Abstract
Purpose of Review
Androgen deprivation therapy (ADT) is the standard of care for the treatment of advanced prostate cancer (PC). ADT, particularly with GnRH agonists, leads to increased risk of cardiovascular disease, including myocardial infarction, hypertension, and stroke. This review discusses the options of ADT, the mechanism of ADT-associated cardiovascular side effects, and potential benefit by using GnRH antagonists.
Recent Findings
GnRH antagonists have relatively less cardiovascular adverse effects compared to GnRH agonists. We highlight on a recently published phase III clinical trial on the oral GnRH antagonist, relugolix, and its comparative benefit to traditional GnRH agonist regarding development of cardiovascular disease.
Summary
Recent data reinforces that GnRH antagonists have a more favorable cardiovascular outcomes compared to GnRH agonists yet maintain a similar efficacy profile. From the data we reviewed, GnRH antagonists may be the preferred method of ADT for PC, but further data with primary cardiovascular outcomes are warranted.
Keywords: Prostate cancer, Androgen deprivation therapy, GnRH antagonist, Coronary artery disease, Cardiovascular toxicity, Cardio-oncology
Introduction
Prostate cancer (PC) is the most common cancer among men in the USA, accounting for more than one in five new cancer diagnoses among men in 2020 [1]. Androgen deprivation therapy (ADT) is the standard of care for patients with intermediate to high-risk localized prostate cancer and for patients with distant metastatic disease [2, 3]. However, ADT has been associated with side effects and risks, including an increase in major adverse cardiovascular events (MACE) [4]. Coronary artery disease (CAD) causes almost as many deaths in men with PC as the cancer itself does, and approximately two out of three men with PC are independently at high risk for coronary artery disease [5, 6•]. Since CAD is the leading cause of death worldwide, and the risk of CAD is increased in men with PC, the relationship between PC treatment and MACE is of high importance [7]. This review analyzes the cardiovascular toxicity in GnRH agonists and GnRH antagonists. We offer recommendations for clinicians treating men with PC and with risk factors, history, or active cardiovascular disease.
Androgen Deprivation Therapy
The benefits of ADT in men with PC were first discovered in 1941 by surgical castration of patients who had PC with skeletal metastases [8]. Despite its cost-effectiveness and immediate reduction in testosterone to castrate level, surgical castration has a tremendous psychological impact on patients. Thus, medical castration is the most widely accepted treatment modality in the USA and Europe. Medical ADT can be achieved through gonadotropin-releasing hormone (GnRH) agonism, GnRH antagonism, anti-androgens, and inhibitors of steroid synthesis. The surgical and medical approaches to ADT as well as their advantages and disadvantages are listed in Table 1.
Table 1.
Different approaches to androgen deprivation therapy
| Method/drug class | Examples | Advantages | Disadvantages | |
|---|---|---|---|---|
| Medical castration | GnRH agonists |
Leuprolide Goserelin |
Reversible, less frequent administrations (up to q12 months depending on formulation) | Initial testosterone surge/flare, castration not achieved for weeks |
| GnRH antagonist |
Degarelix Relugolix* |
Reversible, no testosterone surge, rapid decrease in testosterone level, fewer cardiovascular side effects | Higher frequency of injection-site reactions, more frequent administrations (monthly) | |
| Surgical castration | Bilateral orchiectomy | Low cost, no adherence issue, immediate decrease in testosterone level | Not reversible, psychological impact |
*Recently approved by FDA
GnRH agonists stimulate the GnRH receptor, leading to the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Synthetic GnRH agonists do not readily dissociate from their receptors, so are thought to lead to the downregulation of GnRH receptor and its signaling and androgen synthesis. GnRH agonists can lead to transient increase in release of testosterone from Leydig cells, referred to as a “flare” period [9]. This flare of testosterone may cause transient worsening of symptoms in advanced PC patients [10]. Anti-androgens such as bicalutamide can be used in the beginning of treatment with GnRH agonists to counter the flare in testosterone [11]. However, testosterone surges throughout treatment have been observed after each dose of use, in what is referred to as “microsurges” [12]. It was also observed that GnRH agonists can lead to prolonged secretion of FSH, a potential mechanism for increased CVS toxicity [12].
On the other hand, GnRH antagonists do not cause flares or testosterone surges/microsurges [10]. GnRH antagonists bind to GnRH receptors and cause an immediate competitive blockade, inhibiting the release of FSH or LH and thus preventing the initial rise in testosterone seen with GnRH agonists. GnRH antagonist degarelix has also been shown to suppress testosterone faster and produce a more sustained FSH suppression than leuprolide [13]. Thus, GnRH antagonists seem more ideal mechanistically than GnRH agonists for the treatment of prostate cancer. Degarelix was the only FDA-approved injectable GnRH antagonists with a main drawback of requiring monthly injection and is associated with higher frequency of injection-site reactions [13]. Relugolix, a novel GnRH antagonist, is an oral medication that also leads to rapid and sustained testosterone level reduction and recently gained FDA approval in December 2020 [14].
Androgen Deprivation Therapy Increases Cardiovascular Risks
The initial data relating ADT with an increased risk of CAD were equivocal; however, this relationship has now been accepted. A pooled analysis of six trials in which patients were randomized to a GnRH agonist or a GnRH antagonist showed that men with pre-existing CAD had a significantly lower risk of cardiac events on GnRH antagonists (6.5%) compared to GnRH agonists (14.7%) in 1 year of follow-up (p = 0.002) [15]. Of note, however, among men without a history of CAD, no difference in MACE events was observed [15]. The absolute risk reduction for receiving a GnRH antagonist was 8.2% in the first year, with a number needed to treat of 12 [15].
In another study comparing GnRH agonists to antagonists in PC patients with pre-existing CAD, 20% of patients who received GnRH agonists had a MACE, defined by death, MI, CVA, or percutaneous revascularization, compared to 3% of patients who received an antagonist in a follow-up period of 1 year [16••]. Patients in the two arms had similar baseline characteristics, and all of the men had baseline documented history of cardiovascular disease [16••]. All cardiovascular events defined by death, MI, CVA, a transient ischemic attack, percutaneous coronary intervention, and cardiac-related hospitalization occurred in 33% of the GnRH agonist group compared to 5% in the GnRH antagonist group (p=0.001) [16]. In an analysis of cardiac biomarkers, N-terminal pro-brain natriuretic peptide (NT-proBNP) levels differed in the study period among patients with and without a MACE [16••]. NT-proBNP levels were stable over time in patients without cardiac events but increased over time among patients with cardiac events in the study period [16••]. Interestingly, the NT-proBNP levels peaked at the time when most cardiac events occurred in the study, between 6 and 12 months, and then declined [16••]. Further studies following NT-proBNP in patients on ADT are warranted to refine this hypothesis. Of note, changes in d-dimer, high sensitivity troponin, and C-reactive protein (CRP) were not associated with cardiac events [16••]. This study was limited by its relatively small sample size (80 patients) [16••]. Additionally, twice as many patients with diabetes were in the agonist arm, compared to the antagonist arm, and may have contributed to the increased number of cardiac events [16••]. Larger phase III studies are warranted to confirm these hypotheses.
The injectable GnRH antagonist degarelix was approved in 2008. Its limitations include administering monthly subcutaneous injections and frequent injection site reactions, although they are typically mild to moderate [13]. Of note, cardiovascular adverse effects were observed in 13% of patients on leuprolide compared to 9% of patients on degarelix regardless of pre-existing CAD risk [13]. Ischemic heart disease was the most frequent cardiac event observed in the trial between leuprolide and degarelix and occurred in 10% of the leuprolide patients versus 4% of the degarelix patients [13]. Degarelix has been shown to have less cardiovascular toxicity than GnRH agonists, and it should be considered a treatment option for PC patients, especially those who have a history of CAD.
Mechanism of Androgen Deprivation Therapy-Associated Cardiovascular Side Effects
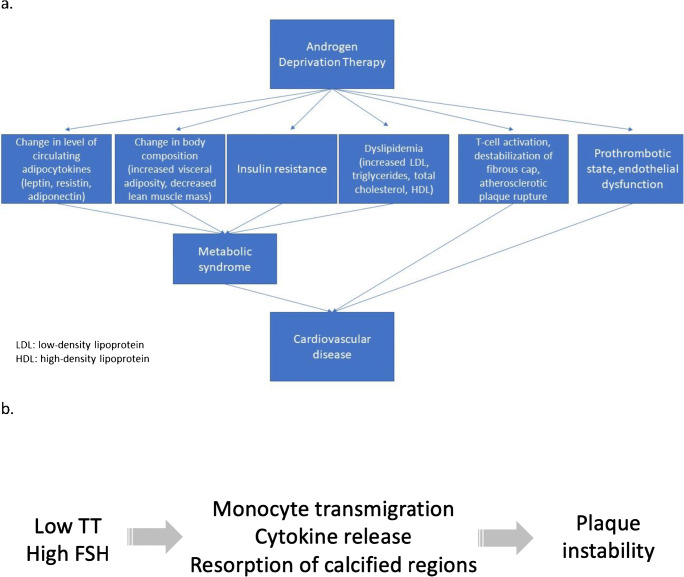
There are ongoing studies to identify the mechanisms for the increased cardiovascular risk associated with ADT, which is most likely multifactorial. Treatment with ADT has been shown to lead to the development of metabolic syndrome through many factors including body composition, lipid profiles, and insulin resistance (Figure 1a) [4, 17]. Physiologic effects of ADT include weight gain, loss of muscle mass, and increased fat mass due to the suppression of androgen synthesis and signaling [17]. In addition, ADT has been shown to increase total cholesterol and triglycerides and decrease HDL levels [18]. Impaired insulin sensitivity can occur as early as 3 months after starting ADT and can progressively worsen throughout course of treatment, eventually causing some patients to develop type 2 diabetes [17]. Further metabolic effects of ADT on glucose metabolism include altered circulating levels of the adipocytokines, leptin, and adiponectin [4, 17, 18]. Specifically, ADT therapy may increase leptin, which is thought to lead to insulin resistance and increased cardiac risk and decrease adiponectin, which is cardioprotective [18].
Figure 1.
a Mechanisms of androgen deprivation therapy leading to cardiovascular disease. b Proposed mechanism for plaque instability in men with prostate cancer. TT, testosterone; FSH, follicle-stimulating hormone.
The mechanism of ADT therapy inducing type 2 diabetes has been shown in murine hepatocytes—knockout (KO) of the androgen receptors (AR) in hepatocytes increased activation of gluconeogenic pathways and decreased activation of glycolytic pathways [19]. Hepatic insulin resistance itself has been shown to lead to dyslipidemia, but androgen deprivation has further direct effects on lipid metabolism as well [19]. Androgen deprivation has been shown to increase levels of circulating lipids, alter lipid metabolism in white adipose tissue, and cause excessive deposition of lipids in non-adipose tissues, including the liver and muscle [19]. AR-KO demonstrated a clear upregulation in production of multiple substrates involved in de novo fatty acid synthesis as well as a reduction in fatty acid oxidation [19].
Another possible mechanism by which ADT may increase cardiac disease risk is through decreased testosterone. Testosterone (TT) has been shown to be atheroprotective by decreasing monocyte adhesion to and transmigration across the vascular endothelium [20]. Low TT levels have been associated with a slight increased risk of MACE [21, 22]. A prior study on men with low TT showed that normalization of TT with TT replacement was associated with a reduction in all-cause mortality, myocardial infarction (MI) [23]. However, this study was retrospective and observational [23]. Conversely, other studies have found that TT replacement has led to an increased risk of cardiac events [24, 25]. Therefore, additional research efforts are needed to elucidate this hypothesis.
Another likely mechanism by which ADT increases CAD risk is through its interaction with the vascular endothelium. Follicle-stimulating hormone (FSH) receptors are expressed on vascular endothelial cells [26]. Since GnRH agonists primarily suppress LH, the increase in FSH observed with these agents may lead to endothelial cell activation. This endothelial cell activation could then lead to increased atherosclerotic plaque instability and inflammation (Figure 1b) [5, 26]. In a study of atherosclerotic plaques in mice, increased atherosclerotic plaque necrosis was observed in leuprolide-treated mice (11.0%) when compared to degarelix-treated mice (0.2%) and the control group (0.6%) [5].
Moreover, GnRH agonists stimulate Th1 cells to secrete pro-inflammatory cytokines including IFN-gamma, which then promotes secretion of collagenases that disrupt the integrity of an existing atherosclerotic plaque and make it more prone to rupture [26]. Th1 cells stimulated by FSH can also release RANKL and promote maturation of osteoclasts, which then resorb calcified regions in the atherosclerotic plaque and further compromise the stability of the plaque [26]. This increased inflammation and destabilization of plaque in pre-existing atherosclerosis could be the mechanism by which GnRH agonists increase CAD risk in men with prostate cancer when compared to GnRH antagonists.
Novel Oral GnRH Antagonist and Its Potential Influence in Cardio-oncology
Despite the well-studied advantages of degarelix as an effective treatment for prostate cancer, it is still an underused treatment option, likely due to injection-site reactions and the need for monthly injections. An oral regimen for PC may work to circumvent some, if not all, of the shortcomings of degarelix while maintaining similar efficacy and safety profiles compared to other GnRH antagonists.
Relugolix is an oral GnRH antagonist which was just recently approved by the FDA for use in PC. It is intended as a once-daily medication. A recent randomized, open-label, parallel-group phase II clinical trial studied testosterone levels in intermediate and high-risk PC patients undergoing external beam radiotherapy (EBRT) plus relugolix compared to degarelix [27•]. Results showed that conventional castration rates in the relugolix and degarelix arms were 95% and 89%, respectively, with time to castration in as few as 4 days in the relugolix group [27•]. This study demonstrated similar median prostate volume reductions as well as median PSA level reductions, with a ≥ 50% reduction in PSA levels seen in ≥ 97% of subjects within 12 weeks [27•]. This study confirmed the efficacy of oral relugolix in the setting of localized prostate cancer.
A recent randomized phase III clinical trial, HERO, compared relugolix to leuprolide in patients with advanced PCa [28••]. In this study, a prespecified analysis defined major adverse cardiovascular events as nonfatal MI, nonfatal stroke, and death from any cause [28••]. However, the findings on major adverse cardiovascular events were a secondary analysis, and the trial’s primary endpoint was anti-cancer efficacy. Castrate levels of testosterone suppression were profound and maintained throughout the trial in the relugolix group, with a mean testosterone level of 38 ng/dL on day 4, compared to a testosterone surge in the leuprolide group with a mean testosterone level of 625 ng/dL at day 4 and castrate levels not occurring until day 29 [28••]. The incidence of MACEs in the relugolix arm was 2.9% compared with 6.2% of patients who received leuprolide, with a 54% lower risk in the relugolix group compared to the leuprolide group [28••]. Among patients who had pre-existing CVD, incidence of adverse cardiac events was 3.6% in the relugolix group and 17.8% in the leuprolide group [28••]. This study was limited by the relatively short duration of follow-up (48 weeks) along with its unblinded study design [28••]. Another limitation of this study was that it identified major adverse cardiovascular events through safety reporting rather than as a pre-specified outcomes, thereby potentially missing cardiac events that would affect the results [28••]. The HERO trial could support the hypothesis that GnRH antagonists may be a better treatment choice in men with prostate cancer and cardiac risk factors; however, it is hypothesis-generating, and additional studies are warranted.
In a recent study by Margel et al., the risk of cardiovascular events and development of cardiovascular disease among patients receiving GnRH agonists or antagonists were compared in individuals with elevated baseline cardiac biomarkers, including pro-BNP, troponin, D-dimer, and CRP [29]. This study showed that baseline-elevated pro-BNP and troponin were positively associated with an increased risk of developing new CV events in patients taking GnRH agonists, but not GnRH antagonists [29]. Results showed that 46% of patients with a baseline pro-BNP > 125 pg/mL suffered a CV event while on a GnRH agonist, while only 6% of patients with a baseline pro-BNP > 125 pg/mL suffered a CV event while on a GnRH antagonist (p = 0.008) [18]. Similarly, 64% of baselines with a baseline troponin > 14 suffered a CV event while on a GnRH agonist, while only 5% of patients suffered a CV event while on a GnRH antagonist (p = 0.004) [16••].
A vital study in the field of cardio-oncology is the PRONOUNCE trial, which is the first prospective study in prostate cancer with a primary endpoint of cardiovascular outcomes as opposed to anticancer efficacy [30]. This blinded study randomized men with prior CAD to GnRH antagonist degarelix or GnRH agonist leuprolide for 1 year [30]. Its primary endpoint is time from randomization to first MACE, defined as a composite endpoint of all-cause death, nonfatal MI, or nonfatal stroke [30]. Initial plans were to enroll 900 patients; however, the trial was closed early due to the COVID-19 pandemic. Data analysis on the 545 patients is currently ongoing. Exploratory analyses of cardiac biomarkers such as NT-BNP, hsCRP, and hsTn will be performed [30].
Androgen Receptor Signaling Inhibitors
Another class of anti-androgenic medications used for men with PCa are the second-generation androgen receptor signaling inhibitors. They are used with ADT. Research has demonstrated that these agents in combination of ADT lead to improved overall survival compared to ADT alone [31••]. However, these agents have been shown to have a higher risk of cardiovascular events [32]. For example, enzalutamide is an oral androgenic receptor inhibitor used in combination with ADT to treat nonmetastatic, castration-resistant prostate cancer and has been shown to lead to a lower risk of metastases or death compared with ADT alone [31••, 33]. However, patients who received enzalutamide had an increased incidence of hypertension (18% vs placebo 6%), increased cardiovascular events (including hemorrhagic central nervous system vascular conditions, ischemic central nervous system vascular conditions, and cardiac failure, 6% vs placebo 2%), and increased events of ischemic heart disease (including myocardial infarction and other ischemic heart disease (6% vs 2%) [31••].
The mechanism for enzalutamide-induced hypertension remains unclear but generally thought to be similar to ADT since enzalutamide induces a state of deeper androgen deprivation. It was observed that there were no significant EKG changes such as QTc prolongation associated with enzalutamide. It was not associated with higher rates of hyperglycemia, hyperlipidemia, or weight gain [34, 35]. Thus, it is possible that the development hypertension seen with enzalutamide is through another mechanism besides metabolic syndrome [35].
Another novel agent, abiraterone, has a distinct mechanism for cardiovascular toxicity. Abiraterone inhibits CYP17, an enzyme involved in cortisol synthesis, resulting in low cortisol, which stimulates hypothalamic-pituitary-adrenal (HPA) axis [36]. Stimulation of HPA axis leads to increase in aldosterone, causing fluid retention, hypertension, and hypokalemia [36]. Abiraterone has been linked to a significantly increased risk of multiple cardiac events including ischemic heart disease, myocardial infarction, supraventricular tachyarrhythmias, ventricular tachyarrhythmias, cardiac failure, and hypertension [34]. Another study showed that abiraterone was linked to elevated cardiac biomarkers including troponin and BNP [37]. Patients with a prior history of hypertension were more likely to develop cardiac complications from abiraterone [37].
Since these agents are now being used earlier in the disease course, and patients are living longer with PC, cardiovascular side effects should be of high concern to the clinician. For example, the most frequent adverse event leading to death in the PROSPER trial was cardiovascular events, which occurred in 2% (14 events out of 930 patients) of the enzalutamide group compared to <1% (2 events out of 465 patients) in the placebo group [31••]. Of note, 10 out of 14 of the patients in the enzalutamide group who had cardiovascular events leading to death had a history significant for cardiovascular disease [31••]. These data suggest that patients who have a significant cardiovascular history and planning to take enzalutamide may benefit from more aggressive cardiovascular disease treatment and more vigilant follow-up for monitoring of cardiovascular events.
Conclusions
ADT is part of the standard of care of PC. Evolving data suggests that GnRH antagonists have more favorable cardiovascular outcomes compared to GnRH agonists while maintaining a similar efficacy profile. Men with PC are at higher risk of CAD, and the most likely non-oncologic cause of death in men with PC is CAD. From the data we reviewed, it is suggested that GnRH antagonists might be the preferred method of ADT in men with PC. The oral GnRH antagonist, relugolix, could be superior for the treatment of men with PC and risk factors, history, or active cardiovascular disease, pending further clinical trials. Results of the PRONOUNCE trial, with a primary endpoint of cardiovascular outcomes, could support this hypothesis. While the second-generation androgen receptor signaling inhibitors in combination with ADT have shown to have a survival benefit in late stage of PC, we emphasize more aggressive cardiovascular disease treatment and close follow-up for monitoring of cardiovascular events in these patients. We emphasize that since men are living longer with PC, and these agents are being used earlier in the disease course, minimizing cardiovascular toxicities is critical to optimize outcomes.
Declarations
Conflict of Interest
The authors have no conflict of interest to report.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Cardio-Oncology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Julia Boland, Email: jboland@gwu.edu.
William Choi, Email: wwchoi5@gwu.edu.
Maximillian Lee, Email: malee@mfa.gwu.edu.
Jianqing Lin, Email: Jilin@mfa.gwu.edu.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Bekelman JE, Rumble RB, Chen RC, Pisansky TM, Finelli A, Feifer A, Nguyen PL, Loblaw DA, Tagawa ST, Gillessen S, Morgan TM, Liu G, Vapiwala N, Haluschak JJ, Stephenson A, Touijer K, Kungel T, Freedland SJ. Clinically localized prostate cancer: ASCO clinical practice guideline endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J Clin Oncol. 2018;36(32):3251–3258. doi: 10.1200/JCO.18.00606. [DOI] [PubMed] [Google Scholar]
- 3.Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists' Collaborative Group. Lancet, 2000. 355(9214): p. 1491-8. [PubMed]
- 4.Bhatia N, Santos M, Jones LW, Beckman JA, Penson DF, Morgans AK, Moslehi J. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer: ABCDE steps to reduce cardiovascular disease in patients with prostate cancer. Circulation. 2016;133(5):537–541. doi: 10.1161/CIRCULATIONAHA.115.012519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knutsson A, Hsiung S, Celik S, Rattik S, Mattisson IY, Wigren M, Scher HI, Nilsson J, Hultgårdh-Nilsson A. Treatment with a GnRH receptor agonist, but not the GnRH receptor antagonist degarelix, induces atherosclerotic plaque instability in ApoE(-/-) mice. Sci Rep. 2016;6:26220. doi: 10.1038/srep26220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leong DP, et al. Cardiovascular risk in men with prostate cancer: insights from the RADICAL PC study. J Urol. 2020;203(6):1109–1116. doi: 10.1097/JU.0000000000000714. [DOI] [PubMed] [Google Scholar]
- 7.Collaborators, G.B.D.C.o.D Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016 a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 9.Waxman J, Man A, Hendry WF, Whitfield HN, Besser GM, Tiptaft RC, Paris AM, Oliver RT. Importance of early tumour exacerbation in patients treated with long acting analogues of gonadotrophin releasing hormone for advanced prostatic cancer. Br Med J (Clin Res Ed) 1985;291(6506):1387–1388. doi: 10.1136/bmj.291.6506.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71(6):1001–6. doi: 10.1016/j.urology.2007.12.070. [DOI] [PubMed] [Google Scholar]
- 11.Conn PM, Crowley WF., Jr Gonadotropin-releasing hormone and its analogues. N Engl J Med. 1991;324(2):93–103. doi: 10.1056/NEJM199101103240205. [DOI] [PubMed] [Google Scholar]
- 12.Sharifi R, Browneller R, Leuprolide Study G. Serum testosterone suppression and potential for agonistic stimulation during chronic treatment with monthly and 3-month depot formulations of leuprolide acetate for advanced prostate cancer. J Urol. 2002;168(3):1001–1004. doi: 10.1016/S0022-5347(05)64560-0. [DOI] [PubMed] [Google Scholar]
- 13.Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, Jensen JK, Olesen TK, Schröder FH. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531–1538. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 14.Shore ND. Experience with degarelix in the treatment of prostate cancer. Ther Adv Urol. 2013;5(1):11–24. doi: 10.1177/1756287212461048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65(3):565–573. doi: 10.1016/j.eururo.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Margel D, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202(6):1199–1208. doi: 10.1097/JU.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 17.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91(4):1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 18.Kiwata JL, Dorff TB, Schroeder ET, Gross ME, Dieli-Conwright CM. A review of clinical effects associated with metabolic syndrome and exercise in prostate cancer patients. Prostate Cancer Prostatic Dis. 2016;19(4):323–332. doi: 10.1038/pcan.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu IC, Lin HY, Sparks JD, Yeh S, Chang C. Androgen receptor roles in insulin resistance and obesity in males: the linkage of androgen-deprivation therapy to metabolic syndrome. Diabetes. 2014;63(10):3180–3188. doi: 10.2337/db13-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo C, Pook E, Tang B, Zhang W, Li S, Leineweber K, Cheung SH, Chen Q, Bechem M, Hu JS, Laux V, Wang QK. Androgen inhibits key atherosclerotic processes by directly activating ADTRP transcription. Biochim Biophys Acta Mol basis Dis. 2017;1863(9):2319–2332. doi: 10.1016/j.bbadis.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corona G, Monami M, Boddi V, Cameron-Smith M, Fisher AD, de Vita G, Melani C, Balzi D, Sforza A, Forti G, Mannucci E, Maggi M. Low testosterone is associated with an increased risk of MACE lethality in subjects with erectile dysfunction. J Sex Med. 2010;7(4 Pt 1):1557–1564. doi: 10.1111/j.1743-6109.2009.01690.x. [DOI] [PubMed] [Google Scholar]
- 22.Corona G, Rastrelli G, di Pasquale G, Sforza A, Mannucci E, Maggi M. Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. J Sex Med. 2018;15(9):1260–1271. doi: 10.1016/j.jsxm.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Sharma R, Oni OA, Gupta K, Chen G, Sharma M, Dawn B, Sharma R, Parashara D, Savin VJ, Ambrose JA, Barua RS. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36(40):2706–2715. doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
- 24.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF, Hoover RN. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vigen R, O'Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 26.Crawford ED, Schally AV, Pinthus JH, Block NL, Rick FG, Garnick MB, Eckel RH, Keane TE, Shore ND, Dahdal DN, Beveridge TJR, Marshall DC. The potential role of follicle-stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy. Urol Oncol. 2017;35(5):183–191. doi: 10.1016/j.urolonc.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Dearnaley DP, et al. The oral gonadotropin-releasing hormone receptor antagonist relugolix as neoadjuvant/adjuvant androgen deprivation therapy to external beam radiotherapy in patients with localised intermediate-risk prostate cancer: a randomised, open-label, parallel-group phase 2 trial. Eur Urol. 2020;78(2):184–192. doi: 10.1016/j.eururo.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Shore ND, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187–2196. doi: 10.1056/NEJMoa2004325. [DOI] [PubMed] [Google Scholar]
- 29.Margel, D., et al., Cardiac biomarkers in patients with prostate cancer and cardiovascular disease receiving gonadotrophin releasing hormone agonist vs antagonist. Prostate Cancer Prostatic Dis, 2020. [DOI] [PMC free article] [PubMed]
- 30.Melloni C, et al. Cardiovascular safety of degarelix versus leuprolide for advanced prostate cancer: the PRONOUNCE trial study design. JACC: CardioOncol. 2020;2(1):70–81. doi: 10.1016/j.jaccao.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sternberg CN, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197–2206. doi: 10.1056/NEJMoa2003892. [DOI] [PubMed] [Google Scholar]
- 32.Di Nunno V, et al. New hormonal agents in patients with nonmetastatic castration-resistant prostate cancer: meta-analysis of efficacy and safety outcomes. Clin Genitourin Cancer. 2019;17(5):e871–e877. doi: 10.1016/j.clgc.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, Ivashchenko P, Demirhan E, Modelska K, Phung D, Krivoshik A, Sternberg CN. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465–2474. doi: 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iacovelli R, Ciccarese C, Bria E, Romano M, Fantinel E, Bimbatti D, Muraglia A, Porcaro AB, Siracusano S, Brunelli M, Mazzarotto R, Artibani W, Tortora G. The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourin Cancer. 2018;16(3):e645–e653. doi: 10.1016/j.clgc.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Fléchon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 36.Saltalamacchia G, et al. Renal and cardiovascular toxicities by new systemic treatments for prostate cancer. Cancers (Basel). 2020;12(7). [DOI] [PMC free article] [PubMed]
- 37.Bretagne M, Lebrun-Vignes B, Pariente A, Shaffer CM, Malouf GG, Dureau P, Potey C, Funck-Brentano C, Roden DM, Moslehi JJ, Salem JE. Heart failure and atrial tachyarrhythmia on abiraterone: a pharmacovigilance study. Arch Cardiovasc Dis. 2020;113(1):9–21. doi: 10.1016/j.acvd.2019.09.006. [DOI] [PubMed] [Google Scholar]