Highlights
-
•
The origin of osteosarcoma cells from osteoblasts and mesenchymal stem cells remains controversial.
-
•
Mesenchymal stem cells regulate the development of osteosarcoma by influencing the tumor microenvironment and mediating cell communication.
-
•
Mesenchymal stem cells and exosomes secreted by them can be used as good genes and drug carriers for the treatment of osteosarcoma.
-
•
Mesenchymal stem cells from different tissue sources have different regulatory effects on the development of osteosarcoma.
Keywords: Osteosarcoma, MSCs, TME, Cellular communication, Clinical application
Abbreviations: MSCs, Mesenchymal stem cells; iPSCs, induced pluripotent stem cells; ESCs, embryonic stem cells; AQP1, Aquaporin-1; MCP-1, Monocyte chemoattractant protein-1; TME, Tumor microenvironment; BMSCs, Bone marrow mesenchymal stem cells; ECM, Extracellular matrix; OS, osteosarcoma; CAFs, Carcinoma-associated-fibroblasts; TGF, Transforming growth factor; TNF, Tumor necrosis factor; HCC, hepatocellular carcinoma; CSF, Colony-stimulating factor; SDF-1, Stromal cell-derived factor 1; EVs, Extracellular vesicles; LINE-1, Long interspersing element 1; SD-MSCs, stressed MSCs; MSC-MVs, MSC microvesicles; MSC-Exos, MSC-derived exosomes; BMSC-derived exosomes, BMSC-Exos; TRA2B, Transformer 2β; AD-MSCs, Adipose-derived MSCs; DP-MSCs, Dental pulp-derived MSCs, hUC-MSCs, Human umbilical cord MSCs; 5 FC, 5-fluorocytosine; hASCs, human adipose stem cells; VEGF, Vascular endothelial growth factor; PDGFα, Platelet derived growth factor α; PDGFRα, Platelet derived growth factor receptor α; PDGFRβ, Platelet derived growth factor receptor β; OPG, osteoprotegerin; GBM, Glioblastoma; 3TSR, Three type 1 repeats; S TRAIL, Secretable variant of the TNF-related apoptosis-inducing ligand; DOX, Doxorubicin; CRC, Colorectal cancer; yCD::UPRT, Yeast cytosine deaminase::uracil phosphoribosyl transferase
Abstract
Mesenchymal stem cells (MSCs) are multipotent stem cells with significant potential for regenerative medicine. The tumorigenesis of osteosarcoma is an intricate system and MSCs act as an indispensable part of this, interacting with the tumor microenvironment (TME) during the process. MSCs link to cells by acting on each component in the TME via autocrine or paracrine extracellular vesicles for cellular communication. Because of their unique characteristics, MSCs can be modified and processed into good biological carriers, loaded with drugs, and transfected with anticancer genes for the targeted treatment of osteosarcoma. Previous high-quality reviews have described the biological characteristics of MSCs; this review will discuss the effects of MSCs on the components of the TME and cellular communication and the prospects for clinical applications of MSCs.
1. Mesenchymal stem cells
1.1. Introduction
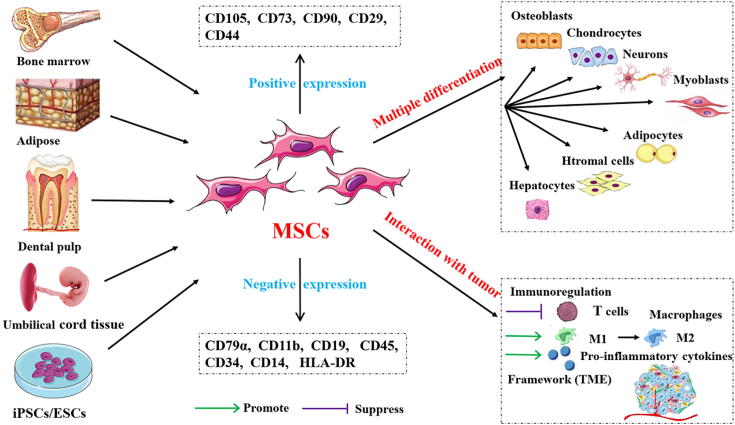
Regenerative medicine, a multidisciplinary field aimed at promoting the repair and regeneration of tissues and organs, has aroused widespread public attention in recent years. It involves using biology and tissue engineering to find feasible, effective treatments that accelerate self-repair and regeneration, or generate new tissues or organs to maintain, improve, and repair damaged bodies. Mesenchymal stem cells (MSCs) are an important source for stem cell therapy in regenerative medicine; they are a kind of adult stem cell, distributed in various tissues of the body, especially in bone marrow, adipose tissue, dental pulp, umbilical cord tissue, and iPSCs/ESCs [1]. Bone marrow MSCs (BMSCs) have shown good results in repairing damaged tissues in various degenerative diseases, in animal models and human clinical trials [2], [3]. The therapeutic potential of stem cells can be attributed to three key mechanisms [4]. The first is homing, which means that they can migrate to the injured site and differentiate into local components there. The second mechanism is the secretion of biologically active factors that affect surrounding tissues because MSCs have the ability to secrete chemokines, cytokines, and growth factors that help tissue regeneration. The third prominent feature of MSCs is their strong proliferation ability and multidirectional differentiation potential: they can differentiate into muscle cells, hepatocytes, osteoblasts, adipocytes, chondrocytes, and stromal cells, inappropriate in vivo or in vitro environments, enabling supplementation or replacement of damaged cells (Fig. 1). MSCs can repair damage to various tissues and cells in the body, such as the lung, liver, heart, and nervous system [5], [6], [7], [8]. Immunomodulation is another critical characteristic of MSCs: they inhibit T cell proliferation and immune response through intercellular interaction and cytokine production, thus having the anti-inflammatory effect of immune reconstitution. However, under the influence of the cellular microenvironment, MSCs can also be induced to differentiate into a pro-inflammatory phenotype, secreting inflammatory cells and aggravating inflammatory damage. As MSCs do not express human leukocyte antigen class II molecules, they have low immunogenicity [9]. MSCs also feature surface antigen expression (positive for CD105, CD73, CD90, CD29, and CD44, and negative for CD79α, CD11b, CD19 or CD45, CD34, CD14, and HLA-DR) [10], [11].
Fig. 1.
Sources of mesenchymal stem cells (MSCs), varieties of differentiation, and interaction with tumor cells. MSCs can be derived from bone marrow, adipose, dental pulp, umbilical cord tissue, and iPSCs/ESCs. Antigens are expressed positively and negatively on the surface of MSCs cells. The most prominent feature of MSCs is that of multiple differentiation: they can differentiate into osteoblasts, chondrocytes, nerve cells, myoblasts, adipocytes, stromal cells, and hepatocytes. MSCs support tumor cell growth through immunoregulation and the construction of a tumor microenvironmental biological framework.
1.2. The MSC–OS relationship
OS is a highly malignant tumor accounting for 60% of all bone sarcomas; it is most likely to affect children and young adults and to occur in the epiphysis of the bone marrow of long bones, such as the femur, tibia, and humerus [12]. One of the major features of OS is its predisposition to early metastasis, mainly to the lung [13]. Moreover, OS can be is resistant to chemotherapy easily, which is the main factor that makes it difficult to cure. According to an epidemiological investigation, the five-year survival rate of OS patients without lung metastasis is 60–70%, but the survival rate of those with lung metastasis is significantly lower [14]. A study on OS histogenesis suggested that it may be accelerated by two types of MSCs, namely, from normal tissue (N-MSCs) and tumor tissue (T–MSCs) [15]. N-MSCs can be recruited to the TME and educated to undergo heterogeneous differentiation into the pro-tumor phenotype. T–MSCs derived from OS tissue dramatically accelerate the abnormal proliferation of OS cells. Other studies have also found that gene mutations, oncogenic stress, and changes in the bone microenvironment at specific stages of differentiation will cause MSCs to differentiate into OS cells [16], [17], [18]. However, several authors have even hypothesized that MSCs are the cell of origin of OS without strong evidence, concluding that osteogenic progenitors rather than undifferentiated MSCs represent the OS cell of origin [19], [20]. Utilizing promoters that were relatively limited to the osteoblast lineages, ranging from the early expression of dual-energy precursors such as Osterix1 and Col1a1 to the expression of promoters that were more restricted to the osteoblast lineages such as osteocalcin, researchers have produced OS mouse models with significantly higher incidence - up to nearly 100% [21], [22], [23]. At present, most researchers use a series of in vivo genetic models to support the hypothesis that the abnormal differentiation of osteoblasts may be the origin of OS cells. The controversy stems from the fact that under physiological conditions, it is difficult to distinguish between MSCs and osteoblasts who are the precursor cells of OS cells, and that MSCs have the potential to differentiate into osteoblasts in a specific bone microenvironment. In addition, there are many subtypes of OS, how to distinguish between MSCs and osteoblasts under some specific differentiation conditions, into different subtypes of OS is also a problem to be solved. And most studies are based on different and usually incomparable experimental models, and there are some problems in the differentiation and comparability between different experimental models. In order to draw conclusions from the experimental model, studies with comparable conditions must be evaluated, otherwise, we will face the risk of simplification and possible misguidance. At present, the gradual maturity of gene modification technology and the improvement of the gene-editing level of cell pedigree may be helpful to explain the origin of OS, providing more possibilities for clinical treatment of OS. MSCs are a double-edged sword for OS; although many studies have confirmed the promotional effect of MSCs on OS, several have found an inhibitory effect, showing significant anti-tumor efficiency.
2. Promotion of OS growth
2.1. Tumor microenvironment
The TME is composed of tumor parenchymal cells, stromal cells, cytokines, and chemokines (Fig. 2). Stromal cells consist of fibroblasts, endothelial cells, immune cells, and MSCs [24]. The progression, resistance to drugs, invasion, and metastasis of tumor parenchymal cells are affected by the bidirectional interaction between tumor cells and TME [25]. The pathological mechanism of a malignant tumor involves not only tumor cells themselves, but also stromal cells, extracellular matrix (ECM), and some normal cells in the TME. Therefore, it is not comprehensive to consider tumor progression, metastasis, and drug resistance only by analyzing tumor cells [26]. The dialogue between tumor cells and cytokines and chemokines in the TME is also indispensable to the processes of a malignant tumor. Only by further understanding and clarifying the mechanism of action between tumor cells and adjacent cells in the TME can the progress of tumor cells be better controlled, and this can provide ideas for the subsequent clinical implementation of effective targeted therapy [24], [27].
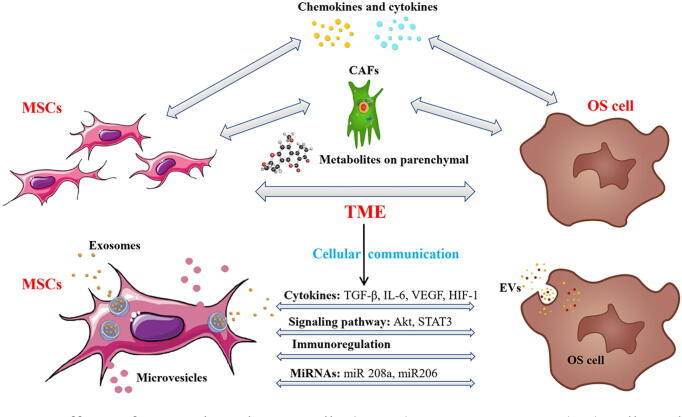
Fig. 2.
Effects of mesenchymal stem cells (MSCs) on osteosarcoma (OS) cells and cellular communication. MSCs promote OS cells mainly through the cross-talk of tumor parenchymal cells, stromal cells (differentiated into carcinoma-associated-fibroblasts), cytokines, and chemokines in the tumor microenvironment. In addition, the communication of MSCs with OS cells in the tumor microenvironment is essential; they do so mainly via secreting extracellular vesicles (mainly composed of microvesicles and exosomes), regulating cytokines, signaling pathways, immunoregulation, and miRNA.
2.1.1. MSCs promote OS cell progression and metastasis
In terms of OS, the proliferation and metastasis of OS cells mainly occur through two processes. On the one hand, tumor cells interact with other cells, especially MSCs, in the TME through autocrine and paracrine signaling, secreting cytokines or activating other signaling pathways, stimulating the proliferation and metastasis of OS cells. On the other, tumor cells to some extent affect the normal expression of genes, which may specifically educate MSCs to differentiate into OS cells, thus achieving the transformation of OS cells. By releasing the paracrine factor IL–8, OS cells form a signaling cross-destruction circuit, stimulating the expression of IL–8 in MSCs and, in turn, receiving the IL–8 released by MSCs, thus accelerating the expression of IL–8 in OS cells [28]. Pelagic, with other researchers [29], exposed OS cells to the conditioned medium from BMSCs and found the level of AQP1 of OS increased after exposure to this medium. It proved that BMSCs recruited to the TME may contribute to OS cell metastasis and invasion by participating in the regulation of the AQP1 level. The process of tumorigenesis is closely related to the abnormal phenotypic transformation of MSCs in the TME. Abnormal gene expression, such as overexpression or silencing, promotes the malignant phenotypic transformation of MSCs. Through exploring the gene expression profile and differential gene expression of overexpressed c–Jun or c–Jun/c–Fos in immortal MSCs, researchers have demonstrated that, under a given condition, certain AP-1 sites are activated and induce MSCs to transform into OS cells [30]. Knocking out a gene may cause the anomalous differentiation of MSCs. An in vivo study successfully transformed human MSCs into an OS-like tumor via a combination of Rb knockdown and c–Myc overexpression in retinoblastoma [31]. More importantly, a comparison of tumor samples from OS patients found that loss of Rb and overexpression of c–Myc were associated with decreased survival [31]. The data strongly suggest that BMSCs transformed in this way are related to clinical OS tissue and patient survival. Saalfrank et al. [32] demonstrated that porcine BMSCs were transformed into abnormal phenotypes with potential tumorigenesis by activating the TP53R167H and K-RASG12D mutations, and c–MYC overexpression promoted the occurrence of OS. The results showed clearly that endogenous TP53- and K-RAS-targeted mutations in primary BMSCs can lead to tumor transformation and promote the occurrence of OS.
2.1.2. MSCs transform into carcinoma-associated fibroblasts
Carcinoma-associated fibroblasts (CAFs) are a crucial component of the TME, contributing to the initiation, progression, and metastasis of various types of cancer, such as colorectal and pancreatic [33]. The differentiation of BMSCs to CAFs is a multistep and complex biological process, which may involve epithelial-mesenchymal transition, a bone marrow-derived progenitor, cellular communication, and cytokines [34]. An in vitro experiment concluded that BMSCs can be transformed into CAFs via contact with OS cells, which further increases the levels of MCP–1, GRO–3a, IL–6, and IL–8 cytokines in the TME [35]. At the same time, researchers have discovered that the mobility and invasiveness of OS cells are increased significantly by MSCs differentiating into CAFs. OS cells stimulated by BMSCs can maintain the migration and invasion of endothelial cells and induce the formation of a capillary network. Therefore, the transformation of BMSCs recruited to the OS site into CAFs, and their subsequent cytokine-induced mesenchymal-to-amoeboid transition, are key to the increase in OS heterogeneity. A follow-up study exposed BMSCs transformed to CAFs can significantly accelerate the proliferation, migration, and invasion of OS cells [33]. An in-depth study disclosed that, remarkably, U2OS could significantly induce the over-expression of IL–6 and phosphorylation of the STAT3 protein in BMSCs. In addition, external intervention to block the IL–6/STAT3 signal pathway in BMSCs can suppress the phenotypic transformation of CAF induced by U2OS. Likewise, Wang et al [36] showed that OS cells could directly induce BMSCs to differentiate into CAFs, with the promotional role of Notch and Akt signal pathways. ECM is a non-cellular component of the TME, constituting its main framework, and is principally produced by CAFs. It is responsible for intercellular communication, cell adhesion, and cell proliferation, promoting malignant phenotypes and increasing tumor heterogeneity [37], [38]. ECM is highly dynamic in that it is continuously deposited, remolded, and degraded during development until maturity, to maintain tissue homeostasis [39]. Cai et al. found that the composition of the ECM varies with cell type, with this varying composition having different effects on the morphology, adhesion, and proliferation of MSCs and MG63 cells. ECMs from normal cells can promote the adhesion and proliferation of MSCs and MG63 cells better than ECMs from MG63 OS cells, suggesting that the ECM may also be a new target for the treatment of OS [40]. Generally speaking, regulating the CAFs transformation of MSCs may have effects on the heterogeneity of osteosarcoma.
2.1.3. MSC-related chemokines and cytokines
As crucial parts of the TME, cytokines, and chemokines, such as transforming growth factor (TGF)–β, tumor necrosis factor (TNF)–α, and interferon–γ, secreted by tissues and cells, have a tremendous effect on tumor ontogeny. Chemokines derived from BMSCs, such as CXCL1, CXCL2, or CXCL12, promote cancer proliferation by binding to receptors CXCR2 and CXCR4 [41]. CXCL12 activates relative signaling pathways by binding to CXCR4 and CXCR7, leading to cell growth and transcriptional regulation of genes, which are central to inflammation, cancer metastasis, and poor overall survival in patients with OS [42]. Han et al. explored the ability of CXCR7 and CXCR4 to induce the invasion of OS The CXCL12/CXCR4 combination was activated in the BMSCs co-culture system, and the invasiveness of OS cells was significantly enhanced [43]. At the same time, it was found that the specific inhibitor AMD3100, which neutralizes antibodies, could effectively inhibit the expression of CXCR4, but the invasiveness of OS cells could not be effectively controlled. This is because the inhibition of the CXCR4 level can lead to CXCR7 up-expression, which itself can still maintain the invasiveness of OS cells. Therefore, suppressing CXCR4 and CXCR7 and regulating the interactions among these two chemokines and MSCs may be a promising strategy to control the invasion of OS. Du et al investigated the effects of MSCs in the TME on the anoikis resistance and pulmonary metastasis of OS cells. They found that MSCs enhance these by regulating the IL–8/CXCR1/Akt pathway, suggesting that MSCs can select OS cells with high metastatic potential in vivo [44]. In the TME, IL–6 activation can act on the target genes of various biological activities [45], OS behavior is adversely affected by osteoclast bone resorption and the level of IL–6 at the tumor site [46], which increases the chemotherapeutic resistance of OS cells [47]. Overexpression of IL–6 activates the STAT3 signaling pathway, which is essential for MSC-induced chemoresistance in OS cells, and further enhances the chemotherapeutic resistance of MSC, while inhibition of this signaling re-sensitizes drug-resistant Saos–2 cells to drug therapy [48]. Extracellular acidosis is a common feature of cancer [49], playing a key role in the progression of many cancers by facilitating chemotherapy or radiation resistance, neovascularization, invasion, and “stemness”. Avnet et al. clarified that OS is characterized by interstitial acidosis, in which interstitial active cells are activated to release mitosis and chemokines [50]. Short-term acidosis transforms MSCs into T–MSCs that secrete factors that promote cloning and migration, thus, OS cells re-encode primitive MSCs into T-MSCs by short-term acidification of the extracellular environment, which promotes their release of excess growth factors, cytokines, and chemokines, and ultimately supports the proliferation, migration, chemotherapy resistance, and stemness of MSCs. It has been shown that myofibroblast-derived SDF–1 recruits endothelial progenitor cells to sites of carcinomas, thereby enhancing angiogenesis and tumor growth [51]. Yu et al. have found that the SDF–1/CXCR4 axis plays an important role in mediating tumor promotion [52]. Their findings proved that recombinant SDF–1 can significantly promote the proliferation of OS cells. Blocking the receptor with AMD3100 is sufficient to prevent the proliferation of OS732 cells mediated by SDF–1. In short, the expression and activation of cytokines and related receptors are of great significance for the differentiation of MSCs and their roles on osteosarcoma
2.2. Cellular communication
TME is a complex three-dimensional “society” composed of different types of cells. Individual cells in this society must coordinate their growth and development in particular ways, therefore, it is necessary for cells to establish intercellular communication. Cellular communication refers to the transmission of information from one cell to another through the medium to produce a corresponding response. The proliferation of tumor cells (the growth and differentiation of stromal cells) requires highly accurate and efficient cellular communication to coordinate all the tissues and cells in the TME, making it a better space for tumor cells to grow. Cellular communication is essential for the genesis and tissue construction of multicellular organisms, coordination of cell functions, and control of cell growth. The interaction, information transfer, and media transfer in the TME are mostly conducted by means of EVs.
2.2.1. Extracellular vesicles
Extracellular vesicles (EVs) are nanoscale vesicles that are actively released by cells and contain nucleic acids (DNA, mRNA, and miRNA), proteins, metabolites, and lipids; they are one of the most important means of cellular communication in the body [53], [54]. In the TME, EVs are generally secreted by the primary tumor [55]. However, cell communication is bidirectional, and MSCs can also affect OS by secreting EVs. EVs can be classified according to their biogenetics, size, and biophysical properties as exosomes, microvesicles, and apoptotic bodies. EVs may also be involved in the premetastatic niche formation of oncoproteins and heat shock proteins that inhibit antitumor immunosuppressive effects [55], [56]. Detection of tumor EVs in the human circulatory system may serve as a diagnostic marker of tumor development [57]. Mannerström et al [58] treated BMSCs with OS cell-derived EVs (OS–EVs) at different times and found that EVs can mediate the interspersing element hypomethylation in BMSCs, which proves that epigenetic regulation occurs in the early stage of the transformation of BMSCs into OS cells. One study [59] concluded that OS cells in MSCs and stressed MSCs (SD-MSC) conditioned media were significantly resistant to apoptosis, and an increased wound-healing rate was observed in cells exposed to either the conditioned media or EVs from MSCs and SD–MSCs. The ability of culture medium and EVs under the SD-MSC condition to improve survival rate and reduce apoptosis is related to the ability of EVs to provide proteins, metabolites, and microRNAs that support tumor growth. A follow-up study revealed that EVs secreted by highly malignant OS cells selectively incorporate a membrane-associated form of TGF–β, which induces pro-inflammatory IL–6 production by MSCs [60]. Tumor-EVs educate MSCs to promote tumor growth, accompanied by intratumor STAT3 activation and lung metastasis formation, which was not observed with control MSCs. OS cells release TGF–β-rich EVs, inducing a pro-metastatic phenotype characterized by high IL–6 production in MSCs. Therefore, inhibition of IL–6 and TGF–β may be a potential new therapeutic approach for OS. Additionally, EVs have the ability to immunomodulate, which can affect the action of immune cells in the TME, which alleviates the inhibitory effect of immune cells on tumor cells, causing tumor cells to escape from autologous immune surveillance, thus promoting the proliferation and metastasis of tumor cells. Lagerweij et al. conducted research, which concluded that EVs secreted by OS cells may modify immune cells in the TME and educate MSCs to differentiate into tumor-supporting phenotypes [61]. Immune regulation and tumor immune escape are the key mechanisms of malignant progression. The secretion of EVs by OS cells may affect innate or adaptive immune components directly or indirectly through MSCs, thus promoting the proliferation of tumor cells. Hypoxia is a common feature of a variety of cancers, including OS, and is related to drug resistance in clinical treatments such as chemotherapy and radiotherapy [62]. Cellular adaptation and peritumor hypoxic angiogenesis are considered important events in cancer progression [63]. Lin revealed that MSC microvesicles (MSC–MVs) have a strong effect, promoting proliferation and anti-apoptosis, on U2OS cells under hypoxia in vitro and can promote the formation and growth of tumors in vivo [64]. MSC–MVs promote the expression of hypoxia-inducible factor 1 in peripheral tissues and target genes such as VEGF and GLUT1 in tumor cells by increasing the phosphorylation of Akt and promote the proliferation, migration, and colony formation of tumor cells. These results offer a new perspective on the role of MSCs in tumor progression and development.
2.2.2. Exosomes
Exosomes are small, lipid-membrane EVs, which are formed by endocytosis, integration, and efflux; they have a diameter of 30–150 nm, are stable in a variety of biological fluids, such as urine, plasma, and serum [65], and can be used as drug carriers. Mediating cellular communication is the main role of exosomes because they can be released by one cell and captured by neighboring cells through ligand–receptor or direct binding [66]. The biogenesis of exosomes occurs via the endocytosis–ectopic pathway when cells absorb a small amount of intracellular fluid in specific membrane regions and form early endosomes. Currently, MSC studies mainly focus on MSC-derived exosomes (MSC–Exos); MSCs are the most prolific exosome producers [67], and such exosomes have similar morphological characteristics, isolation methods, and preservation conditions to those from other cell types. The composition of exosomes depends on their cellular origin. MSC–Exos have biological functions similar to MSCs but have a smaller volume, can penetrate biofilm, have low immunogenicity, and can be stored [68]. Various studies have reported that MSCs-Exos can promote tumor growth and metastasis in a variety of tumors, including OS [69]. Huang et al revealed that such exosomes could promote autophagy of OS cells, thus prompting OS proliferation, metastasis, and invasiveness [70]. Exosomes can mediate local and distant cellular communication by transporting contents containing specific miRNAs [71]. miRNAs have been shown to participate in a variety of biological processes such as cell proliferation, migration, invasion, autophagy, and apoptosis by regulating the expression of target genes at the post-transcriptional level [72]. Exosomes also contain mRNA, which, upon endocytosis by the recipient cell, modulates protein synthesis and cell function. Exosomal miRNAs have been reported to serve as markers to assess tumor aggressiveness and might participate in the progression of different kinds of tumors [73]. Qin et al explored the impact of microRNA 208a (miR–208a)-enriched BMSC–Exos on OS cells [74]. BMSCs communicate with OS cells through exosomes, and the ectopic expression of miR–208a can improve the survival, migration, and cloning ability of OS cells. A similar study concluded that BMSC–Exos could carry and transport miR-206 to inhibit the proliferation, migration, and invasion of OS cells, inducing apoptosis of OS cells [75]. A follow-up study showed that BMSC–Exos encapsulate long non-coding RNA plasmacytoma variant translocation 1 protein and transfect it to OS cells, while this transfected protein promotes tumor growth and metastasis by inhibiting ubiquitin and upregulating oncogenic protein ERG expression in OS cells [76]. This implies that BMSCs-Exos promote the growth and metastasis of OS cells by inhibiting ubiquitin and ERG ubiquitin, providing new insights into the mechanism of how BMSC–Exos affect the progression of OS. Adipose-derived MSCs (AD-MSCs), similar to BMSCs, are of great importance to the progression of OS. The latest study found that COLGALT2 is closely related to the occurrence and development of OS [77]. AD-MSC-derived exosomes can promote the invasion, migration, and proliferation of OS cells by increasing the expression of COLGALT2, which can increase expression of vimentin and the MMP gene, whereas inhibition of COLGALT2 in MG63 cells can inhibit their success [78]. A growing body of research considers adipose tissue to be the largest endocrine organ in the body [79]. Tumor-related adipocytes and adipogenic cells, such as AD-MSCs, are recruited into the TME and, by secreting EVs and exosomes, they establish effective communication with the microenvironment to promote the proliferation and evolution of tumor cells, making them more malignant. However, at present, studies on the effects of AD-MSCs on the gene expression of tumor cells and the related effects of signaling pathways and chemokines are not sufficient; these need to be studied further.
To sum up, MSCs, mainly through their interaction with the TME, mediate cellular communication to establish contact with OS cells, promoting the proliferation, invasion, and metastasis of the latter. Therefore, targeted therapy can be carried out according to different pathomechanisms to alleviate the malignant tumor. However, the current research has mainly focused on the effect of BMSCs on the malignant lesions of OS, with few related studies on other tissue-derived MSCs, such as AD-MSCs, dental pulp-derived MSCs (DP-MSCs), and human umbilical cord (HUC–MSCs) or embryonic stem cell-derived MSCs.
3. Inhibition of OS growth
Note that even though various studies have shown that MSCs support the growth of OS, a few have demonstrated that MSCs can effectively alleviate and inhibit OS recurrence, proliferation, and metastasis. One study used an OS-bearing mouse that expressed luciferase and surgically removed the primary OS. MSCs were delivered to the primary OS site via direct and intravenous injections [80]. Notably, the direct injection suppressed local recurrence, expansion of the recurrent tumor, and inhibited the growth of the remaining OS cells. However, the intravenous injection promoted lung metastasis of OS cells, which suggests that MSCs may be differentiated into tumor growth-supporting phenotypes under the influence of the organism's internal environment during the circulation process, and secretion of corresponding cytokines may affect the growth of tumors, but the specific mechanism is still unclear. A similar effect has been demonstrated in AD-MSCs: Lee and colleagues showed that the local injection of differing concentrations of AD-MSCs into the tumor site causes different effects [81]. To a certain extent, a low concentration of AD–MSCs had an inhibitory effect on cancer, while a higher concentration could stimulate tumor growth (AD-MSCs combined with the osteosarcoma cell in low proportions of 5%, 10%, 15%, and high proportions of 25%). It showed the co-injection of AD-MSCs and OS cells resulted in the most death of AD-MSCs eventually. So, the anti- or pro-cancer effect of AD-MSCs seems to be more likely because of their early humoral effects [82], such as the production of various cytokines and chemokines, rather than because of the direct effect of stem cell proliferation. One experiment evaluated the ability of BMSCs expressing the cytosine deaminase/5-fluorocytosine prodrug (5–FC, hence CD/5–FC MSCs) to target the human OS cell line, CAL72 [83]. The MSCs inhibited tumor growth compared to control mice subcutaneously injected only with CAL72 cells, indicating that CD/5–FC MSCs may be a novel target for the treatment of human OS. An analogous in vitro and in vivo study co-cultured Saos–2 cancer cells with human adipose stem cells (hASCs) to explore the potential effect of cancer cells on hASC differentiation [84]. The results clearly show that, compared with hASCs alone, Saos–2 can induce proliferation of hASCs. Surprisingly, in hASCs, Saos–2 can down-regulate the expression of angiogenic factors (including CD34, PDGFα, PDGFRα, PDGFRβ, and VEGF), implying MSCs cannot differentiate in vitro under the induction of tumor cells and do not support tumor angiogenesis in vivo. DP-MSCs have also shown a certain inhibitory effect on tumors. Compared with BMSCs, DP–MSCs showed increased osteogenic potential, decreased adipogenic potential, formed a dentin pulp-like complex, and were resistant to tumor transformation [85]. These differences in the commitment of the two cell lineages and their tumorigenesis are explained by the difference in phosphatase and tensin homolog (PTEN) expression, mediated by PTEN methylation. BMSCs had higher DNA methylation levels and H3K9Me2 enrichment at the promoter region of PTEN, which were mediated by elevated levels of DNMT3B and G9a, respectively. The characteristics of DP-MSCs, including their extremely low tumorigenic potential and unusual cell fate, mean that they have great value in the treatment of OS, regenerative medicine, and future clinical applications. However, the specific mechanism requires further study.
Taken together, MSCs have differing tissue sources and directions and exhibit disparate effects on the growth of OS cells. BMSCs are currently of great interest in this field, while other tissue-derived MSCs are studied relatively rarely. Additionally, the pro-tumor effect of MSCs on OS cells has been thoroughly covered, while few studies have focused on inhibiting the growth of OS cells. The interaction and communication between MSCs, OS cells, the TME, and stromal cells are extremely complex and perform a decisive role in their overall pro- or anti-effect on OS, determining the evolutionary direction of OS cells. Therefore, a deeper understanding of the molecular mechanisms of OS and MSCs can provide better clinical strategies for OS prevention and treatment. The specific influences of MSCs on OS are shown in (Table1).
Table 1.
Some roles of MSCs in OS.
| Function | Source of MSCs or other related cells | Type of OS cells | Relevant molecules or genes | Reference | |
|---|---|---|---|---|---|
| Promote | |||||
| TME | Progression and metastasis | Human | MG63 | IL-8, FAK and down-regulate Akt signaling pathway | 28 |
| Bone marrow | U2OS | Up-regulate the level of AQP1 protein | 29 | ||
| Human | – | c-JUN and c-FOS, activating protein-1 | 30 | ||
| Porcine's Bone marrow | – | Activate TP53R167H and K-RASG12D, MYC up-regulation | 32 | ||
| Transformation into CAFs | Bone marrow | SaOS2, MG63 and HOS | Up-regulate expression of MCP-1, GRO- 3a, IL-6, and IL-8, MAT transformation | 35 | |
| Bone marrow | U2OS | Up-regulation of IL-6 and phosphorylation of STAT3 signaling pathway | 36 | ||
| Bone marrow | MG63 and U2OS | Notch and Akt signaling pathway | 37 | ||
| Human | MG63 | ECMs derived from normal cells promoted the grouth both MSCs and MG63 cells than that from MG63 OS cells | 40 | ||
| Chemokines | Bone marrow | MG63 and U2OS | Activation of CXCL12, CXCR4 and CXCR7 | 43 | |
| Mouse | SaOS2 | IL-8/CXCR1/Akt signaling pathway | 44 | ||
| Mouse | SaOS2 and U2OS | Up-regulation P-STAT3, MRP, MDR-1; STAT3 activation by IL-6 | 48 | ||
| Human | MG63, SaOS2, and HOS | Up-regulation RelA, RelB, NF-κB and down-regulation CSF2 / GM-CSF, CSF3 /G-CSF, BMP2, CCL5, CXCL5, IL6, IL-8, CXCR4 | 50 | ||
| Bone marrow | MG63 and OS732 | SDF-1/CXCR4 axis | 52 | ||
| Cellular communication | EVs | Bone marrow | HOS143b | OS-EVs mediates LINE-1 hypomethylation | 58 |
| Human | KHOS | Down-regulation of hsa-miR-195 and hsa-miR-124; Up-regulation of hsa-miR-148a | 59 | ||
| Human | – | STAT3 signaling; proinflammatory IL-6 induced by up-regulation TGF-β | 60 | ||
| Bone marrow | – | IL-6/STAT3 signaling pathway | 61 | ||
| Human | U2OS | HIF-1α, VEGF, GLUT1, Bax and cleaved-caspase3; PI3K/AKT and HIF-1α pathway | 64 | ||
| Exosomes | Bone marrow | HOS and MG63 | Autophagy-related gene 5 (ATG5) | 72 | |
| Bone marrow | MG63 and Saos2 | MiR-208a down-regulating PDCD4 and activating the ERK1/2 pathway | 74 | ||
| Bone marrow | KHOS, U2OS, and MG63 | Up-regulation of TRA2B and down-regulation of miR-206 | 75 | ||
| Bone marrow | MNNG, HOS, SaOS2, MG63 | PVT1 inhibiting ubiquitin and increasing the expression of ERG | 76 | ||
| Adipose | MG63, U2OS | Up-regulation COLGALT2 vimentin and MMP. | 77 | ||
| Inhibit | Bone marrow | DLM8 | Suppressed the local recurrence, expansion of the recurrent tumor | 80 | |
| Adipose | UMR-106 | Low proportions of AD-MSCs inhibit the OS and high promote | 81 | ||
| Bone marrow | Cal72 | CD/5-FC | 83 | ||
| Human adipose stem cell | SAOS2 | Up-regulation of CD34, OCT3/4, Nanog, Sox2 and leptin; decrease of CD31, PDGFα, PDGFRα, PDGFRβ and VEGF | 84 | ||
| Dental pulp | – | PTEN/PI3K/AKT pathway; increased DNMT3B and G9a levels mediated H3K9Me2 enrichment | 85 | ||
4. The prospects of MSCs in clinical applications
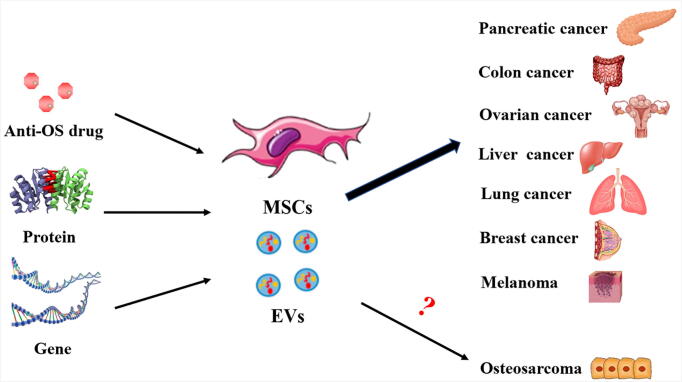
The chemotaxis of cells to the target site through systemic circulation is first manifested by the homing of leukocytes to the inflammatory site. MSCs are thought to use a similar mechanism to migrate to tissue damage sites, including the TME [86], [87]. There are many high-quality reviews in the literature concerning the basic concept that MSCs have a good targeting transport effect, so they can be used as carriers to target the tumor growth site for effective clinical functions (Fig. 3).
Fig. 3.
Clinical application prospects of mesenchymal stem cells (MSCs) and extracellular vesicles (EVs). MSCs and their secreted EVs can serve as excellent carriers of drugs, genes, and proteins. Under the effect of homing in vivo, the loaded cargo can be delivered to the tumorigenesis site for targeted therapy. MSCs or EVs as carriers have been used in the treatment of colon, liver, lung, and breast cancers, as well as other tumors. However, there is a large gap in the current research on osteosarcoma treatment, and more research is needed to support investigation into the treatment of osteosarcoma.
4.1. Loading MSCs with genes to target OS
The characteristics of MSCs mean that they can carry therapeutic anti-cancer genes, making them a unique and promising choice in cancer treatment, part of their recently recognized potential in a wide variety of applications. The most essential factors in the initiation of cancer are the epigenetic changes and genetic mutations in proto-oncogenes and tumor suppressor genes [87]. Therefore, OS can be treated by loading tumor suppressor genes, using the unique characteristics of MSCs. The molecular triad RANKL/RANK/osteoprotegerin (OPG) is the key regulator not only for normal but also pathological bone metabolism [88]. Therefore, RANKL/RANK/OPG has recently become a new therapeutic target for primary and metastatic bone tumors [89]. Qiao and colleagues [90] conducted an experiment that showed that MSCs transfected with OPG gene adenovirus (MSC-OPG) could migrate to the tumor site and express OPG protein and that MSC-OPGs can reduce tumor growth and inhibit bone destruction in vivo. That transgenic MSCs can directly introduce anti-tumor proteins into tumor tissues as carriers have been demonstrated in a number of studies [91]. MSCs loaded with anti-angiogenic genes NK4 and TSP-1, pro-apoptotic genes TRAIL, TNF–α, IL–24, and IL–25, and soluble suppression of tumorigenesis-2 and IL–10 have been tested in other cancers, including melanoma, breast carcinoma, glioblastoma (GBM), lung cancer, and liver cancer, inhibiting the growth of tumor cells in the TME [92], [93], [94], [95], [96], [97], [98]. However, there are currently too few studies on the treatment of OS by MSCs transfected with tumor suppressor genes.
4.2. Loading MSCs with drugs to target OS
The development of MSCs as cell-based drug delivery vectors to respond to numerous clinical indications, including tumors, has significant promise. Exposing MSCs to the drug paclitaxel results in its incorporation into the MSCs [99]. Paclitaxel-induced cells showed dose-dependent anti-angiogenesis and antitumor activity in vitro. However, the overexpression of p-glycoprotein and other drug efflux transporters limits the application of MSCs as cytotoxic drug carriers, resulting in poor drug-loading capacity. Moreover, some underlying problems that prevent the drug-loading of MSCs from achieving their desired effect need to be solved. In the homing process of drug-loaded MSCs, the deposition of MSCs in non-target tissue cells will cause significant toxicity to the surrounding normal cells, resulting in their injury and apoptosis. The concentration of MSCs infiltrated and recruited into the TME is difficult to control. Therefore, the concentration of drugs, biological effects, release kinetics, the quantity of drug that can be loaded per MSC, and effect on tumor inhibition all require further exploration [100]. Methods to enhance either tumor-selective delivery or the specificity of the targeted agent are of critical interest. The existing strategy has been to infect MSCs with the measles virus or an oncolytic adenovirus that selectively replicates in tumor cells, which has the added advantage of increasing the anti-tumor effect with subsequent rounds of infection and lysis. This problem may be solved through genetic engineering modification of MSCs or the application of bioengineered scaffolds. However, in recent years, there has been no research on the application of MSC drug-loading in the treatment of OS.
4.3. MSC-derived exosomes to target OS
Due to the limitations of MSC drug-loading capacity, researchers have focused on the nanoscale EVs secreted by MSCs. These exosomes can be a good solution to the problem of cargo-loading in MSCs and have become promising carriers for the treatment of tumors. MSC–Exos have a variety of therapeutic effects, such as suppressing apoptosis, promoting cell regeneration and migration, regulating immune and inflammatory responses, and supporting angiogenesis, nerve regeneration, and tissue repair and regeneration all while having the advantages of high biocompatibility, high loading ability, long life span in circulation, low immunogenicity, no cytotoxicity, and capacity to cross biological barriers [101]. Compared with MSCs, MSC–Exos have many unique advantages. First, exosomes are easy to collect; a variety of types of MSCs can produce exosomes, and each secretes 1000 to 10,000 of them. Their production is, therefore, simpler and less costly, and time-consuming than that of MSCs. Second, exosomes are stable in long-term storage; with a size one-millionth that of MSCs, they are relatively simple. Third, MSC–Exos have better safety than MSCs for clinical applications. Existing MSC-based therapies have problems with cell survival, regenerative ability, immune response, and the possibility of differentiation into tumors. These problems can be prevented by utilizing exosomes as a cell-free therapy; for instance, as exosomes cannot proliferate, there is no possibility of tumorigenesis [102]. Abello and colleagues conducted an experiment that labeled exosomes derived from hUC–MSCs, which were injected in vivo [103]. The results showed that exosomes from hUC–MSCs would accumulate continuously in the tumor for more than 24–48 h and had the potential to localize tumor or drug delivery. These labeled exosomes have tumor-targeting characteristics and can be used as effective, negatively charged, drug carriers to inhibit OS cells effectively. However, it is not clear whether the cancer parenchyma or stromal cells specifically receive deposits from exocrine bodies derived from hUC–MSCs, and the potential effects on the TME need to be further studied. Targeted therapy of cancers via MSC–Exos has been applied in a variety of clinical treatments, such as for breast, lung, ovarian, colon, liver, and pancreatic cancers, and melanoma [104], [105], [106], [107], [108], [109]. Nevertheless, there have only been a few studies on the application of exosomes secreted by MSCs in OS. Targeting therapy for OS by using exosomes to load drugs or genetically modified anti-cancer RNAs and DNAs has great prospects for clinical applications and merits more in-depth study.
4.4. Cancer immunotherapy of MSCs
Immunotherapy is attracting more and more attention, emerged as a novel option for cancer therapy, eradicating cancer cells by enhancing or modifying the innate immune system [110]. Targeted cytokine immunotherapy to conquer the heterogeneity of malignant and cancer cell defense is also a hot and difficult topic. MSCs have received great attention in the field of cancer immunotherapy for their excellent targeting and secretory functions, playing an indispensable role in cancer immunomodulatory [111], [112]. On the one hand, MSCs and secreted exosomes can be used as biological carriers of genetic engineering to achieve the therapeutic effect by loading related immune cytokines. On the other hand, MSCs and secreted exosomes can regulate the immune response of TME, measuring the expression of T cells and affecting the growth of cancer by secreting cytokines. At present, there are relatively little researches on MSCs in the field of cancer immunotherapy [113], especially osteosarcoma, which is a potential research direction in the future.
5. Conclusions
Currently, MSCs are attracting increasing attention in the treatment of cancer. The mechanisms underlying their effects may include induced differentiation, immune regulation, cell fusion, and paracrine effects. In this review, we discussed the current application of MSCs from various tissue sources in OS, mainly showing the promotional and inhibitory effects of MSCs on the heterogenicity of OS cells. MSCs affect tumor cells through three-dimensional dialogue with tumor cells, stromal cells, and cytokines, and chemokines. In addition, abnormal gene expression performs a critical role in the differentiation of MSCs. At present, the main clinical applications of MSCs have focused on two areas; the first is the use of MSCs themselves through secretory regulation to achieve the activation or inhibition of a target signal pathway and the secretion of related cytokines to limit tumor growth. The second is their use as a carrier to achieve targeted therapy to the tumor site but, due to the limitations of MSCs, the EVs secreted by them, especially exosomes, have a broader development prospect as a molecular drug or gene carrier, although their possible off-target effects have not been studied. MSC–Exos' biodistribution, toxicity, clearance after injecting, and safety verification all require study. In the process of OS evolution, MSCs will be recruited into the TME. The interactions between these recruited MSCs from different sources and the tumor cells are not clear. Additionally, most of the existing studies have explored the effect of single tissue-derived MSCs on OS; there has been no functional comparison between different tissue-derived MSCs. Moreover, based on previous nonmalignant orthopedic diseases [114], [115], [116], [117], animal models injected OS patient-derived xenograft cells, via percutaneous application, intravenous or with graft, and what type of graft structural/non-structural +/− additional factors or expression of genes to influence MSC effects, need further research. Furthermore, in patients with OS are there already factors present in circulation which may interfere with the local signaling in vitro, which could be investigated further more. Generally speaking, MSCs have great prospects for application in the clinical treatment of tumors, and more experiments are needed to study the molecular mechanism of their effects on tumor cells. Only by addressing a series of challenges and difficulties can the therapeutic potential of exosomes be realized.
Acknowledgments
Acknowledgements
Not applicable.
Authors’ contributions
Xingyu Chang and Zhanjun Ma contributed to the investigation and wrote the original draft of the manuscript. Guomao Zhu contributed to the methodology. Jingjing Yang contributed to the conceptualization. All authors read and approved the final manuscript.
Funding
This work was financially supported by the Cuiying Scientific Training Program for Undergraduates of Lanzhou University Second Hospital (CYXZ2020-02; CYXZ2020-03) and Chinese Medicine Administration Research Project of Gansu province (GZK-2019-46).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Author details
1. The First Clinical Medical College, Lanzhou University, No.1 Donggangxi Street, Lanzhou, Gansu 730030, China; 2. The Second Clinical Medical College, Lanzhou University, No.82 Cuiyingmen Street, Lanzhou, Gansu 730030, China.
References
- 1.Main H., Munsie M., O’Connor M.D. Managing the potential and pitfalls during clinical translation of emerging stem cell therapies. Clin. Transl. Med. 2014;3:10. doi: 10.1186/2001-1326-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawai T., Katagiri W., Osugi M., Sugimura Y., Hibi H., Ueda M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy. 2015;17:369–381. doi: 10.1016/j.jcyt.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Kim H.K., Lee S.G., Lee S.W., Oh B.J., Kim J.H., Kim J.A., Lee G., Jang J.D., Joe Y.A. A subset of paracrine factors as efficient biomarkers for predicting vascular regenerative efficacy of mesenchymal stromal/stem cells. Stem Cells. 2019;37:77–88. doi: 10.1002/stem.2920. [DOI] [PubMed] [Google Scholar]
- 4.Rüster B., Göttig S., Ludwig R.J., Bistrian R., Müller S., Seifried E., Gille J., Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz L.A., Gambelli F., McBride C., Gaupp D., Baddoo M., Kaminski N., Phinney D.G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato Y., Araki H., Kato J., Nakamura K., Kawano Y., Kobune M., Sato T., Miyanishi K., Takayama T., Takahashi M., Takimoto R., Iyama S., Matsunaga T., Ohtani S., Matsuura A., Hamada H., Niitsu Y. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 7.Ji J.F., He B.P., Dheen S.T., Tay S.S. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–427. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- 8.Wu G.D., Nolta J.A., Jin Y.S., Barr M.L., Yu H., Starnes V.A., Cramer D.V. Migration of mesenchymal stem cells to heart allografts during chronic rejection. Transplantation. 2003;75:679–685. doi: 10.1097/01.Tp.0000048488.35010.95. [DOI] [PubMed] [Google Scholar]
- 9.Le Blanc K., Ringdén O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2005;11:321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Murray I.R., West C.C., Hardy W.R., James A.W., Park T.S., Nguyen A., Tawonsawatruk T., Lazzari L., Soo C., Péault B. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol. Life Sci. 2014;71:1353–1374. doi: 10.1007/s00018-013-1462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan A., Deeb G., Rahal R., Atwi K., Mondello S., Marei H.E., Gali A., Sleiman E. Mesenchymal stem cells in the treatment of traumatic brain injury. Front. Neurol. 2017;8:28. doi: 10.3389/fneur.2017.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Ann Oncol. 23 Suppl 7 (2012) vii100-9. https://doi.org/10.1093/annonc/mds254. [DOI] [PubMed]
- 13.Broadhead M.L., Clark J.C., Myers D.E., Dass C.R., Choong P.F. The molecular pathogenesis of osteosarcoma: a review. Sarcoma. 2011;2011 doi: 10.1155/2011/959248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kempf-Bielack B., Bielack S.S., Jürgens H., Branscheid D., Berdel W.E., Exner G.U., Göbel U., Helmke K., Jundt G., Kabisch H., Kevric M., Klingebiel T., Kotz R., Maas R., Schwarz R., Semik M., Treuner J., Zoubek A., Winkler K. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J. Clin. Oncol. 2005;23:559–568. doi: 10.1200/jco.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 15.Sun Z., Wang S., Zhao R.C. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J. Hematol. Oncol. 2014;7:14. doi: 10.1186/1756-8722-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Y., Wang G., Chen R., Hua Y., Cai Z. Mesenchymal stem cells in the osteosarcoma microenvironment: their biological properties, influence on tumor growth, and therapeutic implications. Stem Cell Res. Ther. 2018;9:22. doi: 10.1186/s13287-018-0780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu T., Ishikawa T., Sugihara E., Kuninaka S., Miyamoto T., Mabuchi Y., Matsuzaki Y., Tsunoda T., Miya F., Morioka H., Nakayama R., Kobayashi E., Toyama Y., Kawai A., Ichikawa H., Hasegawa T., Okada S., Ito T., Ikeda Y., Suda T., Saya H. c-MYC overexpression with loss of Ink4a/Arf transforms bone marrow stromal cells into osteosarcoma accompanied by loss of adipogenesis. Oncogene. 2010;29:5687–5699. doi: 10.1038/onc.2010.312. [DOI] [PubMed] [Google Scholar]
- 18.A.B. Mohseny, K. Szuhai, S. Romeo, E.P. Buddingh, I. Briaire-de Bruijn, D. de Jong, M. van Pel, A.M. Cleton-Jansen, P.C. Hogendoorn, Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2, J Pathol. 219 (2009) 294–305. DOI:10.1002/path.2603. [DOI] [PubMed]
- 19.Walkley C.R., Qudsi R., Sankaran V.G., Perry J.A., Gostissa M., Roth S.I., Rodda S.J., Snay E., Dunning P., Fahey F.H., Alt F.W., McMahon A.P., Orkin S.H. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008;22:1662–1676. doi: 10.1101/gad.1656808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubio R., Gutierrez-Aranda I., Sáez-Castillo A.I., Labarga A., Rosu-Myles M., Gonzalez-Garcia S., Toribio M.L., Menendez P., Rodriguez R. The differentiation stage of p53-Rb-deficient bone marrow mesenchymal stem cells imposes the phenotype of in vivo sarcoma development. Oncogene. 2013;32:4970–4980. doi: 10.1038/onc.2012.507. [DOI] [PubMed] [Google Scholar]
- 21.Lin P.P., Pandey M.K., Jin F., Raymond A.K., Akiyama H., Lozano G. Targeted mutation of p53 and Rb in mesenchymal cells of the limb bud produces sarcomas in mice. Carcinogenesis. 2009;30:1789–1795. doi: 10.1093/carcin/bgp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berman S.D., Calo E., Landman A.S., Danielian P.S., Miller E.S., West J.C., Fonhoue B.D., Caron A., Bronson R., Bouxsein M.L., Mukherjee S., Lees J.A. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11851–11856. doi: 10.1073/pnas.0805462105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutsaers A.J., Ng A.J., Baker E.K., Russell M.R., Chalk A.M., Wall M., Liddicoat B.J., Ho P.W., Slavin J.L., Goradia A., Martin T.J., Purton L.E., Dickins R.A., Walkley C.R. Modeling distinct osteosarcoma subtypes in vivo using Cre:lox and lineage-restricted transgenic shRNA. Bone. 2013;55:166–178. doi: 10.1016/j.bone.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Meurette O., Mehlen P. Notch signaling in the tumor microenvironment. Cancer Cell. 2018;34:536–548. doi: 10.1016/j.ccell.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartmann S., Bhola N.E., Grandis J.R. HGF/met signaling in head and neck cancer: impact on the tumor microenvironment. Clin. Cancer Res. 2016;22:4005–4013. doi: 10.1158/1078-0432.Ccr-16-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klemm F., Joyce J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawano M., Tanaka K., Itonaga I., Iwasaki T., Tsumura H. Interaction between human osteosarcoma and mesenchymal stem cells via an interleukin-8 signaling loop in the tumor microenvironment. Cell Commun Signal. 2018;16:13. doi: 10.1186/s12964-018-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelagalli A., Nardelli A., Fontanella R., Zannetti A. Inhibition of AQP1 hampers osteosarcoma and hepatocellular carcinoma progression mediated by bone marrow-derived mesenchymal stem cells. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou H., Zarubin T., Ji Z., Min Z., Zhu W., Downey J.S., Lin S., Han J. Frequency and distribution of AP-1 sites in the human genome. DNA Res. 2005;12:139–150. doi: 10.1093/dnares/12.2.139. [DOI] [PubMed] [Google Scholar]
- 31.Wang J.Y., Wu P.K., Chen P.C., Lee C.W., Chen W.M., Hung S.C. Generation of osteosarcomas from a combination of Rb silencing and c-Myc overexpression in human mesenchymal stem cells. Stem Cells Transl. Med. 2017;6:512–526. doi: 10.5966/sctm.2015-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saalfrank A., Janssen K.P., Ravon M., Flisikowski K., Eser S., Steiger K., Flisikowska T., Müller-Fliedner P., Schulze É., Brönner C., Gnann A., Kappe E., Böhm B., Schade B., Certa U., Saur D., Esposito I., Kind A., Schnieke A. A porcine model of osteosarcoma. Oncogenesis. 2016;5 doi: 10.1038/oncsis.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin L., Huang K., Guo W., Zhou C., Wang G., Zhao Q. Conditioned medium of the osteosarcoma cell line U2OS induces hBMSCs to exhibit characteristics of carcinoma-associated fibroblasts via activation of IL-6/STAT3 signalling. J. Biochem. 2020;168:265–271. doi: 10.1093/jb/mvaa044. [DOI] [PubMed] [Google Scholar]
- 34.Zhu H., Guo S., Zhang Y., Yin J., Yin W., Tao S., Wang Y., Zhang C. Proton-sensing GPCR-YAP signalling promotes cancer-associated fibroblast activation of mesenchymal stem cells. Int. J. Biol. Sci. 2016;12:389–396. doi: 10.7150/ijbs.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pietrovito L., Leo A., Gori V., Lulli M., Parri M., Becherucci V., Piccini L., Bambi F., Taddei M.L., Chiarugi P. Bone marrow-derived mesenchymal stem cells promote invasiveness and transendothelial migration of osteosarcoma cells via a mesenchymal to amoeboid transition. Mol. Oncol. 2018;12:659–676. doi: 10.1002/1878-0261.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y.M., Wang W., Qiu E.D. Osteosarcoma cells induce differentiation of mesenchymal stem cells into cancer associated fibroblasts through Notch and Akt signaling pathway. Int. J. Clin. Exp. Pathol. 2017;10:8479–8486. [PMC free article] [PubMed] [Google Scholar]
- 37.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J. Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu R., Zhang Y.W., Li H.M., Lv W.C., Zhao L., Guo Q.L., Lu T., Weiss S.J., Li Z.Y., Wu Z.Q. LW106, a novel indoleamine 2,3-dioxygenase 1 inhibitor, suppresses tumour progression by limiting stroma-immune crosstalk and cancer stem cell enrichment in tumour micro-environment. Br. J. Pharmacol. 2018;175:3034–3049. doi: 10.1111/bph.14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker C., Mojares E., Del Río Hernández A. Role of extracellular matrix in development and cancer progression. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai R., Kawazoe N., Chen G. Influence of surfaces modified with biomimetic extracellular matrices on adhesion and proliferation of mesenchymal stem cells and osteosarcoma cells. Colloids Surf. B Biointerfaces. 2015;126:381–386. doi: 10.1016/j.colsurfb.2014.11.050. [DOI] [PubMed] [Google Scholar]
- 41.Halpern J.L., Kilbarger A., Lynch C.C. Mesenchymal stem cells promote mammary cancer cell migration in vitro via the CXCR2 receptor. Cancer Lett. 2011;308:91–99. doi: 10.1016/j.canlet.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y.J., Dai Y.L., Zhang W.B., Li S.J., Tu C.Q. Clinicopathological and prognostic significance of chemokine receptor CXCR4 in patients with bone and soft tissue sarcoma: a meta-analysis. Clin. Exp. Med. 2017;17:59–69. doi: 10.1007/s10238-015-0405-y. [DOI] [PubMed] [Google Scholar]
- 43.Y. Han, C. Wu, J. Wang, N. Liu, CXCR7 maintains osteosarcoma invasion after CXCR4 suppression in bone marrow microenvironment, Tumour Biol. 39 (2017) 1010428317701631. DOI:10.1177/1010428317701631. [DOI] [PubMed]
- 44.Du L., Han X.G., Tu B., Wang M.Q., Qiao H., Zhang S.H., Fan Q.M., Tang T.T. CXCR1/Akt signaling activation induced by mesenchymal stem cell-derived IL-8 promotes osteosarcoma cell anoikis resistance and pulmonary metastasis. Cell Death Dis. 2018;9:714. doi: 10.1038/s41419-018-0745-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walter M., Liang S., Ghosh S., Hornsby P.J., Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28:2745–2755. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avnet S., Longhi A., Salerno M., Halleen J.M., Perut F., Granchi D., Ferrari S., Bertoni F., Giunti A., Baldini N. Increased osteoclast activity is associated with aggressiveness of osteosarcoma. Int. J. Oncol. 2008;33:1231–1238. [PubMed] [Google Scholar]
- 47.Duan Z., Lamendola D.E., Penson R.T., Kronish K.M., Seiden M.V. Overexpression of IL-6 but not IL-8 increases paclitaxel resistance of U-2OS human osteosarcoma cells. Cytokine. 2002;17:234–242. doi: 10.1006/cyto.2001.1008. [DOI] [PubMed] [Google Scholar]
- 48.Tu B., Zhu J., Liu S., Wang L., Fan Q., Hao Y., Fan C., Tang T.T. Mesenchymal stem cells promote osteosarcoma cell survival and drug resistance through activation of STAT3. Oncotarget. 2016;7:48296–48308. doi: 10.18632/oncotarget.10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sedlakova O., Svastova E., Takacova M., Kopacek J., Pastorek J., Pastorekova S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front. Physiol. 2014;4:400. doi: 10.3389/fphys.2013.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avnet S., Di Pompo G., Chano T., Errani C., Ibrahim-Hashim A., Gillies R.J., Donati D.M., Baldini N. Cancer-associated mesenchymal stroma fosters the stemness of osteosarcoma cells in response to intratumoral acidosis via NF-κB activation. Int. J. Cancer. 2017;140:1331–1345. doi: 10.1002/ijc.30540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chou A.J., Geller D.S., Gorlick R. Therapy for osteosarcoma: where do we go from here? Paediatr Drugs. 2008;10:315–327. doi: 10.2165/00148581-200810050-00005. [DOI] [PubMed] [Google Scholar]
- 52.Yu F.X., Hu W.J., He B., Zheng Y.H., Zhang Q.Y., Chen L. Bone marrow mesenchymal stem cells promote osteosarcoma cell proliferation and invasion. World J. Surg. Oncol. 2015;13:52. doi: 10.1186/s12957-015-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 54.Perut F., Roncuzzi L., Baldini N. The emerging roles of extracellular vesicles in osteosarcoma. Front. Oncol. 2019;9:1342. doi: 10.3389/fonc.2019.01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shao H., Im H., Castro C.M., Breakefield X., Weissleder R., Lee H. New technologies for analysis of extracellular vesicles. Chem. Rev. 2018;118:1917–1950. doi: 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhuang G., Wu X., Jiang Z., Kasman I., Yao J., Guan Y., Oeh J., Modrusan Z., Bais C., Sampath D., Ferrara N. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. Embo J. 2012;31:3513–3523. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chalmin F., Ladoire S., Mignot G., Vincent J., Bruchard M., Remy-Martin J.P., Boireau W., Rouleau A., Simon B., Lanneau D., De Thonel A., Multhoff G., Hamman A., Martin F., Chauffert B., Solary E., Zitvogel L., Garrido C., Ryffel B., Borg C., Apetoh L., Rébé C., Ghiringhelli F. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest. 2010;120:457–471. doi: 10.1172/jci40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mannerström B., Kornilov R., Abu-Shahba A.G., Chowdhury I.M., Sinha S., Seppänen-Kaijansinkko R., Kaur S. Epigenetic alterations in mesenchymal stem cells by osteosarcoma-derived extracellular vesicles. Epigenetics. 2019;14:352–364. doi: 10.1080/15592294.2019.1585177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vallabhaneni K.C., Hassler M.Y., Abraham A., Whitt J., Mo Y.Y., Atfi A., Pochampally R. Mesenchymal stem/stromal cells under stress increase osteosarcoma migration and apoptosis resistance via extracellular vesicle mediated communication. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baglio S.R., Lagerweij T., Pérez-Lanzón M., Ho X.D., Léveillé N., Melo S.A., Cleton-Jansen A.M., Jordanova E.S., Roncuzzi L., Greco M., van Eijndhoven M.A.J., Grisendi G., Dominici M., Bonafede R., Lougheed S.M., de Gruijl T.D., Zini N., Cervo S., Steffan A., Canzonieri V., Martson A., Maasalu K., Köks S., Wurdinger T., Baldini N., Pegtel D.M. Blocking tumor-educated MSC paracrine activity halts osteosarcoma progression. Clin. Cancer Res. 2017;23:3721–3733. doi: 10.1158/1078-0432.Ccr-16-2726. [DOI] [PubMed] [Google Scholar]
- 61.Lagerweij T., Pérez-Lanzón M., Baglio S.R. A preclinical mouse model of osteosarcoma to define the extracellular vesicle-mediated communication between tumor and mesenchymal stem cells. J. Vis. Exp. 2018 doi: 10.3791/56932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Semenza G.L. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol. Med. 2012;18:534–543. doi: 10.1016/j.molmed.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samanta D., Semenza G.L. Metabolic adaptation of cancer and immune cells mediated by hypoxia-inducible factors. Biochim. Biophys. Acta Rev. Cancer. 1870;2018:15–22. doi: 10.1016/j.bbcan.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Lin S., Zhu B., Huang G., Zeng Q., Wang C. Microvesicles derived from human bone marrow mesenchymal stem cells promote U2OS cell growth under hypoxia: the role of PI3K/AKT and HIF-1α. Hum. Cell. 2019;32:64–74. doi: 10.1007/s13577-018-0224-z. [DOI] [PubMed] [Google Scholar]
- 65.Milane L., Singh A., Mattheolabakis G., Suresh M., Amiji M.M. Exosome mediated communication within the tumor microenvironment. J. Control Release. 2015;219:278–294. doi: 10.1016/j.jconrel.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 66.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 67.Yu B., Zhang X., Li X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nawaz M., Fatima F., Vallabhaneni K.C., Penfornis P., Valadi H., Ekström K., Kholia S., Whitt J.D., Fernandes J.D., Pochampally R., Squire J.A., Camussi G. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016:1073140. doi: 10.1155/2016/1073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kidd S., Spaeth E., Dembinski J.L., Dietrich M., Watson K., Klopp A., Battula V.L., Weil M., Andreeff M., Marini F.C. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang Y., Liu W., He B., Wang L., Zhang F., Shu H., Sun L. Exosomes derived from bone marrow mesenchymal stem cells promote osteosarcoma development by activating oncogenic autophagy. J. Bone Oncol. 2020;21 doi: 10.1016/j.jbo.2020.100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun Z., Shi K., Yang S., Liu J., Zhou Q., Wang G., Song J., Li Z., Zhang Z., Yuan W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol. Cancer. 2018;17:147. doi: 10.1186/s12943-018-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rupaimoole R., Slack F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 73.Kinoshita T., Yip K.W., Spence T., Liu F.F. MicroRNAs in extracellular vesicles: potential cancer biomarkers. J. Hum. Genet. 2017;62:67–74. doi: 10.1038/jhg.2016.87. [DOI] [PubMed] [Google Scholar]
- 74.Qin F., Tang H., Zhang Y., Zhang Z., Huang P., Zhu J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J. Cell Physiol. 2020;235:4734–4745. doi: 10.1002/jcp.29351. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H., Wang J., Ren T., Huang Y., Liang X., Yu Y., Wang W., Niu J., Guo W. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020;490:54–65. doi: 10.1016/j.canlet.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Zhao W., Qin P., Zhang D., Cui X., Gao J., Yu Z., Chai Y., Wang J., Li J. Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived exosomes promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183-5p. Aging (Albany NY). 2019;11:9581–9596. doi: 10.18632/aging.102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hennet T. Collagen glycosylation. Curr. Opin. Struct. Biol. 2019;56:131–138. doi: 10.1016/j.sbi.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y., Chu Y., Li K., Zhang G., Guo Z., Wu X., Qiu C., Li Y., Wan X., Sui J., Zhang D., Xiang H., Chen B. Exosomes secreted by adipose-derived mesenchymal stem cells foster metastasis and osteosarcoma proliferation by increasing COLGALT2 expression. Front. Cell Dev. Biol. 2020;8:353. doi: 10.3389/fcell.2020.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng T., Lyon C.J., Bergin S., Caligiuri M.A., Hsueh W.A. Obesity, inflammation, and cancer. Annu. Rev. Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 80.Aanstoos M.E., Regan D.P., Rose R.J., Chubb L.S., Ehrhart N.P. Do mesenchymal stromal cells influence microscopic residual or metastatic osteosarcoma in a murine model? Clin. Orthop Relat. Res. 2016;474:707–715. doi: 10.1007/s11999-015-4362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee S.W., Jeon T.J., Biswal S. Effect of local treatment with adipose tissue-derived mesenchymal stem cells in the early tumorigenesis of osteosarcoma. Oncol. Rep. 2015;33:1381–1387. doi: 10.3892/or.2015.3711. [DOI] [PubMed] [Google Scholar]
- 82.Ghannam S., Bouffi C., Djouad F., Jorgensen C., Noël D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res. Ther. 2010 doi: 10.1186/scrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.NguyenThai Q.A., Sharma N., Luong do H., Sodhi S.S., Kim J.H., Kim N., Oh S.J., Jeong D.K. Targeted inhibition of osteosarcoma tumor growth by bone marrow-derived mesenchymal stem cells expressing cytosine deaminase/5-fluorocytosine in tumor-bearing mice. J. Gene Med. 2015;17:87–99. doi: 10.1002/jgm.2826. [DOI] [PubMed] [Google Scholar]
- 84.Paino F., La Noce M., Di Nucci D., Nicoletti G.F., Salzillo R., De Rosa A., Ferraro G.A., Papaccio G., Desiderio V., Tirino V. Human adipose stem cell differentiation is highly affected by cancer cells both in vitro and in vivo: implication for autologous fat grafting. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2016.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen W.C., Lai Y.C., Li L.H., Liao K., Lai H.C., Kao S.Y., Wang J., Chuong C.M., Hung S.C. Methylation and PTEN activation in dental pulp mesenchymal stem cells promotes osteogenesis and reduces oncogenesis. Nat. Commun. 2019;10:2226. doi: 10.1038/s41467-019-10197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Becker A., Riet I.V. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J. Stem Cells. 2016;8:73–87. doi: 10.4252/wjsc.v8.i3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nitzsche F., Müller C., Lukomska B., Jolkkonen J., Deten A., Boltze J. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells. 2017;35:1446–1460. doi: 10.1002/stem.2614. [DOI] [PubMed] [Google Scholar]
- 88.Tat S.K., Padrines M., Theoleyre S., Couillaud-Battaglia S., Heymann D., Redini F., Fortun Y. OPG/membranous–RANKL complex is internalized via the clathrin pathway before a lysosomal and a proteasomal degradation. Bone. 2006;39:706–715. doi: 10.1016/j.bone.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 89.Lamoureux F., Richard P., Wittrant Y., Battaglia S., Pilet P., Trichet V., Blanchard F., Gouin F., Pitard B., Heymann D., Redini F. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007;67:7308–7318. doi: 10.1158/0008-5472.Can-06-4130. [DOI] [PubMed] [Google Scholar]
- 90.Qiao B., Shui W., Cai L., Guo S., Jiang D. Human mesenchymal stem cells as delivery of osteoprotegerin gene: homing and therapeutic effect for osteosarcoma. Drug Des. Dev. Ther. 2015;9:969–976. doi: 10.2147/dddt.S77116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sage E.K., Kolluri K.K., McNulty K., Lourenco Sda S., Kalber T.L., Ordidge K.L., Davies D., Gary Lee Y.C., Giangreco A., Janes S.M. Systemic but not topical TRAIL-expressing mesenchymal stem cells reduce tumour growth in malignant mesothelioma. Thorax. 2014;69:638–647. doi: 10.1136/thoraxjnl-2013-204110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kanehira M., Xin H., Hoshino K., Maemondo M., Mizuguchi H., Hayakawa T., Matsumoto K., Nakamura T., Nukiwa T., Saijo Y. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007;14:894–903. doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- 93.Sasportas L.S., Kasmieh R., Wakimoto H., Hingtgen S., van de Water J.A., Mohapatra G., Figueiredo J.L., Martuza R.L., Weissleder R., Shah K. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc. Natl. Acad. Sci. U.S.A. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tyciakova S., Matuskova M., Bohovic R., Polakova K., Toro L., Skolekova S., Kucerova L. Genetically engineered mesenchymal stromal cells producing TNFα have tumour suppressing effect on human melanoma xenograft. J. Gene Med. 2015;17:54–67. doi: 10.1002/jgm.2823. [DOI] [PubMed] [Google Scholar]
- 95.Choi S.H., Tamura K., Khajuria R.K., Bhere D., Nesterenko I., Lawler J., Shah K. Antiangiogenic variant of TSP-1 targets tumor cells in glioblastomas. Mol. Ther. 2015;23:235–243. doi: 10.1038/mt.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Piri E.A., Hajikhanmirzaei Z.M. Interleukin-25 as a candidate gene in immunogene therapy of pancreatic cancer. J. Med. Hypotheses Ideas. 2012 [Google Scholar]
- 97.Martínez-González I., Roca O., Masclans J.R., Moreno R., Salcedo M.T., Baekelandt V., Cruz M.J., Rello J., Aran J.M. Human mesenchymal stem cells overexpressing the IL-33 antagonist soluble IL-1 receptor-like-1 attenuate endotoxin-induced acute lung injury. Am. J. Respir. Cell Mol. Biol. 2013;49:552–562. doi: 10.1165/rcmb.2012-0406OC. [DOI] [PubMed] [Google Scholar]
- 98.Niu J., Yue W., Song Y., Zhang Y., Qi X., Wang Z., Liu B., Shen H., Hu X. Prevention of acute liver allograft rejection by IL-10-engineered mesenchymal stem cells. Clin. Exp. Immunol. 2014;176:473–484. doi: 10.1111/cei.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pessina A., Bonomi A., Coccè V., Invernici G., Navone S., Cavicchini L., Sisto F., Ferrari M., Viganò L., Locatelli A., Ciusani E., Cappelletti G., Cartelli D., Arnaldo C., Parati E., Marfia G., Pallini R., Falchetti M.L., Alessandri G. Mesenchymal stromal cells primed with paclitaxel provide a new approach for cancer therapy. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krueger T.E.G., Thorek D.L.J., Denmeade S.R., Isaacs J.T., Brennen W.N. Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl. Med. 2018;7:651–663. doi: 10.1002/sctm.18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baek G., Choi H., Kim Y., Lee H.C., Choi C. Mesenchymal stem cell-derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Transl. Med. 2019;8:880–886. doi: 10.1002/sctm.18-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marote A., Teixeira F.G., Mendes-Pinheiro B., Salgado A.J. MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential. Front. Pharmacol. 2016;7:231. doi: 10.3389/fphar.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abello J., Nguyen T.D.T., Marasini R., Aryal S., Weiss M.L. Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics. 2019;9:2325–2345. doi: 10.7150/thno.30030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Munagala R., Aqil F., Jeyabalan J., Agrawal A.K., Mudd A.M., Kyakulaga A.H., Singh I.P., Vadhanam M.V., Gupta R.C. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017;393:94–102. doi: 10.1016/j.canlet.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morishita M., Takahashi Y., Nishikawa M., Ariizumi R., Takakura Y. Enhanced Class I tumor antigen presentation via cytosolic delivery of exosomal cargos by tumor-cell-derived exosomes displaying a pH-sensitive fusogenic peptide. Mol. Pharm. 2017;14:4079–4086. doi: 10.1021/acs.molpharmaceut.7b00760. [DOI] [PubMed] [Google Scholar]
- 106.Hadla M., Palazzolo S., Corona G., Caligiuri I., Canzonieri V., Toffoli G., Rizzolio F. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine (Lond) 2016;11:2431–2441. doi: 10.2217/nnm-2016-0154. [DOI] [PubMed] [Google Scholar]
- 107.Li Y., Gao Y., Gong C., Wang Z., Xia Q., Gu F., Hu C., Zhang L., Guo H., Gao S. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine. 2018;14:1973–1985. doi: 10.1016/j.nano.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 108.Li H., Yang C., Shi Y., Zhao L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J. Nanobiotechnol. 2018;16:103. doi: 10.1186/s12951-018-0429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Altanerova U., Jakubechova J., Benejova K., Priscakova P., Repiska V., Babelova A., Smolkova B., Altaner C. Intracellular prodrug gene therapy for cancer mediated by tumor cell suicide gene exosomes. Int. J. Cancer. 2021;148:128–139. doi: 10.1002/ijc.33188. [DOI] [PubMed] [Google Scholar]
- 110.Mosallaei M., Simonian M., Ehtesham N., Karimzadeh M.R., Vatandoost N., Negahdari B., Salehi R. Genetically engineered mesenchymal stem cells: targeted delivery of immunomodulatory agents for tumor eradication. Cancer Gene Ther. 2020 doi: 10.1038/s41417-020-0179-6. [DOI] [PubMed] [Google Scholar]
- 111.Poggi A., Varesano S., Zocchi M.R. How to hit mesenchymal stromal cells and make the tumor microenvironment immunostimulant rather than immunosuppressive. Front. Immunol. 2018 doi: 10.3389/fimmu.2018.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Razmkhah M., Abtahi S., Ghaderi A. Mesenchymal stem cells, immune cells and tumor cells crosstalk: a sinister triangle in the tumor microenvironment. Curr Stem Cell Res. Ther. 2019 doi: 10.2174/1574888X13666180816114809. [DOI] [PubMed] [Google Scholar]
- 113.Sineh Sepehr K., Razavi A., Hassan Z.M., Fazel A., Abdollahpour-Alitappeh M., Mossahebi-Mohammadi M., Yekaninejad M.S., Farhadihosseinabadi B., Hashemi S.M. Comparative immunomodulatory properties of mesenchymal stem cells derived from human breast tumor and normal breast adipose tissue. Cancer Immunol. Immunother. 2020 doi: 10.1007/s00262-020-02567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hernigou P., Mathieu G., Poignard A., Manicom O., Beaujean F., Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Surgical technique. J. Bone Joint Surg. Am. 2006 doi: 10.2106/JBJS.F.00203. [DOI] [PubMed] [Google Scholar]
- 115.Hernigou P., Guissou I., Homma Y., Poignard A., Chevallier N., Rouard H., Flouzat Lachaniette C.H. Percutaneous injection of bone marrow mesenchymal stem cells for ankle non-unions decreases complications in patients with diabetes. Int. Orthop. 2015 doi: 10.1007/s00264-015-2738-2. [DOI] [PubMed] [Google Scholar]
- 116.P. Douras, T. Tosounidis, P.V. Giannoudis. Application of the 'diamond concept' with fast bone marrow aspirate concentration for the treatment of medial malleolus non-union Injury. 2018 DOI:10.1016/j.injury.2018.11.013. [DOI] [PubMed]
- 117.Hernigou P., Trousselier M., Roubineau F., Bouthors C., Chevallier N., Rouard H., Flouzat-Lachaniette C.H. Stem cell therapy for the treatment of hip osteonecrosis: a 30-year review of progress. Clin. Orthop Surg. 2016 doi: 10.4055/cios.2016.8.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.