (7) Staphylococcus saprophyticus (3) | |
| 154 | CLARE | No growth | Staphylococcus epidermidis (28) | Staphylococcus epidermidis (3) Staphylococcus hominis (18) | |
| 6 | IK | Not available | No growth | Staphylococcus epidermidis (42) | |
| 19b | IK | Staphylococcus hominis (1) | Staphylococcus hominis (1) | Staphylococcus epidermidis (1) | |
This subject had worn the lenses for 22 days, outside of the 14-day wear schedule.
These participants had stored the contact lenses in the Biotrue contact lens solution rather than the PBS provided in the red eye kit.
All nine participants’ upper bulbar conjunctival swabs were available for microbiological analysis (Table 1). Of these, four samples (eye swabs for two Mel4-coated lens wearers and two control lens wearers) did not show any microbial growth. The majority of the control lens conjunctival swabs grew coagulase-negative staphylococci. No Gram-negative bacteria or fungi were isolated from any of the upper bulbar conjunctival swabs. One Gram-negative bacterium, Acinetobacter lwoffii, was isolated from the lid of an eye that wore a control lens (Table 1). All other lid swabs of eyes that wore the control lens grew various types of coagulase-negative staphylococci. None of the lid swabs from eyes that wore the Mel4-coated lenses grew any microbes. No fungi were isolated from any of the lower lid swabs.
Susceptibility of the Gram-Negative Bacterial Isolates to Mel4 and Mel4-Coated Contact Lenses
The Gram-negative bacteria isolated from participants with CIEs were susceptible to Mel4 in solution, with minimum inhibitory concentrations ranging between 15.6 and 62.5 µg/mL (Table 2). The Mel4-coated contact lenses reduced the adhesion of these Gram-negative bacteria by ≥2.1 log10 CFU/lens (Table 2).
Table 2.
Susceptibility of Gram-Negative Bacteria to Mel4 Peptide and Inhibition of Adhesion to Mel4-Coated Lenses
| Organism | Minimum Inhibitory Concentration (µg/mL) | Inhibition of Adhesion (log10) |
|---|---|---|
| Citrobacter diversus | 15.6 | 2.14 |
| Acinetobacter haemolyticus | 62.5 | 2.10 |
| Acinetobacter lwoffii | 62.5 | 2.25 |
Discussion
This study for the first time, to our knowledge, investigated the ability of an antimicrobial peptide-coated contact lens to reduce the incidence of CIEs during extended human wear. Mel4-coated etafilcon A lenses were associated with 50% to 69% fewer CIEs when compared with control uncoated etafilcon A lenses. The lack of a significant reduction in CIEs was most likely due to the low rate of CIEs observed during the study in the eyes that wore the control lenses. The study sample size was calculated based on a 65% reduction in CIEs from a previous study at the same study site which reported a 6.7% rate of CIEs within a period of 3 months34 in the same population wearing a silicone hydrogel lens. This is considerably higher than the rate of 1.3 CIEs per 100 participant-months (or 3.4% of the population) in the current study. Another study, again using the same population, this time in a bilateral extended-wear hydrogel (etafilcon A) lens study conducted in the 1990s,38 found a CIE event rate of 3.7 CIEs per 100 participant-months, again higher than the current study (1.3 CIEs per 100 participant months).
It is interesting to speculate why there was such a reduction in the incidence of CIEs in the current study. The current study used a contralateral design, whereas other studies have used bilateral designs. It is possible that participants in the current study swapped lenses between eyes, although a protocol had been put in place to prevent this from occurring. Participants were given differently colored lens vials and told which color vial should contain each eye's lens. Also, participants were reminded at each visit to be careful about remembering which lens from which vial should go in which eye. Also, approximately 50% of the study population had different refractive errors in each eye, which would make it unlikely that they would have swapped lenses, as doing so would compromise their vision. Even so, we cannot totally rule out the possibility of switching lenses between eyes. The study from which the sample size calculation data were obtained investigated silicone hydrogel lenses. Reports have suggested that adverse event rates in silicone hydrogel lenses are approximately twice the rate of those in hydrogel lenses.18,41 The current study used etafilcon A hydrogel lenses, so the apparent adverse event rate may have been reduced by half compared with that used in the sample size calculation, which might have reduced the adverse event rate to 3.5 per 100 participant-months, still considerably higher than the 1.3 CIEs per 100 participant-months in the current study.
A previous silicone hydrogel study39 designed to examine the risk factors for CIEs found that people who worked outdoors or in places where they were exposed to wind, dust, fumes, and water splashes were predisposed to inflammatory events (19.2% of such people experienced CIEs). The current study recruited 82% of people who would have been classified as working in ideal environments (68% of students and 14% hospital workers),39 so this may have contributed to the reduced CIE event rate. The use of soft contact lenses in teens and young adults increases the rate of CIEs.42,43 The age range sits almost exactly within the age range (15–25 years) reported previously to increase the rate of adverse events during contact lens wear,42,43 so this should not have resulted in a lessening of the CIE rate. However, the studies by Chalmers et al.41–43 were conducted on a US population, which may not be directly comparable to an Indian population. People with corneal vascularization may have higher adverse event rates,39 but the current study excluded participants with corneal vascularization. Another factor that might affect the CIE rate is lens movement.39 In the current study, 94% of participants had an optimal lens movement of 0.5 mm, and the remaining 6% had more than 0.5 mm of lens movement.
In addition, LVPEI is a center of excellence for eye care in India. In the current study, 25% of the study participants had previously participated in contact lens trails at LVPEI. Another 20% of the study participants were optometry trainees in the hospital for their internship and fellowship programs, as well as first- to third-year students who were studying optometry. Therefore, 45% of the participants were well aware of the care regimens required and had good training in the management of contact lenses.
Other factors that might have contributed to the very low CIE rate in the current study include changes in the socioeconomic status of the population from which the participants were drawn. The population of Hyderabad increased from 6.73 million in 2011 to 10.86 million in 2017, when the study was conducted. The gross domestic product of India increased from US$1.827 trillion in 2012 to US$2.602 trillion in 2017. Previous studies have demonstrated that the socioeconomic status of populations can affect the rates of microbial keratitis15,44,45 and CIEs.46 Given that the incidence of CIEs in the control eyes was less than anticipated, a larger clinical trial with approximately 350 participants (if using a contralateral design, 3 months of lens wear, or 1050 participant-months) to find a statistical reduction in CIEs is necessary.
This study showed that three lenses collected from CIEs were contaminated with S. hominis, A. haemolyticus, or C. diversus. All of the other lenses collected from infiltrative events were sterile. The three participants with CIEs reported to the clinic with their lenses in lens cases containing the Biotrue multipurpose disinfecting solution, despite repeated instructions to use the red eye kit and sterile saline. Although this was a protocol violation, this violation would not have affected the rates of CIEs in the current study. The fact that Gram-negative bacteria on lenses were associated with CLARE events confirms the results of other studies.7–9 The Mel4-coated lenses reduced bacterial adhesion by 2.14 log10 CFU/lens, which is greater than the levels of inhibition produced by Mel4 coatings on the adhesion of P. aeruginosa (1.3 log10), Stenotrophomonas maltophilia (1.1 log10), Elizabethkingia meningoseptica, (0.7 log10), Delftia acidovorans (1.1 log10), and Burkholderia cepacia (0.5 log10).30,31 One Mel4-coated lens was contaminated with C. diversus during an incidence of CLARE. The CLARE event was observed at the end of the wear period (day 13 of lens wear). Moreover, all of the CIEs associated with Mel4-coated lenses that were not the result of a protocol violation were reported on the 13th day (or more for one participant) of extended wear, perhaps suggesting that the lenses retained antimicrobial activity from the earlier wear period; however, this would require confirmation.
There are possible limitations to this study. First, the study sample size was found to be insufficient given the low level of CIEs found in the population during the study. Second, this study investigated the antimicrobial activity of a Mel4 coating on hydrogel etafilcon A lenses rather than silicone hydrogel lenses. As approximately 50% of contact lens wearers worldwide use silicone hydrogel lenses, such lenses should be included in future studies. Finally, the contralateral study design may have resulted in subjects swapping lenses between eyes. To overcome this limitation, future studies should consider the use of different study designs.
Conclusions
To our knowledge, this is the first randomized human clinical trial with Mel4-coated contact lenses to show that these lenses have the capacity to reduce CIEs by at least 50% compared with uncoated control lenses during extended wear over 3 months. However, the reduction did not reach statistical significance due to the low rate of CIEs observed with the control lenses in the studied population at the time.
Supplementary Material
Acknowledgments
The authors thank Johnson & Johnson Vision for providing study contact lenses as part of an investigator-initiated study; Thomas John Naduvilath, PhD, for statistical guidance; study participants; contact lens research optometrists; and Jhaveri Microbiology Centre, L V Prasad Eye Institute, Hyderabad, India, for their assistance.
Supported by a National Health and Medical Research Council development grant (APP1076206), by the Hyderabad Eye Research Foundation, and by CooperVision, Inc.
Disclosure: P. Kalaiselvan, None; N. Konda, None; N. Pampi, None; P.K. Vaddavalli, None; S. Sharma, None; F. Stapleton, None; N. Kumar, None; M.D.P. Willcox, None; D. Dutta, None
References
- 1. Sankaridurg PR, Sharma S, Willcox M, et al.. Bacterial colonization of disposable soft contact lenses is greater during corneal infiltrative events than during asymptomatic extended lens wear. J Clin Microbiol . 2000; 38(12): 4420−4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keay L, Stapleton F, Schein O.. Epidemiology of contact lens-related inflammation and microbial keratitis: a 20-year perspective. Eye Contact Lens . 2007; 33(6 pt 2): 346−353, discussion 362−343. [DOI] [PubMed] [Google Scholar]
- 3. Szczotka-Flynn L, Jiang Y, Raghupathy S, et al.. Corneal inflammatory events with daily silicone hydrogel lens wear. Optom Vis Sci . 2014; 91(1): 3−12. [DOI] [PubMed] [Google Scholar]
- 4. Steele KR, Szczotka-Flynn L.. Epidemiology of contact lens-induced infiltrates: an updated review. Clin Exp Optom . 2017; 100(5): 473−481. [DOI] [PubMed] [Google Scholar]
- 5. Houang E, Lam D, Fan D, Seal D.. Microbial keratitis in Hong Kong: relationship to climate, environment and contact-lens disinfection. Trans R Soc Trop Med Hyg . 2001; 95(4): 361−367. [DOI] [PubMed] [Google Scholar]
- 6. Konda N, Motukupally SR, Garg P, Sharma S, Ali MH, Willcox MD.. Microbial analyses of contact lens-associated microbial keratitis. Optom Vis Sci . 2014; 91(1): 47−53. [DOI] [PubMed] [Google Scholar]
- 7. Holden BA, La Hood D, Grant T, et al.. Gram-negative bacteria can induce contact lens related acute red eye (CLARE) responses. CLAO J. 1996; 22(1): 47−52. [PubMed] [Google Scholar]
- 8. Sankaridurg PR, Willcox MD, Sharma S, et al.. Haemophilus influenzae adherent to contact lenses associated with production of acute ocular inflammation. J Clin Microbiol . 1996; 34(10): 2426−2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Willcox M, Sharma S, Naduvilath TJ, Sankaridurg PR, Gopinathan U, Holden BA.. External ocular surface and lens microbiota in contact lens wearers with corneal infiltrates during extended wear of hydrogel lenses. Eye Contact Lens . 2011; 37(2): 90−95. [DOI] [PubMed] [Google Scholar]
- 10. Jalbert I, Willcox MD, Sweeney DF.. Isolation of Staphylococcus aureus from a contact lens at the time of a contact lens-induced peripheral ulcer: case report. Cornea . 2000; 19(1): 116−120. [DOI] [PubMed] [Google Scholar]
- 11. Cheng KH, Leung SL, Hoekman HW, et al.. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet . 1999; 354(9174): 181−185. [DOI] [PubMed] [Google Scholar]
- 12. Morgan PB, Efron N, Hill EA, Raynor MK, Whiting MA, Tullo AB.. Incidence of keratitis of varying severity among contact lens wearers. Br J Ophthalmol . 2005; 89(4): 430−436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poggio EC, Glynn RJ, Schein OD, et al.. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med . 1989; 321(12): 779−783. [DOI] [PubMed] [Google Scholar]
- 14. Seal DV, Kirkness CM, Bennett HG, Peterson M, Keratitis Study Group. Population-based cohort study of microbial keratitis in Scotland: incidence and features. Cont Lens Anterior Eye . 1999; 22(2): 49−57. [DOI] [PubMed] [Google Scholar]
- 15. Stapleton F, Keay L, Edwards K, et al.. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology . 2008; 115(10): 1655−1662. [DOI] [PubMed] [Google Scholar]
- 16. Brennan NA, Coles ML, Comstock TL, Levy B.. A 1-year prospective clinical trial of balafilcon a (PureVision) silicone-hydrogel contact lenses used on a 30-day continuous wear schedule. Ophthalmology . 2002; 109(6): 1172−1177. [DOI] [PubMed] [Google Scholar]
- 17. Levy B, McNamara N, Corzine J, Abbott RL.. Prospective trial of daily and extended wear disposable contact lenses. Cornea . 1997; 16(3): 274−276. [PubMed] [Google Scholar]
- 18. Szczotka-Flynn L, Diaz M.. Risk of corneal inflammatory events with silicone hydrogel and low Dk hydrogel extended contact lens wear: a meta-analysis. Optom Vis Sci . 2007; 84(4): 247−256. [DOI] [PubMed] [Google Scholar]
- 19. Szczotka-Flynn L, Lass JH, Sethi A, et al.. Risk factors for corneal infiltrative events during continuous wear of silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci . 2010; 51(11): 5421−5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sankaridurg P, Chen X, Naduvilath T, et al.. Adverse events during 2 years of daily wear of silicone hydrogels in children. Optom Vis Sci . 2013; 90(9): 961−969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sankaridurg PR, Sweeney DF, Holden BA, et al.. Comparison of adverse events with daily disposable hydrogels and spectacle wear: results from a 12-month prospective clinical trial. Ophthalmology . 2003; 110(12): 2327−2334. [DOI] [PubMed] [Google Scholar]
- 22. Willcox MDP, Hume EBH, Vijay AK, Petcavich R.. Ability of silver-impregnated contact lenses to control microbial growth and colonisation. J Optom . 2010; 3(3): 143−148. [Google Scholar]
- 23. Mathews SM, Spallholz JE, Grimson MJ, Dubielzig RR, Gray T, Reid TW.. Prevention of bacterial colonization of contact lenses with covalently attached selenium and effects on the rabbit cornea. Cornea . 2006; 25(7): 806−814. [DOI] [PubMed] [Google Scholar]
- 24. Ozkan J, Zhu H, Willcox M.. Efficacy and clinical performance of selenium antibacterial silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci . 2009; 50: 5632. [Google Scholar]
- 25. Zhu H, Kumar A, Ozkan J, et al.. Fimbrolide-coated antimicrobial lenses: their in vitro and in vivo effects. Optom Vis Sci . 2008; 85(5): 292−300. [DOI] [PubMed] [Google Scholar]
- 26. Cole N, Hume EBH, Vijay AK, Sankaridurg P, Kumar N, Willcox MDP.. In vivo performance of melimine as an antimicrobial coating for contact lenses in models of CLARE and CLPU. Invest Ophthalmol Vis Sci . 2010; 51(1): 390−395. [DOI] [PubMed] [Google Scholar]
- 27. Dutta D, Vijay AK, Kumar N, Willcox MDP.. Melimine-coated antimicrobial contact lenses reduce microbial keratitis in an animal model. Invest Ophthalmol Vis Sci . 2016; 57(13): 5616−5624. [DOI] [PubMed] [Google Scholar]
- 28. Dutta D, Ozkan J, Willcox MDP.. Biocompatibility of antimicrobial melimine lenses: rabbit and human studies. Optom Vis Sci . 2014; 91(5): 570−581. [DOI] [PubMed] [Google Scholar]
- 29. Dutta D, Kamphuis B, Ozcelik B, et al.. Development of silicone hydrogel antimicrobial contact lenses with Mel4 peptide coating. Optom Vis Sci . 2018; 95(10): 937−946. [DOI] [PubMed] [Google Scholar]
- 30. Dutta D, Kumar N, Willcox MDP. Antimicrobial activity of four cationic peptides immobilised to poly-hydroxyethylmethacrylate. Biofouling . 2016; 32(4): 429−438. [DOI] [PubMed] [Google Scholar]
- 31. Dutta D, Zhao T, Cheah KB, Holmlund L, Willcox MDP.. Activity of a melimine derived peptide Mel4 against Stenotrophomonas, Delftia, Elizabethkingia, Burkholderia and biocompatibility as a contact lens coating. Cont Lens Anterior Eye. 2017; 40(3): 175−183. [DOI] [PubMed] [Google Scholar]
- 32. Dutta D, Cole N, Kumar N, Willcox MDP.. Broad spectrum antimicrobial activity of melimine covalently bound to contact lenses. Invest Ophthalmol Vis Sci . 2013; 54(1): 175−182. [DOI] [PubMed] [Google Scholar]
- 33. Dutta D, Willcox MDP.. A laboratory assessment of factors that affect bacterial adhesion to contact lenses. Biology . 2013; 2(4): 1268−1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ozkan J, Willcox MDP, Lazon de la Jara P, et al.. The effect of daily lens replacement during overnight wear on ocular adverse events. Optom Vis Sci . 2012; 89(12): 1674−1681. [DOI] [PubMed] [Google Scholar]
- 35. Dupont WD. PS power and sample size program available for free on the Internet. Control Clin Trials . 1997; 18: 274. [Google Scholar]
- 36. Terry RL, Schnider CM, Holden BA, et al.. CCLRU standards for success of daily and extended wear contact lenses. Optom Vis Sci . 1993; 70(3): 234−243. [DOI] [PubMed] [Google Scholar]
- 37. Sweeney DF, Jalbert I, Covey M, et al.. Clinical characterization of corneal infiltrative events observed with soft contact lens wear. Cornea . 2003; 22(5): 435−442. [DOI] [PubMed] [Google Scholar]
- 38. Sankaridurg PR, Sweeney DF, Sharma S, et al.. Adverse events with extended wear of disposable hydrogels: results for the first 13 months of lens wear. Ophthalmology . 1999; 106(9): 1671−1680. [DOI] [PubMed] [Google Scholar]
- 39. Ozkan J, Mandathara P, Krishna P, et al.. Risk factors for corneal inflammatory and mechanical events with extended wear silicone hydrogel contact lenses. Optom Vis Sci . 2010; 87(11): 847−853. [DOI] [PubMed] [Google Scholar]
- 40. Ozkan J, Zhu H, Gabriel M, Holden BA, Willcox MDP.. Effect of prophylactic antibiotic drops on ocular microbiota and physiology during silicone hydrogel lens wear. Optom Vis Sci . 2012; 89(3): 326−335. [DOI] [PubMed] [Google Scholar]
- 41. Chalmers RL, Hickson-Curran SB, Keay L, Gleason WJ, Albright R.. Rates of adverse events with hydrogel and silicone hydrogel daily disposable lenses in a large postmarket surveillance registry: the TEMPO Registry. Invest Ophthalmol Vis Sci . 2015; 56(1): 654−663. [DOI] [PubMed] [Google Scholar]
- 42. Chalmers RL, McNally JJ, Schein OD, et al.. Risk factors for corneal infiltrates with continuous wear of contact lenses. Optom Vis Sci . 2007; 84(7): 573−579. [DOI] [PubMed] [Google Scholar]
- 43. Chalmers RL, Wagner H, Mitchell GL, et al.. Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the Contact Lens Assessment in Youth (CLAY) study. Invest Ophthalmol Vis Sci . 2011; 52(9): 6690−6696. [DOI] [PubMed] [Google Scholar]
- 44. Stapleton F, Keay L, Jalbert I, Cole N.. The epidemiology of contact lens related infiltrates. Optom Vis Sci . 2007; 84(4): 257−272. [DOI] [PubMed] [Google Scholar]
- 45. Stapleton F, Carnt N.. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond) . 2012; 26(2): 185−193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stapleton F, Dart JK, Minassian D.. Risk factors with contact lens related suppurative keratitis. CLAO J . 1993; 19(4): 204−210. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.