Highlights
-
•
The genomes and proteomes of 12 Bifidobacterium and 46 Lactobacillus were reviewed and then compared for bacteriocin identification.
-
•
NCBI-Genome, UniProt-Proteome, Bactibase, and BAGL4 databases, as well as BLASTP, and Clustal Omega can be used for bacteriocin mining.
-
•
Lactobacillus species have more diversity and abundance of bacteriocin compared to Bifidobacterium species.
-
•
Notably, L. sakei, L. plamtarum, L. reuteri, L. fermentum, and L. casei had the highest pathogen inhibition (E. coli MG 1655); respectively.
-
•
A set of Lactobacillus bacteria including L. sakei, L. reuteri, L. fermentum, and L. casei can be proposed as a biosecure and safe solution to control gastrointestinal pathogens.
Keywords: Probiotics, Genome, Proteome, Bacteriocin, Pathogen inhibition
Abstract
Bacteriocins are a large family of bacterial peptides or proteins, ribosomally synthesized with antimicrobial activity against other bacteria. We investigated and compared the genomes and proteomes of 12 Bifidobacterium and 46 Lactobacillus species for bacteriocins using NCBI-Genome, UniProt-Proteome, Bactibase, and BAGL4 databases. Selected Lactobacillus species were examined for bile salt resistance, acid and pH resistance, pepsin and trypsin enzyme resistance, and antibiotic resistance. Also, the antimicrobial activity of selected Lactobacillus species was evaluated against E. coli MG 1655. Results showed that Lactobacillus species have more diversity and abundance of bacteriocin compared to Bifidobacterium species. Notably, L. sakei, L. plamtarum, L. reuteri, L. fermentum, and L. casei had the highest pathogen inhibition; respectively. Therefore, a combination of these Lactobacillus species can be suggested as a biochemical and safe solution to control gastrointestinal pathogens and suitable alternatives to antibiotics and chemicals in food technology.
1. Introduction
The World Health Organization (WHO) and the Food and Agriculture Organization (FAO) have defined probiotics as live microorganisms that, when consumed adequately, can exhibit health effects in the host body. Several isolates of lactic acid bacteria (LAB) are used in food preservatives due to their antimicrobial properties. [1,2]. These bacteria can be used to control human pathogens. Lactobacillus are gram-positive, rod-shaped, non-spores-free, flagellate-free, and anaerobic bacteria or microaerophile [3]. Lactobacillus species are the largest LAB group with the ability to convert lactose and other sugars to lactic acid. Lactobacillus species include both homofermentative and heterofermentative species; homofermentative species ferment sugars predominantly into lactic acid (more than 90%) and do not produce gas. Heterofermentative species, on the other hand, ferment sugar (glucose) into lactic acid besides other substances such as acetic acid and produce CO2 [4,5]. A differentiating factor between Lactobacillus species is the amount of lactic acid, which differs among them. Notably, different Lactobacillus species may produce different types of lactic acid as follows: L-lactate, D-lactate, or a mixture of both [6]. Bifidobacterium species are gram-positive, rod-shaped, anaerobic, catalase-negative bacteria that belong to the branch of actinobacteria. The ideal pH for the growth of Bifidobacterium species is 6-7, and at pH about 4.5-5 and above 8-8.5, no growth is observed. Bifidobacterium species have been found in human beings, warm-blooded animals, and bees. The abundance of Bifidobacterium species depends on age and diet. They settle in the gastrointestinal tract shortly after birth and form the dominant strain in the gastrointestinal tract after birth, and their number decreases with age. [7,8]. The main difference between Bifidobacterium species and other Lactobacillus species is in the source of nitrogen, in a way that Bifidobacterium can grow in the environments containing ammonium (mineral nitrogen), in contrast, other lactic acid bacteria need an organic nitrogen source such as peptides to multiply [9,10].
Various studies conducted on probiotic strains have reported different results, such as the strains used must have specific characteristics to have beneficial effects [10]. In this regard, they are not pathogenic and belong to the GRAS group (Generally recognized as safe) under essential conditions [11]. The main characteristics of probiotic bacteria are a) survival under conditions exposed to stomach acids and bile salts [12], b) ability to adhere to the mucosal surface of the gastrointestinal tract to prevent leaching by smoky bowel movements [13], c) antagonistic effect on a specific pathogen by producing antimicrobial substances, etc. [14], d) antibiotic resistance [15], and e) the ability to stimulate the immune system without causing inflammation.
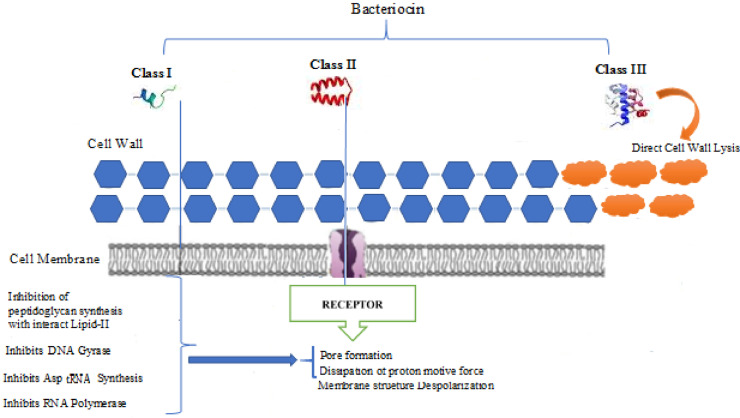
Bacteriocins are protein metabolites, usually with a molecular weight of less than 10 kDa containing 30-70 amino acids and are helical amphiphilic [16,17]. These compounds differ in terms of molecular weight of genetic origin, type of action, and biochemical properties [18]. Bacteriocins are synthesized by ribosomes and are active against bacteria closely related to the produced bacteria [19,20]. Extensive research has shown that many bacteria and archaea can produce bacteriocin [21], [22], [23]. Numerous bacteriocins have received a great deal of attention due to their significant potentials as food preservatives, therapeutic agents, or biological controllers [24]. These antimicrobial peptides have been reported in many bacteria to act against microbial pathogens in humans and animals without showing any toxicity [25]. Therefore, the most important benefits of bacteriocins are their physical stability and non-toxicity [26,27]. Different classes of bacteriocins have different mechanisms against gram-positive and gram-negative bacteria [28]. Bacteriocins can attach to cell wall components, including lipids or molecular binding sites, through a specific or nonspecific receptor, which disrupts or lyses the cell and consequently causes cell death by depleting the bacterial proton locomotor system [29]. Nisin attacks the cytoplasmic membrane of gram-negative bacteria when combined with EDTA (ethylene diamine tetra-acetic acid). Mersacidin kills gram-positive bacteria by inhibiting cell wall synthesis and increasing its activity with calcium ions [30]. Lysostaphin of class III bacteriocins kills the Staphylococcus aureus cell wall by lysis [31]. Colicin can also kill gram-negative bacteria by forming cavities. Pesticin, which is a high molecular weight ba cteriocin (39.9 kDa), kills the gram-negative bacteria Yersinia spp. and Escherichia coli by loosening the cell wall through breaking glycosidic bonds from the cell wall. Bifidobacterium bifidum produces two bacteriocins, bifidin and bifidocin B. Bifidobacterium infantis BCRC 14602 produces bifidin I which consists of 8 amino acid residues. The antibacterial activity of bifidin I and its ability to inhibit the growth of pathogens can prevent food spoilage and food-borne diseases, thus greatly contributing to food safety. These examples show that each class of bacteriocin can kill gram-positive and gram-negative bacteria in a variety of well-known ways. Lacticin 3147 contains two peptides, named LtnA1 and LtnA2, which kill Listeria by inhibiting cell wall synthesis and cavity formation [28]. Many molecules that interact with the cell surface, such as the mannose phosphotransferase and lipid II system, are known as molecules interacting with class I bacteriocins [32]. Class I molecules can directly penetrate the cell membrane and consequently affecting cell integrity. Also, some class I molecules inhibit cell wall synthesis in interaction with lipids. Class II molecules bind to the pore receptor system, such as mannose phosphotransferase, and depolarize cell membranes. Class III molecules directly cause cell lysis. Figure 1 schematically shows different classes of bacteriocins as well as their mechanisms. Sub-Ib antimicrobial peptides are distinguished by their rigid and spherical nature, and DNA / RNA inhibition is performed by protein or cell wall synthesis by binding with electrostatic adsorption to a membrane or by specific binding to some membrane components such as the mannose phosphotransferase system [33,34]. Nisins operate in a dual state as follows: 1- They can inhibit cell wall synthesis by binding to lipid II (as the primary transporter of peptide glycan subunits from the cytoplasm to the cell wall) which results in cell death. 2- They can remove lipid II from the membrane structure by forming pores in the membrane, which leads to cell death [35]. Antibiotics are known as secondary metabolites made up of multiple and large enzyme complexes during various cellular processes. Moreover, they have a wide range of antimicrobial activities produced by some bacteria and fungi, as well as broad-spectrum effects on other microorganisms [36]. On the other hand, Bacteriocins are antimicrobial peptides synthesized on ribosomes during the early growth phase using a translation process and also have a narrow spectrum of antimicrobial activity, mainly against those closely related species. The bacteriocin mechanism on target cells is diverse and associated with the formation of pores in the outer cell membrane. On the other hand, bacteriocin can inhibit the synthesis of intracellular processes and the replications of DNA and RNA [37], [38], [39].
Figure 1.
Mechanism of action of bacteriocins
2. Materials and methods
2.1. Investigation of bacteriocin in Lactobacillus and Bifidobacterium species
BACTIBASE (http://bactibase.hammamilab.org/main.php) and BAGEL4 (http://bagel4.molgenrug.nl/databases.php), were used to identify the bacteriocins produced by Lactobacillus and Bifidobacterium species. Also, 46 bacteriocins belong to Lactobacillus and one case belongs to Bifidobacterium were identified. BAGEL4 is a database that enables researchers to mine and visualizes bacterial genomic DNA for bacteriocins and ribosome-synthesized and posttranslationally modified peptides (RiPPs) and bacteriocin-producing gene clusters in the genomes [40]. BACTIBASE is a database designed to characterize of bacteriocins and provides a manually curated annotation of bacteriocin sequences. BACTIBASE has various tools for bacteriocin analysis, such as homology search, multiple sequence alignments, and retrieval through taxonomy browser [41]. The search filter was species name.
2.2. Investigation of bacteriocin sequences in the proteomes
173 proteomes references of Lactobacillus and Bifidobacterium species were retrieved from the proteomes section of UniProt (www.uniprot.org/proteomes/). Bacteriocin sequences were identified and retrieved from the BACTIBASE and BAGEL4 biological databases. After preparing library of bacteriocin and related proteomes, BLASTP and BioEdit 7.2 were used for the similarity alignment and determination of the exact and similar sequences. Also, the frequency of bacteriocin sequences was determinated in the genomes of Lactobacillus and Bifidobacterium species using the BLASTN and NCBI Genome (www.ncbi.nlm.nih.gov/genome/).
2.3. Evaluation of probiotic indicators
Lactobacillus species were optained from the (Persian Type Culture Collection (PTCC) and were cultured in De Man, Rogosa and Sharpe (MRS) medium.
2.4. Evaluation of acid and pH resistance
For the assessment of acid and pH resistance, the study of microorganisms' resistance under acidic conditions was performed by culturing microorganisms in a liquid culture medium which pH was chenged. After the incubation at the appropriate temperature and time, the number of microorganisms was counted [42]. Also, following the preparation of the MRS broth medium, they were poured into two separate containers, their pH levels were adjusted to 2.5 and 4 with hydrochloric acid, and then autoclaved. In 50 ml Falcons, 10 ml of the culture medium was added, and then 100 μl of a half-McFarland turbid microbial suspension was inoculated into each one of the Falcons. Subsequently, the falcons were incubated for 3 to 4 hours at 37°C. After the incubation process, 1 ml of the suspension was removed and then inoculated into the MRS Agar culture medium. Next, the plates were incubated for 48 hours at 37°C and after the incubation, the growth of bacteria was examined. The evaluation of resistance to pepsin and trypsin: the evaluation of resistance to acidic stomach conditions was performed by culturing microorganisms in culture media simulated with gastric fluid containing pepsin and trypsin, incubation at appropriate temperature and time, linear culture on appropriate agar medium, and growth and lack of growth were determined.
2.5. Evaluation of bile salt resistance
The evaluation of bile salt resistance was performed by examining the growth rate of selected strains in the presence of bile salts (bile oxalate). To prepare the culture medium, 3 gr of bile oxalate was used in liquid MRS in one liter, which was then sterilized for 15 minutes at 121°C. Subsequently, 100 microliters of microbial suspension were added to the culture medium containing bile and the medium with no bile (as blank), and the optical absorption (OD) of the mediums was then measured at 600 to 650 nm before the incubation process. The media were incubated for 8 hours at 37°C, and the light absorption (OD) of the media was measured again at 600 to 650 nm. The resistance of selected strains to bile was calculated from the following formula:
wherein:
Cinh: Coefficient of Inhibition
Absorption of light in the culture medium without bile, after 8 hours of incubation (T8 Control).
Absorption of light in the culture medium without bile, before the incubation process (T0 Control)
Absorption of light in culture medium containing bile, after 8 hours of incubation (T8 Treatment)
Absorption of light in culture medium containing bile, before the incubation process (T0 Treatment)
The inhibition factor must be equal to or less than 0.4.
2.6. Evaluation of antibiotic susceptibility of probiotic strains
The evaluation of antibiotic susceptibility or antibiogram profile of selected strains was performed by examining the tolerance of these strains to antibiotics. Accordingly, in this test, the Kirby-Bayer disk diffusion method and CLSI (Clinical and Laboratory Standards Institute) standard were used. The antibiotics used in this test included the following: Amoxicillin (AMX), tetracycline (TE), cefotaxime (CTX), gentamicin (GM), vancomycin (V), chloramphenicol (C), and ciprofloxacin (CP). At first, a half-McFarland microbial suspension was grass-fed on Müller-Hinton (MHA) medium, standard antibiotic discs were placed on the surface, and then the plates were incubated for 24 hours at 37°C. At the end of the incubation process, the diameter of the growth inhibition zone (mm) was measured. The obtained results are reported as follows: Strain resistant (R) (15 ≤ mm), semi-sensitive (I) (16 -20 mm), and sensitive (S) (21≤ mm).
2.7. Evaluation of the antimicrobial activity
The well diffusion agar method was used to evaluate the antibacterial activity of of selected strains on Escherichia coli MG1655. Selected Lactobacillus strains were cultured in the MRS broth culture medium and incubated for 48 hours at 37°C. After the incubation period, the falcons containing the microorganisms were centrifuged in a refrigerated centrifuge at 1500 rpm for 15 minutes at 4°C, then the supernatant was transferred to the new falcons. Due to the lack of growth of Escherichia coli in the MRS medium, the nutrient agar medium was used for Escherichia coli. From the microbial suspension of Escherichia coli with the turbidity of half McFarland (CFU⁄mg), the grass culture medium was given on the nutrient agar medium. Next, using a pipette, a well with a 2 mm diameter was created in plates containing the culture medium, and agar-bearing MRS sampler was then added to the wells and selected strains were inoculated from the supernatant into the wells. Afterward, the plates were refrigerated for 2 h (to absorb the solution) and then incubated for 24 h at 37°C. At the end of the incubation process, the diameters were measured. Notably, the presence of a growth inhibition zone with a diameter of more than 2 mm is reported as an antimicrobial effect on pathogenic microorganisms.
3. Results
3.1. In silico study
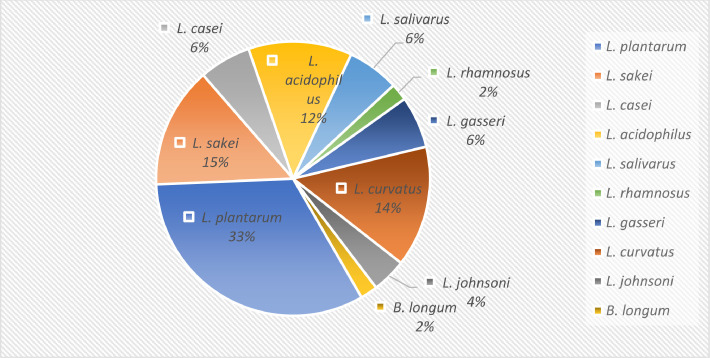
In this study, by identifying the genomes and proteomes of probiotic bacteria and comparing them, it was found that Lactobacillus species have more abundance and diversity of bacteriocins as well as only a limited number of Bifidobacterium that have reported to contain bacteriocins (Figure 2). The results show that several bacteriocins belong to several species of Lactobacillus and bifidobacteria, like L. reuteri, while some bacteriocins were species-specific, like BLD1648, which is important in the use of Lactobacillus and Bifidobacterium as probiotics. According to studies conducted on the genome and proteome of Lactobacillus and Bifidobacterium as well as their ability in producing antimicrobial compounds, these bacteria can fight pathogenic bacteria.
Figure 2.
Abundance and diversity of bacteriocin in Lactobacillus and Bifidobacterium species based on In silico analysis.
3.2. Evaluation of acid and pH resistance
The results show that L. casei, L. sakei, L. reuteri, and L. fermentum strains had the best growth rates at pH 4, 2.5(Table 1).
Table 1.
Counting colonies of Lactobacillus species at 4 and 2.5 pH based on CFU
| CFU (pH 4) | CFU (pH 2.5) | Organism |
|---|---|---|
| >1000 | 925 | L. casei |
| >1000 | 168 | L. paracasei |
| >1000 | 216 | L. sakei |
| >1000 | 335 | L. reuteri |
| >1000 | 825 | L. fermentum |
| >1000 | 454 | L. plantarum |
| >1000 | 100 | L.rhamnosus |
3.3. Evaluation of resistance to pepsin and trypsin
In the presence of pepsin enzyme, L. casei, L. paracasei, L. fermentum, and L. reuteri bacteria, and in the presence of trypsin enzyme, L. sakei, L. paracasei, L. reuteri, and L. rhamnosus had the best growth rates. In general, L. paracasei, L. casei, L. reuteri, and L. fermentum showed the highest growth and resistance rates in the presence of trypsin and pepsin enzymes after 2 and 24 hours (Table 2).
Table 2.
Colony count of Lactobacillus species based on CFU at pH = 2 at 2 and 24 hours
| CFU Try-24hr) | CFU (Try-2hr) | CFU (Pep-4hr) | CFU(Pep-2hr) | Organism |
|---|---|---|---|---|
| 31 | 35 | 126 | >1000 | L.casei |
| 38 | 64 | 236 | >1000 | l.plantarum |
| 44 | 85 | 368 | >1000 | L.paracasei |
| 22 | 42 | 35 | 365 | L.sakei |
| 35 | 52 | 58 | >1000 | L.reuteri |
| 37 | 32 | 67 | >1000 | L.fermentum |
| 26 | 57 | 51 | >1000 | L.rhamnosus |
3.4. Evaluation of bile salt resistance
The results show that L. casei, L. paracasei, L. rhamnosus, and L. sakei showed the lowest inhibition coefficients, and therefore grew better in the presence of bile salts (Table 3).
Table 3.
Bile salt resistance of Lactobacillus species
| Cinh | Treatment | Control | Organism | ||
|---|---|---|---|---|---|
| T8 | T0 | T8 | T0 | ||
| 0.37 | 0.12 | 0.002 | 0.19 | 0.002 | L. casei |
| 0.44 | 0.09 | 0.003 | 0.16 | 0.003 | L. paracasei |
| 0.45 | 0.1 | 0.002 | 0.18 | 0.002 | L. sakei |
| 0.47 | 0.07 | 0.003 | 0.13 | 0.003 | L. reuteri |
| 0.47 | 0.08 | 0.003 | 0.15 | 0.003 | L. fermentum |
| 0.35 | 0.13 | 0.002 | 2 | 0.002 | L. plantarum |
| 0.58 | 0.06 | 0.003 | 0.14 | 0.003 | L.rhamnosus |
3.5. Evaluation of antibiotic susceptibility or antibiogram profile
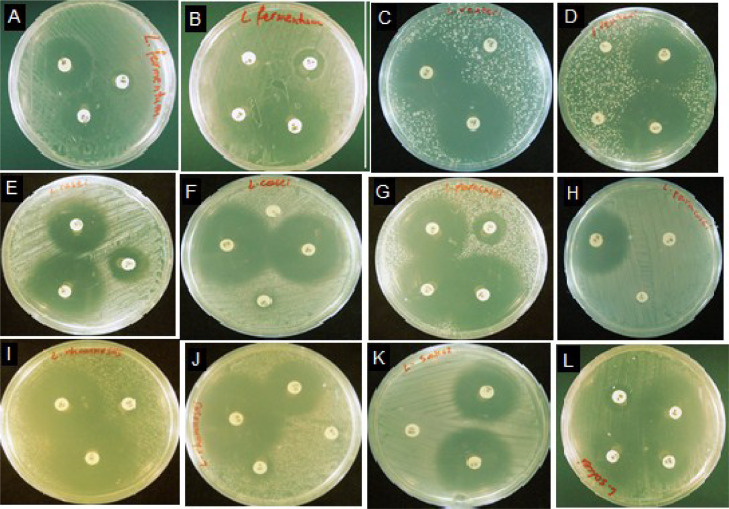
The results were reported according to the standard as mentioned by Ruiz-Moyano et al. [43]. All strains are resistant to GM, CP, and V and also are sensitive to AMX, C, CTX, and TE (Figure 3).
Figure 3.
Evaluation of antibiogram susceptibility of probiotic strains. There are probiotics on both sides. L. fermentum (A, B). L. reuteri, (C, D). L. casei. (E, F) L. paracasei. (G, H). L. rhamnosus (I, J). L. sakei (K, L).
3.6. Evaluation of the antimicrobial activity
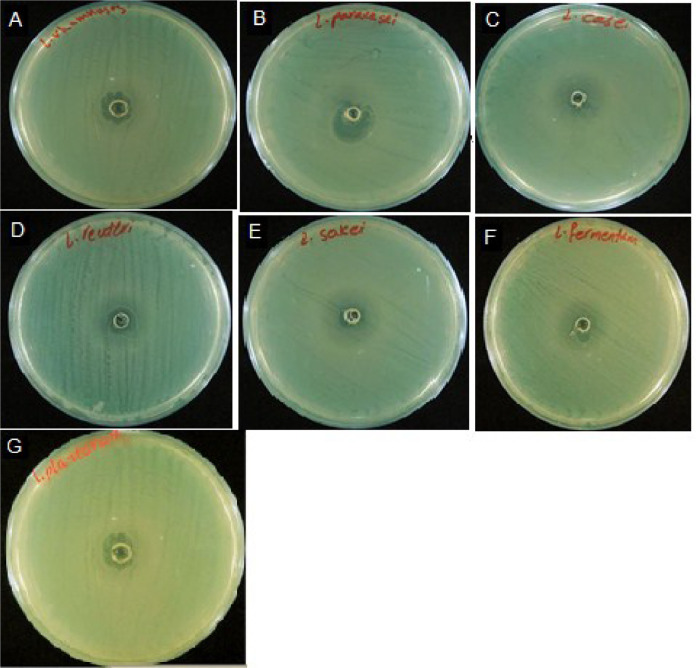
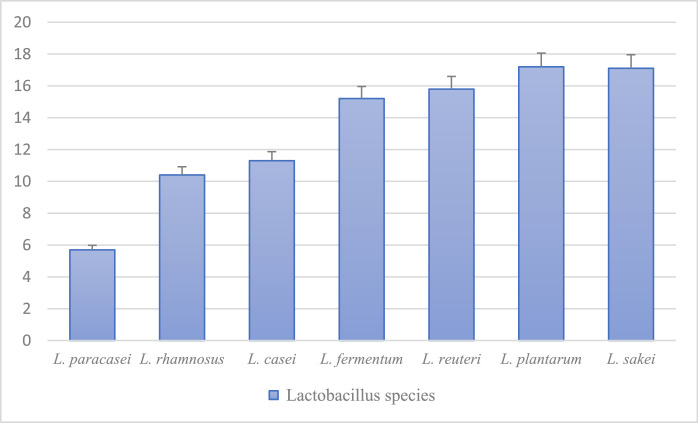
Antimicrobial activity of selected strains were performed with 3 replications, which showed that L. sakei, L. reuteri, L. plantarum, and L. fermentum had the best resistance rates against Escherichia coli MG1655 (Figure 4). The size of the halo diameters are shown in Figure 5.
Figure 4.
Observation of the antimicrobial activity of probiotic strains against Escherichia coli MG1655. L. rhamnosus (A), L. paracasei. (B), L. casei. (C), L. reuteri, (D), L. sakei (E), L. fermentum (F), L. plantarum (G).
Figure 5.
Measurement of growth of lactobacillus strains in mm against Escherichia coli MG1655. According to the diagram, L. sakei, L. plantarum, L. reuteri, L. fermentum had the best resistance against Escherichia coli MG1655.
4. Discussion
This study aimed to determine the antibacterial activity of the strains of Lactobacillus and Bifidobacterium and their roles in the inhibition of E. coli in two parts as follows: in silico and in vivo. By identifying the genomes and proteomes of probiotic bacteria and comparing them, we found that Lactobacillus species have a greater abundance and diversity of bacteriocins than Bifidobacterium species. Coneonnier et al. (1998) indicated that the use of the supernatant cultures of L. casei, L. fermentum, and L. acidophilus bacteria has an antimicrobial effect on a wide range of gram-positive and gram-negative bacteria [44]. In this regard, our research showed that L. sakei, L reuteri, L. fermentum, and L. casei have good inhibitory effects on Gram-negative Escherichia coli. Anas et al. (2008) stated that the culture of Lactobacillus plantarum has a strong antimicrobial effect on Escherichia coli and Staphylococcus aureus [45]. The results of our study show that the Lactobacillus plantarum has the highest frequency and diversity in terms of bacteriocin production. Moreover, by examining bacteriocins in bioinformatics studies, it was revealed that the gene of some bacteriocins located in the plasmid of probiotic bacteria, which is one of the prominent features of bacteriocins that require plasmid mediation like Lactobacillus plantarum. A noteworthy point in the present study is that experimental studies are consistent with bioinformatics studies in terms of L. sakei that showed the high diameter of growth inhibition zone against the E. coli among the selected bacteria, which also have more frequency and variety of bacteriocin in bioinformatics studies. Bioinformatics studies have also shown that L. fermentum has the high number of bacteriocins and has the high rank in terms of the diameter of the growth inhibition zone against the pathogen. L. paracasei also showed the lowest drop of the growth inhibition, which is low in terms of bacteriocin production in bioinformatics studies. However, according to experimental studies, L. paracasei has a high resistance to bile salts as well as the enzymes pepsin and trypsin. . In addition, it is notable that bile salts can kill microorganisms by disrupting the structure of the cell wall. Therefore, it can be concluded that despite the low abundance of bacteriocin and the diameter of the halo of low growth against E. coli strain that is resistant to bile salts and gastric aneurysms, as one of the essential properties of probiotics (survival and activity in the small intestine), it is one of the most vital probiotics. Another noteworthy point in bioinformatics studies is that, although some species are low in bacteriocin in terms of abundance and diversity, they have high antimicrobial activities in experimental calculations, For example, although L. reuteri is not able to produce many bacteriocins, it shows excellent antimicrobial activity. Also, L. casei is in the low level of bacteriocin production, but it has very resistance to stomach acid and bile salts. Due to the resistance of the species investigated in this study to vancomycin, ciprofloxacin, and gentamicin, it is possible to use these probiotics simultaneously during the treatment with the above-mentioned antibiotics as together to control the related infectious diseases.
Declaration of Competing Interest
There is no conflict of interest.
Acknowledgments
The authors are thankful to the National Institute of Genetic Engineering and Biotechnology (NIGEB) and Payame Noor University, East Tehran Branch for their supports, facilities, and cooperation. This research was supported by the National Institute of Genetic Engineering and Biotechnology, Tehran, Iran. Grant number (I-703)
Contributor Information
Nasrin Darvishi, Email: ndarvishi6363@gmail.com.
Najaf Allahyari Fard, Email: allahyar@nigeb.ac.ir.
Maryam Sadrnia, Email: msadrnia@yahoo.com.
References
- 1.Kaur I.P., Chopra K., Saini A. Probiotics: potential pharmaceutical applications. European Journal of Pharmaceutical Sciences. 2002;15(1):1–9. doi: 10.1016/s0928-0987(01)00209-3. [DOI] [PubMed] [Google Scholar]
- 2.Kopp-Hoolihan L. Prophylactic and therapeutic uses of probiotics: a review. Journal of the American Dietetic Association. 2001;101(2):229–241. doi: 10.1016/S0002-8223(01)00060-8. [DOI] [PubMed] [Google Scholar]
- 3.Salvetti E., Torriani S., Felis G.E. The genus Lactobacillus: a taxonomic update. Probiotics and antimicrobial proteins. 2012;4(4):217–226. doi: 10.1007/s12602-012-9117-8. [DOI] [PubMed] [Google Scholar]
- 4.Allen H.K. Finding alternatives to antibiotics. Ann NY Acad Sci. 2014;1323(1):91–100. doi: 10.1111/nyas.12468. [DOI] [PubMed] [Google Scholar]
- 5.Lee J.W. Immunomodulatory and antitumor effects in vivo by the cytoplasmic fraction of Lactobacillus casei and Bifidobacterium longum. Journal of veterinary science. 2004;5(1):41–48. [PubMed] [Google Scholar]
- 6.Nahaisi M. Lactobacillus acidophilus: therapeutic properties, products and enumeration. Developments in food microbiology. 1986 [Google Scholar]
- 7.Langhendries J.P. Effect of a fermented infant formula containing viable bifidobacteria on the fecal flora composition and pH of healthy full-term infants. Journal of pediatric gastroenterology and nutrition. 1995;21(2):177–181. doi: 10.1097/00005176-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Chopra L. Sonorensin: a new bacteriocin with potential of an anti-biofilm agent and a food biopreservative. Scientific reports. 2015;5:13412. doi: 10.1038/srep13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes A.M., Malcata F.X., Klaver F.A. Growth enhancement of Bifidobacterium lactis Bo and Lactobacillus acidophilus Ki by milk hydrolyzates. Journal of Dairy Science. 1998;81(11):2817–2825. doi: 10.3168/jds.S0022-0302(98)75840-0. [DOI] [PubMed] [Google Scholar]
- 10.Guarner F., Malagelada J.-R. Gut flora in health and disease. The Lancet. 2003;361(9356):512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 11.Sgorbati, B., B. Biavati, and D. Palenzona, The genus Bifidobacterium, in The genera of lactic acid bacteria 1995, Springer. p. 279-306.
- 12.Saarela M. Probiotic bacteria: safety, functional and technological properties. Journal of biotechnology. 2000;84(3):197–215. doi: 10.1016/s0168-1656(00)00375-8. [DOI] [PubMed] [Google Scholar]
- 13.Bibiloni R., Pérez P.F., De Antoni G.L. Will a high adhering capacity in a probiotic strain guarantee exclusion of pathogens from intestinal epithelia? Anaerobe. 1999;5(5):519–524. [Google Scholar]
- 14.Soomro A., Masud T., Anwaar K. Role of lactic acid bacteria (LAB) in food preservation and human health-a review. Pakistan Journal of Nutrition. 2002;1(1):20–24. [Google Scholar]
- 15.Salminen S. Demonstration of safety of probiotics—a review. International journal of food microbiology. 1998;44(1-2):93–106. doi: 10.1016/s0168-1605(98)00128-7. [DOI] [PubMed] [Google Scholar]
- 16.Pieterse R., Todorov S.D. Bacteriocins: exploring alternatives to antibiotics in mastitis treatment. Brazilian Journal of Microbiology. 2010;41(3):542–562. doi: 10.1590/S1517-83822010000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pometto A. CRC Press; 2005. Food biotechnology. [Google Scholar]
- 18.Morin K. Recombinant expression of indolicidin concatamers in Escherichia coli. Applied microbiology and biotechnology. 2006;70(6):698–704. doi: 10.1007/s00253-005-0132-5. [DOI] [PubMed] [Google Scholar]
- 19.Inglis R.F. The role of bacteriocins as selfish genetic elements. Biology letters. 2013;9(3) doi: 10.1098/rsbl.2012.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAuliffe O., Ross R.P., Hill C. Lantibiotics: structure, biosynthesis and mode of action. FEMS microbiology reviews. 2001;25(3):285–308. doi: 10.1111/j.1574-6976.2001.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 21.Micenková L. Bacteriocin-encoding genes and ExPEC virulence determinants are associated in human fecal Escherichia coli strains. BMC microbiology. 2014;14(1):109. doi: 10.1186/1471-2180-14-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley M.A., Chavan M.A. Springer; 2007. Bacteriocins. [Google Scholar]
- 23.Riley M.A., Wertz J.E. Bacteriocins: evolution, ecology, and application. Annual Reviews in Microbiology. 2002;56(1):117–137. doi: 10.1146/annurev.micro.56.012302.161024. [DOI] [PubMed] [Google Scholar]
- 24.Sobrino-López A., Martín-Belloso O. Use of nisin and other bacteriocins for preservation of dairy products. International Dairy Journal. 2008;18(4):329–343. [Google Scholar]
- 25.Cintas L. Bacteriocins of lactic acid bacteria. Food Science and Technology International. 2001;7(4):281–305. [Google Scholar]
- 26.Manosroi A. Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. International Journal of Pharmaceutics. 2010;392(1-2):304–310. doi: 10.1016/j.ijpharm.2010.03.064. [DOI] [PubMed] [Google Scholar]
- 27.Morgan S.M. Sequential actions of the two component peptides of the lantibiotic lacticin 3147 explain its antimicrobial activity at nanomolar concentrations. Antimicrobial agents and chemotherapy. 2005;49(7):2606–2611. doi: 10.1128/AAC.49.7.2606-2611.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cascales E. Colicin biology. Microbiology and molecular biology reviews. 2007;71(1):158–229. doi: 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clardy J., Fischbach M.A., Walsh C.T. New antibiotics from bacterial natural products. Nature biotechnology. 2006;24(12):1541–1550. doi: 10.1038/nbt1266. [DOI] [PubMed] [Google Scholar]
- 30.Böttiger T. Influence of Ca2+ ions on the activity of lantibiotics containing a mersacidin-like lipid II binding motif. Applied and environmental microbiology. 2009;75(13):4427–4434. doi: 10.1128/AEM.00262-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gründling A., Schneewind O. Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus. Journal of bacteriology. 2006;188(7):2463–2472. doi: 10.1128/JB.188.7.2463-2472.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kjos M., Nes I.F., Diep D.B. Class II one-peptide bacteriocins target a phylogenetically defined subgroup of mannose phosphotransferase systems on sensitive cells. Microbiology. 2009;155(9):2949–2961. doi: 10.1099/mic.0.030015-0. [DOI] [PubMed] [Google Scholar]
- 33.van Belkum M.J., Worobo R.W., Stiles M.E. Double-glycine-type leader peptides direct secretion of bacteriocins by ABC transporters: colicin V secretion in Lactococcus lactis. Molecular microbiology. 1997;23(6):1293–1301. doi: 10.1046/j.1365-2958.1997.3111677.x. [DOI] [PubMed] [Google Scholar]
- 34.Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature reviews microbiology. 2005;3(3):238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 35.Gut I.M., Blanke S.R., Van Der Donk W.A. Mechanism of inhibition of Bacillus anthracis spore outgrowth by the lantibiotic nisin. ACS chemical biology. 2011;6(7):744–752. doi: 10.1021/cb1004178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Netz D.J.A. Biochemical characterisation and genetic analysis of aureocin A53, a new, atypical bacteriocin from Staphylococcus aureus. Journal of molecular biology. 2002;319(3):745–756. doi: 10.1016/S0022-2836(02)00368-6. [DOI] [PubMed] [Google Scholar]
- 37.Cleveland J. Bacteriocins: safe, natural antimicrobials for food preservation. International journal of food microbiology. 2001;71(1):1–20. doi: 10.1016/s0168-1605(01)00560-8. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad V. Antimicrobial potential of bacteriocins: in therapy, agriculture and food preservation. International Journal of Antimicrobial Agents. 2017;49(1):1–11. doi: 10.1016/j.ijantimicag.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Behrens H.M. The therapeutic potential of bacteriocins as protein antibiotics. Emerging Topics in Life Sciences. 2017;1(1):65–74. doi: 10.1042/ETLS20160016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Heel A.J. BAGEL4: a user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic acids research. 2018;46(W1):W278–W281. doi: 10.1093/nar/gky383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammami R. BACTIBASE second release: a database and tool platform for bacteriocin characterization. BMC Microbiology. 2010;10(1):1–5. doi: 10.1186/1471-2180-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang B. Screening of probiotic activities of lactobacilli strains isolated from traditional Tibetan Qula, a raw yak milk cheese. Asian-Australasian journal of animal sciences. 2016;29(10):1490. doi: 10.5713/ajas.15.0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruiz-Moyano S. Screening of autochthonous lactic acid bacteria strains from artisanal soft cheese: probiotic characteristics and prebiotic metabolism. LWT. 2019;114 [Google Scholar]
- 44.Coconnier M.-H. Antagonistic activity againstHelicobacter infection in vitro and in vivo by the humanLactobacillus acidophilus strain LB. Applied and Environmental Microbiology. 1998;64(11):4573–4580. doi: 10.1128/aem.64.11.4573-4580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anas M., Eddine H.J., Mebrouk K. Antimicrobial activity of Lactobacillus species isolated from Algerian raw goat's milk against Staphylococcus aureus. World J Dairy Food Sci. 2008;3(2):39–49. [Google Scholar]