Highlights
-
•
SC-CO2 at temperatures > 60 °C was effective to inactivate spores in lipid emulsions.
-
•
C. butyricum spores were more resistant to the SC-CO2 treatments than A. niger spores.
-
•
HPU intensified the inactivation of C. butyricum spores while no effect was found on A. niger.
-
•
Higher temperatures increase the SC-CO2 + HPU spore inactivation.
-
•
Pressure increase improved SC-CO2 + HPU spore inactivation up to 350 bar.
Keywords: Spores, Inactivation, Supercritical fluids, Ultrasound, Emulsions
Abstract
For the first time, this study addresses the intensification of supercritical carbon dioxide (SC-CO2) treatments using high-power ultrasound (HPU) for the inactivation of fungal (Aspergillus niger) and bacterial (Clostridium butyricum) spores in oil-in-water emulsions. The inactivation kinetics were analyzed at different pressures (100, 350 and 550 bar) and temperatures (50, 60, 70, 80, 85 °C), depending on the microorganism, and compared to the conventional thermal treatment. The inactivation kinetics were satisfactorily described using the Weibull model.
Experimental results showed that SC-CO2 enhanced the inactivation level of both spores when compared to thermal treatments. Bacterial spores (C. butyricum) were found to be more resistant to SC-CO2 + HPU, than fungal (A. niger) ones, as also observed in the thermal and SC-CO2 treatments. The application of HPU intensified the SC-CO2 inactivation of C. butyricum spores, e.g. shortening the total inactivation time from 10 to 3 min at 85 °C. However, HPU did not affect the SC-CO2 inactivation of A. niger spores. The study into the effect of a combined SC-CO2 + HPU treatment has to be necessarily extended to other fungal and bacterial spores, and future studies should elucidate the impact of HPU application on the emulsion’s stability.
1. Introduction
The inactivation of fungal and bacterial spores plays a relevant role in the food and pharmaceutical industry due to the fact that spores may cause product spoilage and related diseases. Spores are known to be highly resistant to many processing treatments, such as heating, drying, radiation or chemicals, among others.
The most common technology used to inactivate all types of microorganisms, including spores, has been moist heat at high temperatures (≥121 °C). However, many disadvantages are linked to the use of high temperatures, such as changes in the nutritive or organoleptic properties of the treated products.
The use of supercritical CO2 (SC-CO2) has been investigated as an alternative technology for the purposes of microbial inactivation [1]. CO2 is non-toxic, nonflammable, cheap, and its critical temperature (31 °C) and pressure (73.8 bar) are easy to reach. Moreover, SC-CO2 has liquid-like density, gas-like diffusivity and viscosity, and zero surface tension, which provides CO2 with excellent transport properties. SC-CO2 has been seen to perform well in the inactivation of vegetative cells, such as E. coli, S. cerevisiae [2] or B. diminuta, at mild temperatures (35–50 °C) [3]. The inactivation mechanisms of SC-CO2 on vegetative cells have been extensively studied. In short, SC-CO2 dissolves in the media causing acidification that modifies the membrane of the microbial cells, increasing the permeability; thus, SC-CO2 easily diffuses into the inner cell. As a result, the vital intracellular components of the cell are extracted, which leads to cell death [4]. However, SC-CO2 inactivation mechanisms in spores are not yet fully elucidated. Spore structure is different compared to that of vegetative cells, with one of the main differences being the extreme dehydration of the spores [5], [6].
In order to maintain product quality standards in SC-CO2 treatments, it is advisable to operate at the lowest possible temperature and pressure for the shortest time, while preserving product safety. However, the use of SC-CO2 at mild temperatures (<50 °C) is insufficient to inactivate fungal and bacterial spores and high pressures and temperatures and long times are required [7], [8], [9]. Consequently, the damage of heat sensitive components in the product and the increase in the process cost hinders the use of SC-CO2 for spore inactivation.
SC-CO2 treatment can be intensified by the application of high-power ultrasound (HPU). The effect of HPU on microbial inactivation is mainly linked to the violent collapse of microbubbles, known as cavitation [10]. Locally intense high temperatures and pressures, with significant shearing and turbulence effects, are caused by cavitation [11], which can affect microbial integrity. In addition, both the contact between SC-CO2 and the surface of the cells and the SC-CO2 penetration into the cell are enhanced [12]. The coupling of HPU to the SC-CO2 treatment has been demonstrated to shorten the inactivation time for vegetative cells located in fat-free media [13], [14]. However, there are no references to the combined SC-CO2 + HPU treatment for the inactivation of fungal and bacterial spores in lipid media. Only Michelino et al. [15] dealt with the SC-CO2 + HPU inactivation of bacterial spores naturally present in a solid product (coriander) and revealed an enhanced inactivation when HPU was applied. It is well known that oils/fats can hinder SC-CO2 microbial inactivation; thus, due to the importance of oil-in-water emulsions in the food and pharmaceutical industry, it would be of great interest to find alternative non-thermal treatments able to achieve a noticeable spore reduction in this type of product. Therefore, the aim of this study was to assess the feasibility of intensifying the SC-CO2 inactivation of fungal (Aspergillus niger) and bacterial (Clostridium butyricum) spores in oil-in-water emulsions by using high-power ultrasound. A. niger is a spore-forming mesophilic and aerobic filamentous fungi, common in contaminated food and pharmaceutical products [16] and widely distributed in the environment. It is also an opportunistic fungus causing otomycosis and implicated in nosocomial infections [17]. C. butyricum is an anaerobic gram-positive bacterium, which forms spores as a mechanism of resistance to stress factors. It is a spoilage bacterium capable of growing and forming butyric acid in food and pharmaceutical products [18].
2. Materials and methods
2.1. Preparation of the oil-in-water emulsion
The treated samples were oil-in-water emulsions with 20% soybean oil content. The emulsions were prepared in three stages: mixing with an Ultra-Turrax, sonication and homogenization. Firstly, the lipid phase, consisting of soybean oil and egg phospholipid, was mixed using a disperser device (IKA T25 Digital; tool S25N − 25G, Staufen, Germany) at 14000 rpm for 2 min, 10200 rpm for 4 min and 10600 rpm for 4 min. Subsequently, the lipid phase was slowly added to the water phase (deionized water and glycerol), while being mixed again at 14000 rpm. Afterwards, samples were sonicated for 5 min (UP400S, Hielscher, Teltow, Germany) using the H22-type sonotrode. Finally, the product was homogenized in two stages (50 bar; 550 bar) using a high-pressure homogenizer (PANDA Plus 2000, GEA Niro Soavi, Parma, Italy).
2.2. Preparation of the Aspergillus niger and Clostridium butyricum spore suspension
The lyophilized strains of Aspergillus niger CECT 2807 and Clostridium butyricum CECT 361T used in this study were obtained from the Spanish Type Culture Collection (CECT, Valencia, Spain).
A. niger was cultured on Potato Dextrose Agar (PDA, Scharlab, Barcelona, Spain) at 25 °C for 7 days. Afterwards, the spores were rubbed from the surface of the agar with 10 mL of 0.1% (v/v) Tween 80 and collected. The suspension was kept in a sterile container at 4 °C until use. Finally, prior to each treatment, 5 mL of the A. niger spore suspension were inoculated in a 20% soybean emulsion (60 mL) until an A. niger spore concentration of 106–107 CFU/mL. C. butyricum was sporulated following the methodology of Mafart et al. [19] with modifications. A single colony of C. butyricum was anaerobically pre-cultivated in Reinforced Clostridial Medium (RCM, Scharlab, Barcelona, Spain) at 37 °C until the stationary phase was reached (36 h). Anaerobic conditions were achieved with incubation containers with a CO2 gas generating system (Oxoid, Thermo Fisher Scientific, Waltham, Massachusetts, USA). An anaerobic indicator (Oxoid, Thermo Fisher Scientific, Waltham, Massachusetts, USA) was used to monitor the anaerobic conditions. 100 µL of the C. butyricum culture were poured into Reinforced Clostridial Agar (RCA, Scharlab, Barcelona, Spain) enriched with MnSO4 (40 mg/L) and CaCl2 (100 mg/L) to enhance the sporulation. The plates were anaerobically incubated at 37 °C for 5–6 days, during which time spores were formed (determined with a Thoma counting chamber and an optical microscope). Afterwards, spores were collected by scraping the surface of the agar, suspended in 2 mL of sterile distilled water, and washed three times by centrifugation (8000×g for 15 min) (Medifriger BL-S, JP Selecta, Barcelona, Spain). The pellet was resuspended in 2 mL of ethanol (50% v/v) and kept at 4 °C for 12 h to eliminate vegetative non-sporulated bacteria. Lastly, the suspension was washed again three times by centrifugation, distributed into sterile Eppendorf microtubes and kept at 4 °C until use. Before being treated, the microtubes were heat-shocked at 80 °C for 15 min to eliminate vegetative cells and cooled again at 4 °C. Prior to each treatment, the spore suspension was added (2 mL) to the autoclaved emulsion (60 mL) to reach a cell concentration of 104–105 CFU/mL.
2.3. Thermal treatment
The thermal treatments for A. niger inoculated in the emulsion were performed at 50, 60 and 70 °C. The thermal treatments for C. butyricum inoculated in the emulsion were performed at 70 and 85 °C in a temperature-controlled water bath (1812, Bunsen, Madrid, Spain). 1.5 mL of sample (emulsion with a concentration of 106 –107 CFU/mL of A. niger or 104-105 CFU/mL of C. butyricum) were poured into borosilicate glass tubes, 8 mm in diameter and 70 mm in length (Fiolax, DWK, Wertheim/Main, Germany). The tubes were taken out of the bath after different times, ranging from 5 s to 30 min, depending on the microorganism and the temperature of the treatment. The samples were placed in ice until analysed. The experiments were carried out in triplicate.
2.4. Ultrasonic-assisted supercritical fluid treatments
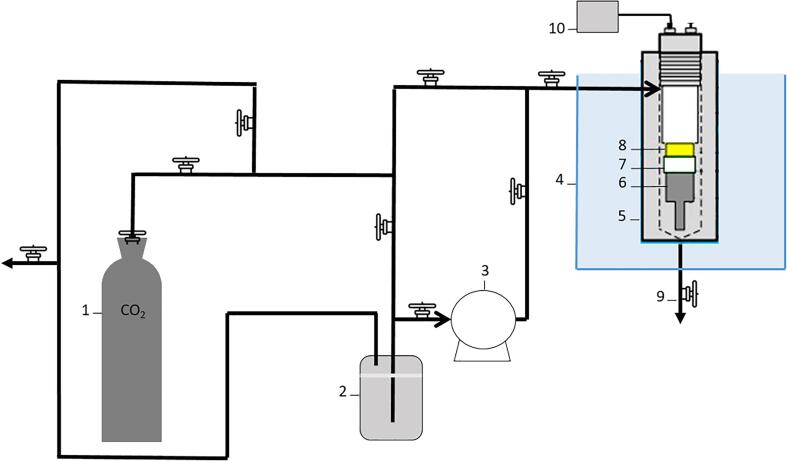
The supercritical carbon dioxide (SC-CO2) treatments for the purposes of inactivating A. niger and C. butyricum were carried out using batch lab-scale equipment already described by Gomez-Gomez et al. [3](Fig. 1). Briefly, the system consisted of a CO2 tank (1, Fig. 1), a chiller reservoir (2, Fig. 1); a diaphragm metering pump (LDB, LEWA, Tokyo, Japan) (3, Fig. 1), a thermostatic water bath (4, Fig. 1) and an inactivation vessel (5, Fig. 1). Additionally, a high-power ultrasound (HPU) transducer [20] was attached to the vessel lid to perform the combined SC-CO2 + HPU treatments. The ultrasound system mainly consisted of a high power (>1W/cm2) piezoelectric transducer (6, Fig. 1), a sonotrode and a power generation unit (10, Fig. 1). The power was 50 ± 5 W (I = 250 ± 10 mA; U = 220 ± 5 V), and the frequency was 30 ± 2 kHz (WT210, Yokogawa Electric Corporation, Tokyo, Japan).
Fig. 1.
Supercritical CO2 treatment system. (1-CO2 tank, 2-Reservoir, 3-Pump, 4-Bath, 5-Treatment vessel, 6-Transducer, 7-Insulation joint, 8-Ceramics, 9-Sample extraction, 10-Power Generation Unit).
Treatments were extended up to 50 min and samples of 2 mL were taken at different time intervals, ranging from 5 s to 20 min. Both treatment and sampling time were chosen depending on the microorganisms and process conditions. The treated samples were immediately cooled in ice before analysis.
For A. niger, SC-CO2 and SC-CO2 + HPU inactivation treatments were carried out combining two different pressures (100 and 350 bar) and temperatures (50 and 60 °C). The lowest pressure (100 bar) was chosen because it is close to the critical pressure (73.8 bar) and the highest (350 bar) as it is a common pressure used in SC-CO2 inactivation studies [21]. 50 °C was selected as a mild temperature that has little thermal effect on the inactivation of the studied microorganism [22] and 60 °C was selected to study the effect a higher temperature has on the inactivation. Moreover, treatments at 70 °C were tested at different pressure levels (100, 350 and 550 bar) to explore more extreme conditions, which could provide larger inactivation levels. In the case of C. butyricum, and due to the greater thermal resistance of bacterial spores than fungal ones [21], more extreme conditions of temperature and pressure were tested. Thus, SC-CO2 and SC-CO2 + HPU inactivation treatments were performed at 60, 70, 80 and 85 °C and 100, 350 and 550 bar. All the experiments were performed in triplicate.
2.5. Microbiological analyses
A. niger and C. butyricum spores were quantified by means of standard plate count techniques. Depending on the expected count, appropriate serial dilutions were prepared with sterile distilled water. For A. niger, 100 μL of the dilution were spread on the surface of PDA (Scharlab, Barcelona, Spain) in triplicate and incubated at 25 °C for 72 h. The initial A. niger population in the sample was also determined following the same procedure. For C. butyricum, 500 μL of the dilution were poured on to empty plates in triplicate and melted RCA (Scharlab, Barcelona, Spain) was added to each plate. Plates were anaerobically incubated at 37 °C for 15 h. The initial C. butyricum spore population in the sample was also determined following the same procedure.
2.6. Modelling
Microbial inactivation kinetics are usually considered as first-order kinetics [19], [23]. However, a survival curve is the cumulative temporal distribution of mortality events and can exhibit a wide variety of shapes. Thus, several models have been proposed to describe this behaviour, the Weibull model being a robust one [24]. Therefore, Weibull distribution was used in this study to describe the microbial inactivation kinetics of A. niger and C. butyricum, computing the log-cycle reduction in the number of viable cells (N), using Eq. 1.
| (1) |
where N0 (CFU/mL) represents the initial number of spores in the sample, N is the number of spores in the sample (CFU/mL) at treatment time t, n is the shape factor and b is the rate parameter (min-n). The kinetic constants (b and n) of the model were calculated by minimizing the sum of squared differences between experimental and predicted inactivation level using Solver Microsoft ExcelTM tool. According to this model, an upward concavity is manifested for n < 1 (tailing) and a downward concavity for n > 1 (shoulder). The traditional ‘first-order kinetics’ is just a special case of the model, with n = 1 [25].
The root mean squared error (RMSE, Eq. 2) and the coefficient of determination (R2, Eq. 3) were computed to evaluate the estimation accuracy and the model’s goodness of fit.
| (2) |
| (3) |
where and * are the experimental and the estimated data, respectively; z is the number of experimental values and and are the standard deviations of the estimation and the sample deviation, respectively.
Several authors [25], [26] have compared the parameters (b and n) of the Weibull model as independent values, but they are mathematically related since the units of b rate parameter are min-n. Thus, when comparing two different inactivation treatments, a higher value of b in one of them does not directly involve a faster inactivation, since a lower n can diminish the microbial inactivation rate in favor of the other treatment. In this regard, some authors have fixed the shape parameter (n) at an average value and estimated only the rate parameter b [27], [28]. However, this estimation is only acceptable when there is no influence of the studied conditions (pressure and temperature of the treatment, type of microorganism, treated media) on the shape of the inactivation kinetics. Therefore, in order to use the model to compare the effect of the different variables (temperature, pressure, use of HPU and microorganism) on microbial inactivation, the time required to achieve complete inactivation (tx) was calculated from Eq.1 and the b and n values of Weibull model obtained for each condition (Table 1, Table 2), where × is the average number of log-cycles of total inactivation for every microorganism (6.8 log-cycles for A. niger and 4.8 log-cycles for C. butyricum)
Table 1.
Parameters (b and n), total time for complete inactivation (t6.8; 6.8 log-cycle reduction) and goodness of fit by using the Weibull model for thermal, SC-CO2 and SC-CO2 + HPU inactivation kinetics of A. niger in oil-in-water emulsions under different pressure and temperature conditions. Values in brackets indicate standard errors.
| Treatment | Temperature (°C) | Pressure (bar) | b (min–n) | n | t6.8 (min) | R2 | RMSE |
|---|---|---|---|---|---|---|---|
| Thermal | 50 | – | 0.01 (0.002) | 0.73 (0.06) | 7588.4 | 0.99 | 0.006 |
| Thermal | 60 | – | 5.60 (0.18) | 0.06 (0.01) | 25.4 | 0.99 | 0.059 |
| Thermal | 70 | – | 3.77 (0.88) | 0.52 (0.26) | 3.1 | 0.80 | 1.079 |
| SC-CO2 | 50 | 100 | 0.08 (0.01 | 1.04 (0.04) | 71.6 | 0.99 | 0.064 |
| SC-CO2 | 50 | 350 | 0.29 (0.10) | 0.82 (0.10) | 46.9 | 0.97 | 0.199 |
| SC-CO2 | 60 | 100 | 5.25 (0.03) | 0.07 (0.002) | 40.3 | 0.99 | 0.006 |
| SC-CO2 | 60 | 350 | 5.24 (0.004) | 0.12 (0.0004) | 8.8 | 0.99 | 0.003 |
| SC-CO2 + HPU | 50 | 100 | 0.82 (0.33) | 0.52 (0.11) | 58.4 | 0.98 | 0.246 |
| SC-CO2 + HPU | 50 | 350 | 0.39 (0.19) | 0.75 (0.13) | 45.2 | 0.98 | 0.301 |
| SC-CO2 + HPU | 60 | 100 | 4.77 (0.39) | 0.11 (0.33) | 25.1 | 0.99 | 0.089 |
| SC-CO2 + HPU | 60 | 350 | 6.17 (0.23) | 0.04 (0.02) | 11.4 | 0.99 | 0.119 |
| SC-CO2 + HPU | 70 | 100 | 5.48 (0.17) | 0.18 (0.03) | 3.3 | 0.98 | 0.313 |
| SC-CO2 + HPU | 70 | 350 | * | * | * | * | * |
| SC-CO2 + HPU | 70 | 550 | * | * | * | * | * |
* Not enough experimental data for model fitting.
Table 2.
Parameters (b and n), total time for complete inactivation (t4.8; 4.8 log-cycles of reduction) and goodness of fit by using Weibull model for thermal, SC-CO2 and SC-CO2 + HPU inactivation kinetics of C. butyricum spores in oil-in-water emulsions under different pressure and temperature conditions. Values in brackets indicate standard errors.
| Treatment | Temperature (°C) | P (bar) | b (min-n) | n | t4.8 (min) | R2 | RMSE |
|---|---|---|---|---|---|---|---|
| Thermal | 70 | – | 1.33E-02 | 1.00E-02 | – | 0.50 | 0.004 |
| Thermal | 85 | – | 0.39 (0.09) | 0.27 (0.14) | 9751.8 | 0.85 | 0.086 |
| SC-CO2 | 70 | 550 | 0.02 (0.01) | 1.29 (0.12) | 70.0 | 0.99 | 0.097 |
| SC-CO2 | 85 | 550 | * | * | * | * | * |
| SC-CO2 + HPU | 60 | 550 | 4.07E-04 (0.001) | 2.06 (0.53) | 94.8 | 0.94 | 0.144 |
| SC-CO2 + HPU | 70 | 550 | 0.50 (0.17) | 0.57 (0.10) | 52.9 | 0.95 | 0.342 |
| SC-CO2 + HPU | 80 | 550 | 1.66 (0.60) | 0.39 (0.14) | 15.2 | 0.95 | 0.385 |
| SC-CO2 + HPU | 85 | 550 | * | * | * | * | * |
| SC-CO2 + HPU | 60 | 350 | 0.14 (0.02) | 0.48 (0.04) | 1578.2 | 0.98 | 0.036 |
| SC-CO2 + HPU | 85 | 100 | 0.98 (0.20) | 1.17 (0.16) | 3.9 | 0.99 | 0.171 |
| SC-CO2 + HPU | 85 | 350 | * | * | * | * | * |
* Not enough experimental data for model fitting.
2.7. Statistical analysis
Statgraphics Centurion XVI was used to perform a general linear model (GLM) in order to evaluate the effect of the treatment conditions (pressure, temperature and use of HPU) and the type of microorganism on the inactivation. Fisher's least significant difference (LSD) procedure was used to discriminate among the means with a 95.0% confidence level (p < 0.05).
3. Results and discussion
3.1. SC-CO2 inactivation of A. Niger spores in oil-in-water emulsions
Fig. 2A shows the inactivation of A. niger spores in the 20% oil-in-water emulsion treated with SC-CO2 at different pressures (100 and 350 bar) and temperatures (50 and 60 °C), compared to the conventional thermal treatments at 50 and 60 °C. The experimental variability found in the inactivation treatments may be ascribed to possible pressure and temperature fluctuations and a variability in microbial growth behaviour. The fitting of the Weibull model to the SC-CO2 kinetics was satisfactory, providing R2 of over 0.97 and RMSE of under 0.199 (Table 1). All n Weibull parameter values were lower than 1, which reveals that the shape of the inactivation kinetics was concave-upward, except for the treatment at 100 bar and 50 °C, with an n value of 1.04, which was close to the linear behaviour.
Fig. 2.
Inactivation kinetics of A. niger in oil-in-water emulsion treated using thermal (T.T.) and SC-CO2 treatments (A) and through SC-CO2 + HPU treatments (B) at different pressures (100 and 350 bar) and temperatures (50 and 60 °C). Experimental data (discrete points) and Weibull model (continuous and dashed lines).
3.1.1. SC-CO2 vs thermal treatments
The thermal inactivation of A. niger after 50 min and at 50 °C was negligible (a reduction of<0.2 log-cycles), while at 60 °C, 6.8 log-cycles were achieved after 20 min. In SC-CO2 treatments at 50 °C, an average of 5.1 log-cycles for 100 and 350 bar were inactivated after 40 min (Fig. 2A). However, in the treatments at 60 °C, only the use of 350 bar allowed for a slightly (p < 0.05) faster inactivation than the thermal treatment (Fig. 2A). Ballestra and Cuq [22] demonstrated a greater lethal effect in the case of A. niger spores in Ringer solution with saccharose treated with CO2 at 50 bar and 50 °C (D-value of 46 min), compared to the thermal treatment (D-values of>300 min). On the contrary, when using 60 °C, these authors found no noticeable differences between the thermal and the pressurized CO2 treatments, which coincides with the experimental results depicted in Fig. 2A. Ballestra and Cuq [22] manifested that at high temperatures, the antimicrobial effect of pressurized CO2 could be masked by the lethal effect of heat.
3.1.2. Effects of pressure and temperature
The temperature had a significant (p < 0.05) effect on inactivation in both thermal and SC-CO2 treatments. The time required to reach complete inactivation (on average, 6.8 log-cycles of reduction; t6.8) was computed using the Weibull model in order to compare the performances of the different treatments (Table 1). For the thermal treatments, values of 7588 min and 25.4 min at 50 and 60 °C, respectively, were computed. For the SC-CO2 treatments, the average t6.8 values were 58.3 min at 50 °C and 24.6 min at 60 °C. Therefore, A. niger inactivation kinetics in oil-in-water emulsions was highly temperature dependent. The increase in temperature is known to decrease the CO2 viscosity and, therefore, to facilitate its diffusion in the media. The temperature also affects the integrity of the cell wall of the fungal spores, facilitating the penetration of CO2 and the hydration of the cell structure [29].
The pressure also had a significant (p < 0.05) effect, although milder than the temperature. On average, a rise in pressure from 100 to 350 bar for a treatment of 20 min led to an increase of between 4.2 and 5.1 log-cycles in the inactivation level of A. niger (Fig. 2A). The pressure shortened the t6.8 computed by the Weibull model (Table 1), lasting on average 56.0 min at 100 bar and 27.9 min at 350 bar. The rise in pressure is known to increase the solubility of CO2 in the suspension; therefore, both the acidification of the external medium and the contact between CO2 and the microbial cells are improved, which facilitates the CO2 penetration into the cells [30], [31].
Noman et al. [29] and Shimoda et al. [16] also found that the inactivation of A. niger spores was more noticeable at high temperatures and pressures in distilled water and a saline solution, respectively. However, different results were obtained by Calvo et al. [32] for A. niger inoculated in milled dried cocoa since the increase in the temperature (from 40 to 80 °C) or the pressure (from 130 to 300 bar) did not improve the inactivation. This distinction was probably due to the sizeable differences in the nature of the treated medium, since Calvo et al. [31] conducted the experiments in a solid medium. The composition and nature of the treated medium may modulate its protective effect on the microorganism. In fact, marked protective effects to inactivation treatments were observed in complex physicochemical samples, compared to simpler media [33]. As an example, Noman et al. [29] observed A. niger spores to be completely inactivated (6.0 log-cycles of reduction) in distilled water at 75 °C, 300 bar and after 90 min; in contrast, the maximum inactivation level in a seawater medium and normal saline was 5.5 log-cycles. No references have been found to the inactivation of A. niger in lipid media. However, several authors found there was a protective effect exerted by the oil content in the treating media against subcritical CO2 or SC-CO2 treatments for other microorganisms, such as vegetative bacteria [3], [8].
3.2. SC-CO2 + HPU inactivation of A. niger spores in oil-in-water emulsions
Fig. 2B shows the SC-CO2 + HPU inactivation kinetics of A. niger spores in the oil-in-water emulsion at 50 and 60 °C and 100 and 350 bar. The Weibull model satisfactorily described the inactivation kinetics since R2 was higher than 0.98 and RMSE was lower than 0.301 (Table 1). In the case of the shape parameter (n), all the values were lower than 1 (Table 1), indicating the kinetics were concave-upward shaped. Since the process times required to achieve emulsion sterilization were longer than expected, higher temperatures and pressure levels (70 °C and up to 550 bar) were assessed for the combined SC-CO2 + HPU treatment (Fig. 3).
Fig. 3.
Inactivation kinetics of A. niger in oil-in-water emulsion at 70 °C treated with SC-CO2 + HPU at different pressures (100, 350 and 550 bar) and by thermal treatment (T.T.). Experimental data (discrete points) and Weibull model (continuous and dashed lines).
3.2.1. Effect of temperature and pressure
As in the SC-CO2 inactivation treatments (Fig. 2A), both temperature and pressure significantly (p < 0.05) affected the inactivation level of SC-CO2 + HPU treatments at 50 and 60 °C (Fig. 2B), the temperature effect being greater than that of the pressure. An increase in temperature from 50 to 60 °C implied the shortening of the time needed for total inactivation from 45 to 17.5 min, whereas an increase in pressure from 100 to 350 bar only led to a reduction from 35 to 27.5 min (Fig. 2B). When ultrasound was applied, the effect of the pressure and temperature on inactivation kinetics was milder compared to that in the SC-CO2 treatments. Gomez-Gomez et al. [3] reported similar results for the inactivation of E. coli and B. diminuta in oil-in-water emulsions. However, several studies using SC-CO2 + HPU to inactivate microorganisms found that an increase in both the pressure and temperature of the process did not increase the inactivation rate, probably because the marked influence of HPU masked the effects [13], [34]. In this sense, although Ortuño et al. [4] found that neither pressure (100–350 bar at 36 °C) nor temperature (31–41 °C at 225 bar) had any effect on the inactivation level of E. coli in apple juice, the effects were significant (p < 0.05) for S. cerevisiae. Therefore, the effect of pressure and temperature on SC-CO2 + HPU inactivation depends not only on the treating media but also on the microorganism considered. The effect of the temperature still remained strong at 70 °C, since the total inactivation time was shortened from<3 min (Fig. 3) to the 10–25 min required at 60 °C (Fig. 2B). As for the pressure, A. niger spores were completely inactivated (6.8 log-cycles) after a longer time (3 min) at 70 °C and 100 bar, compared to higher pressures (350 bar and 550 bar), where similar results were found and the complete inactivation was achieved in only 5 s (Fig. 3). Therefore, 5 s treatments at 350 bar could be implemented in the industry for emulsion pasteurization, which could improve the quality of the treated products, properly preserving heat sensitive compounds.
3.2.2. Effect of HPU
Unlike previous studies into inactivation, where the implementation of HPU in the SC-CO2 treatment drastically shortened the processing times [3], [13], [34], in the present study, HPU did not significantly (p > 0.05) affect the SC-CO2 inactivation kinetics of A. niger (Fig. 2B). For example, at 350 bar, 50 °C and after 45 min, similar reductions were achieved without (Fig. 2A) or with HPU (Fig. 2B) (6.2 and 6.8 log-cycles, respectively). Moreover, no microbial count was detected after 10 min at 350 bar and 60 °C, regardless of the use of HPU (Fig. 2AB). As regards the thermal treatment at 70 °C (Fig. 3), a slower rate of inactivation was shown at the beginning of the treatment (until 30 s of process), compared to the SC-CO2 + HPU treatments. However, in the following 30 s of the thermal treatment, the inactivation rate sped up to reach a similar inactivation level as in the SC-CO2 + HPU treatment at 100 bar and, no plate count was detected after 3 min for either treatment. Therefore, at high temperatures (60 and 70 °C), similar inactivation levels are reached in the thermal treatment and the SC-CO2 + HPU treatment at 100 bar.
No references were found to the application of HPU to the SC-CO2 treatment of inoculated filamentous fungi. However, the results obtained would show that the inactivation of the A. niger fungal spores was limited by the CO2 penetration through the A. niger spore structure. In addition, the expected effect of the ultrasound cavitation on the spore integrity was not found. In fact, the A. niger spore could be more resistant to cavitation than other microorganisms due to the differences in the composition of its multilayer cell wall. A. niger conidium cell is composed of a multi layered polysaccharide-rich wall, covered by a proteinaceous, highly hydrophobic layer of rodlets (hydrophobins), which conceals an underlying, dense, pigmented layer, composed of melanin [35]. Melanin is related to an adaptation of fungi whereby they are able to resist environmental stress since this pigment is known to increase cell wall rigidity, which could improve its resistance to ultrasonic mechanical stress.
3.3. SC-CO2 inactivation of C. Butyricum spores in oil-in-water emulsions
Fig. 4 shows the inactivation kinetics of C. butyricum in a 20% oil-in-water emulsion for the SC-CO2 treatment, at 70 °C (A) and 85 °C (B) and 550 bar, compared to the conventional thermal treatment at 70 and 85 °C. The fitting of the Weibull model to the SC-CO2 inactivation kinetics was highly satisfactory at 70 °C, providing R2 higher than 0.99 and RMSE lower than 0.097 (Table 2), while not enough experimental data was obtained for the fitting at 85 °C.
Fig. 4.
Inactivation kinetics of C. butyricum spores in oil-in-water emulsion treated with SC-CO2 at 550 bar and 70 °C (A) and 85 °C (B) and using thermal treatments (T.T.). Experimental data (discrete points) and Weibull model (continuous line).
The fact that C. butyricum spores displayed greater heat resistance than A. niger spores was remarkable. In the thermal treatments at 70 °C, no A. niger count was detected after 3 min, while no inactivation was found for C. butyricum under the same conditions. As for the SC-CO2 treatments, C. butyricum spores were also significantly (p < 0.05) more resistant than A. niger spores. A. niger was completely inactivated (6.8 log-cycles) after 10 min of SC-CO2 treatment at 60 °C and 350 bar, while only 0.5 log-cycles of C. butyricum spores were reduced for the same treatment time at a higher temperature (70 °C) and pressure (550 bar).
3.3.1. SC-CO2 vs thermal treatments
The SC-CO2 treatment at 550 bar significantly (p < 0.05) enhanced the inactivation, compared to the thermal treatment alone (Fig. 4), since no inactivation was obtained in the thermal treatment at 70 °C while 3.2 log-cycles were reduced in the SC-CO2 treatment at 70 °C after 50 min (Fig. 4A). In addition, only 0.7 log-cycles were achieved after 10 min of the thermal treatment at 85 °C, while no microbial count was detected in the SC-CO2 one (Fig. 4B). The results obtained clearly indicated that CO2 has a major role in C. butyricum spore inactivation.
Few studies have addressed the SC-CO2 inactivation of Bacillus spores, such as B. subtilis [7], [22], [36] or B. pumilus [37]. However, for the first time, this study addresses the SC-CO2 inactivation of Clostridium spp. Only Haas et al. [38] studied the inactivation of C. sporogenes spores using pressurized CO2 at low pressure (55 bar).
3.3.2. Effect of temperature
As for the temperature, the use of 70 or 85 °C in the SC-CO2 treatments significantly (p < 0.05) affected to what extent C. butyricum was reduced. In Fig. 4, the SC-CO2 inactivation kinetics showed that after only 10 min, C. butyricum spores were completely inactivated at 85 °C (B), while at 70 °C (A), the inactivation achieved after 10 min was only of 0.5 log-cycles. Haas et al. [38] also found a clear temperature effect when treating C. sporogenes spores (7.8 log CFU/mL) suspended in thioglycolate broth with CO2 at 55 bar, since substantial levels of inactivation were achieved at 70 °C after 120 min (from 0.8 to 7.8 log-cycles, depending on the pH of the media), while no inactivation was found at 60 °C. The effect of high temperatures has been widely investigated for the SC-CO2 inactivation of vegetative bacterial cells, where SC-CO2 is able to penetrate into the cell membranes faster, accelerating the inactivation mechanisms [39]. However, the complex and resistant structure of bacterial spores could not be compatible with those mechanisms [22], [37] because the CO2 penetration and dissolution into the spore could be restrained, as its structure is dehydrated [5]. There is no clear explanation for the inactivation mechanisms for bacterial spores. One of the hypotheses was that spores firstly have to be activated so as to germinate before being inactivated [39]. As Spilimbergo et al. [36] explained, the effect of the CO2 acidification along with a certain spore-dependent temperature could be sufficient to promote the activation, which leads to a destruction of the spore coat and the subsequent hydration of the spore structure, becoming more sensitive to CO2 treatments [7]. Another widely accepted mechanism is related with changes in the spore structure [21] induced by the effect of the temperature along with SC-CO2, causing damage to the spore envelope until the inner membrane is modified and its permeability increased. Thus, the core spore could be hydrated and the spores could lose their resistance [7].
3.4. SC-CO2 + HPU inactivation of C. Butyricum spores in oil-in-water emulsions
Fig. 5, Fig. 6 show the inactivation kinetics of C. butyricum in a 20% oil-in-water emulsion for the combined SC-CO2 + HPU treatment at different temperatures and pressures. The fitting of the kinetics with the Weibull model was appropriate, providing R2 higher than 0.94 and RMSE lower than 0.385 in every case (Table 2).
Fig. 5.
Inactivation kinetics of C. butyricum spores in oil-in-water emulsion treated with SC-CO2 + HPU at 550 bar and four different temperatures (60, 70, 80 and 85 °C). Experimental data (discrete points) and Weibull model (continuous and dashed lines).
Fig. 6.
Inactivation kinetics of C. butyricum spores in oil-in-water emulsion treated with SC-CO2 + HPU at 350 and 550 bar at 60 °C (A) and at 100, 350 and 550 bar at 85 °C (B). Experimental data (discrete points) and Weibull model (continuous line).
3.4.1. Effect of temperature and pressure
As in the SC-CO2 treatments (Fig. 4), the higher the temperature, the higher the level of SC-CO2 + HPU inactivation (Fig. 5). As an example, at the lowest studied temperature (60 °C), a reduction of 1.4 log-cycles was achieved after 50 min, which should be considered as a weak inactivation level for a highly time-consuming treatment with HPU. On the contrary, at 85 °C, no microbial count was achieved after a treatment of only 3 min. The temperature effect was also computed by the t4.8, which shortened as the temperature rose (Table 2), being 94.8, 52.9 and 15.2 at 60, 70 and 80 °C, respectively. In the kinetics at 60 °C and 550 bar (Fig. 5), an initial lag-phase (of around 15 min) was found, a phase which was not observed at higher temperatures (70 and 80 °C). This could mean that at temperatures higher than 60 °C, the heat along with the decrease in the pH of the media exerted by the SC-CO2 and the effect of HPU are able to damage the cortex of the spore immediately, making it accessible for CO2. The lag-phase in the SC-CO2 + HPU treatments at 550 bar (Fig. 5) was also well computed by the n parameter of the Weibull model (Table 2) since it was only higher than 1 for the treatment at 60 °C (2.06).
As regards pressure, whether 350 or 550 bar was used was found to have no significant (p > 0.05) effects on the SC-CO2 + HPU inactivation at 60 °C (Fig. 6A). In addition to 350 bar and 550 bar, a lower pressure (100 bar) was investigated in the treatments at 85 °C (Fig. 6B). In this case, the pressure had a significant (p < 0.05) effect on the inactivation. After 2 min, 2.0 log-cycles of reduction were achieved at 100 bar, 3.2 log-cycles at 350 bar and 4.1 log-cycles at 550 bar. However, after 3 min, no microbial count was obtained either at 350 or 550 bar. Therefore, high pressures barely improved CO2 solubility in oil-in-water emulsions (Fig. 3). Ballestra and Cuq [22] postulated that if the spore structure is already altered by the action of the SC-CO2 and temperature, an increase in pressure can lead to a increase in the amount of CO2 passing through the membrane and to a decrease in the internal pH.
3.4.2. Effect of HPU
The application of HPU had a significant (p < 0.05) effect on the inactivation at both studied temperatures (70 and 85 °C). In the treatments at 70 °C with HPU (Fig. 5), no microbial count was obtained after 50 min, while only 3.2 log-cycles were reduced when HPU was not applied (Fig. 4A). In addition, at 70 °C, the kinetics changed from a downward concavity shape (n > 1) in the treatment without HPU (Fig. 4A) to upward concavity behavior (n < 1) in the treatment with HPU (Fig. 5). At 85 °C, the time required for no microbial count was shortened from 10 (Fig. 4B) to only 3 min (Fig. 5) when ultrasound was applied.
Numerous studies have already demonstrated the high effect of HPU on SC-CO2 treatments in vegetative microorganisms [15], [34], [40]. As for bacterial spores, only Michelino et al. [15] studied the effect of the combined SC-CO2 + HPU (40 W) treatment. An inactivation of 1.6 log-cycles of mesophilic bacterial spores from fresh coriander (with an initial load, naturally present in the product, of 3.6 log CFU/g) was achieved at 40 or 50 °C and 100 bar only during the pressurization (20 min) and depressurization (40 min), while no spore inactivation was obtained when HPU was not applied.
As concerns the effect of HPU on the SC-CO2 treatments, although a remarkable difference was found between the microorganisms analyzed in the present study, it was only remarkable in the case of the inactivation of C. butyricum spores while no significant effect of HPU was found for the inactivation of A. niger spores. Therefore, it seems that the differences between the cell wall of fungal (A. niger) and bacterial spores (C. butyricum) contribute not only to the roles of temperature, pressure and time but also to the effect that HPU has on the inactivation process [29]. Although no previous studies have compared the resistance of bacterial and fungal spores to SC-CO2 + HPU treatments, the objective of Michelino et al. [15] was to compare bacterial spores with the yeast and molds naturally present in coriander; however, yeast and molds were already completely inactivated in the SC-CO2 treatment without HPU. In addition, other authors compared the resistance of different bacterial spores to thermosonication treatments (70–75 °C, up to 60 min) and found a negligible effect on A. acidoterrestris and C. perfringens spores, while for B. cereus the effect was remarkable in beef slurry [41].
4. Conclusions
The present study demonstrated that the industrial application of the low temperature pasteurization (<60 °C) of fungal and bacterial spores in oil-in-water emulsions using SC-CO2 was not feasible due to the low inactivation rate. A. niger spores were more sensitive to the SC-CO2 treatments than C. butyricum spores and the application of HPU only intensified the inactivation of the C. butyricum spores, thus illustrating for the first time the different resistance of bacterial and fungal spores to the combined SC-CO2 + HPU. The performance of the SC-CO2 + HPU inactivation treatments on C. butyricum spores was affected by the temperature (from 60 to 85 °C), while pressure levels above 350 bar did not improve the inactivation. Additional studies should evaluate the effect of the combined SC-CO2 + HPU treatment on other spores and address its effect on the quality properties of the oil-in-water emulsions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the financial support from Fresenius Kabi Deutschland GmbH, Germany.
References
- 1.Omar A.K.M., Tengku Norsalwani T.L., Asmah M.S., Badrulhisham Z.Y., Easa A.M., Omar F.M., Hossain M.S., Zuknik M.H., Nik Norulaini N.A. Implementation of the supercritical carbon dioxide technology in oil palm fresh fruits bunch sterilization: A review. J. CO2 Util. 2018;25:205–215. doi: 10.1016/j.jcou.2018.03.021. [DOI] [Google Scholar]
- 2.Ortuño C., Martínez-Pastor M.T., Mulet A., Benedito J. Supercritical carbon dioxide inactivation of Escherichia coli and Saccharomyces cerevisiae in different growth stages. J. Supercrit. Fluids. 2012;63:8–15. doi: 10.1016/J.SUPFLU.2011.12.022. [DOI] [Google Scholar]
- 3.Gomez-Gomez A., Brito-de la Fuente E., Gallegos C., Garcia-Perez J.V., Benedito J. Non-thermal pasteurization of lipid emulsions by combined supercritical carbon dioxide and high-power ultrasound treatment. Ultrason. Sonochem. 2020;67:105–138. doi: 10.1016/J.ULTSONCH.2020.105138. [DOI] [PubMed] [Google Scholar]
- 4.Ortuño C., Quiles A., Benedito J. Inactivation kinetics and cell morphology of E. coli and S. cerevisiae treated with ultrasound-assisted supercritical CO2. Food Res. Int. 2014;62:955–964. doi: 10.1016/J.FOODRES.2014.05.012. [DOI] [Google Scholar]
- 5.Ishihara Y., Saito H., Takano J. Differences in the surface membranes and water content between the vegetative cells and spores of Bacillus subtilis. Cell Biochem. Funct. 1999;17:9–13. doi: 10.1002/(SICI)1099-0844(199903)17:1<9::AID-CBF803>3.0.CO;2-9. [DOI] [Google Scholar]
- 6.Feofilova E.P., Ivashechkin A.A., Alekhin A.I., Sergeeva Y.E. Fungal spores: Dormancy, germination, chemical composition, and role in biotechnology (review) Appl. Biochem. Microbiol. 2012;48(1):1–11. doi: 10.1134/S0003683812010048. [DOI] [PubMed] [Google Scholar]
- 7.Rao L., Xu Z., Wang Y., Zhao F., Hu X., Liao X. Inactivation of Bacillus subtilis spores by high pressure CO2 with high temperature. Int. J. Food Microbiol. 2015;205:73–80. doi: 10.1016/J.IJFOODMICRO.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Gonzalez L., Geeraerd A.H., Elst K., Van Ginneken L., Van Impe J.F., Devlieghere F. Influence of type of microorganism, food ingredients and food properties on high-pressure carbon dioxide inactivation of microorganisms. Int. J. Food Microbiol. 2009;129(3):253–263. doi: 10.1016/j.ijfoodmicro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Kamihira M., Taniguchi M., Kobayashi T. Sterilization of microorganisms with supercritical carbon dioxide. Agric. Biol. Chem. 1987;51(2):407–412. doi: 10.1080/00021369.1987.10868053. [DOI] [Google Scholar]
- 10.Bi X., Wang X., Chen Y., Chen L., Xing Y., Che Z. Effects of combination treatments of lysozyme and high power ultrasound on the Salmonella typhimurium inactivation and quality of liquid whole egg. Ultrason. Sonochem. 2020;60:104763. doi: 10.1016/j.ultsonch.2019.104763. [DOI] [PubMed] [Google Scholar]
- 11.Cárcel J.A., García-Pérez J.V., Benedito J., Mulet A. Food process innovation through new technologies: Use of ultrasound. J. Food Eng. 2012;110(2):200–207. doi: 10.1016/j.jfoodeng.2011.05.038. [DOI] [Google Scholar]
- 12.Cappelletti M., Ferrentino G., Spilimbergo S. Supercritical carbon dioxide combined with high power ultrasound: An effective method for the pasteurization of coconut water. J. Supercrit. Fluids. 2014;92:257–263. doi: 10.1016/J.SUPFLU.2014.06.010. [DOI] [Google Scholar]
- 13.Ortuño C., Martínez-Pastor M.T., Mulet A., Benedito J. An ultrasound-enhanced system for microbial inactivation using supercritical carbon dioxide. Innov. Food Sci. Emerg. Technol. 2012;15:31–37. doi: 10.1016/J.IFSET.2012.02.006. [DOI] [Google Scholar]
- 14.Ferrentino G., Spilimbergo S. A combined high pressure carbon dioxide and high power ultrasound treatment for the microbial stabilization of cooked ham. J. Food Eng. 2016;174:47–55. doi: 10.1016/J.JFOODENG.2015.11.013. [DOI] [Google Scholar]
- 15.Michelino F., Zambon A., Vizzotto M.T., Cozzi S., Spilimbergo S. High power ultrasound combined with supercritical carbon dioxide for the drying and microbial inactivation of coriander. J. CO2 Util. 2018;24:516–521. doi: 10.1016/J.JCOU.2018.02.010. [DOI] [Google Scholar]
- 16.Shimoda M., Kago H., Kojima N., Miyake M., Osajima I.H. Accelerated death kinetics of Aspergillus niger spores under high-pressure carbonation. Appl. Environ. Microbiol. 2002;68:4162–4167. doi: 10.1128/AEM.68.8.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kar S., Mujumdar A.S., Sutar P.P. Aspergillus niger inactivation in microwave rotary drum drying of whole garlic bulbs and effect on quality of dried garlic powder. Dry. Technol. 2019;37(12):1528–1540. doi: 10.1080/07373937.2018.1517777. [DOI] [Google Scholar]
- 18.Ghoddusi H.B., Sherburn R. Preliminary study on the isolation of Clostridium butyricum strains from natural sources in the UK and screening the isolates for presence of the type E botulinal toxin gene. Int. J. Food Microbiol. 2010;142(1-2):202–206. doi: 10.1016/j.ijfoodmicro.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Mafart P., Couvert O., Gaillard S., Leguerinel I. On calculating sterility in thermal preservation methods: application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002;72(1-2):107–113. doi: 10.1016/S0168-1605(01)00624-9. [DOI] [PubMed] [Google Scholar]
- 20.J. Benedito, M.T. Martínez-Pastor, A. Mulet, C. Ortuño, R. Peña, Procedure of inactivation microorganisms by combination of supercritical fluids and ultrasound, Spain. Pat. (2011).
- 21.Soares G.C., Learmonth D.A., Vallejo M.C., Davila S.P., González P., Sousa R.A., Oliveira A.L. Supercritical CO2 technology: The next standard sterilization technique? Mater. Sci. Eng. C. 2019;99:520–540. doi: 10.1016/J.MSEC.2019.01.121. [DOI] [PubMed] [Google Scholar]
- 22.Ballestra P., Cuq J.-L. Influence of pressurized carbon dioxide on the thermal inactivation of bacterial and fungal spores. LWT - Food Sci. Technol. 1998;31(1):84–88. doi: 10.1006/fstl.1997.0299. [DOI] [Google Scholar]
- 23.Corradini M.G., Peleg M. The kinetics of microbial inactivation by carbon dioxide under high pressure. Dense Phase Carbon Dioxide Food Pharm. Appl. 2012:135–155. [Google Scholar]
- 24.Peleg M. Advanced Quantitative Microbiology For Foods And Biosystems. 2006. Models for predicting growth and inactivation. [Google Scholar]
- 25.Jiao S., Zhang H., Hu S., Zhao Y. Radio frequency inactivation kinetics of Bacillus cereus spores in red pepper powder with different initial water activity. Food Control. 2019;105:174–179. doi: 10.1016/J.FOODCONT.2019.05.038. [DOI] [Google Scholar]
- 26.Deen B., Diez-Gonzalez F. Assessment of Pediococcus acidilactici ATCC 8042 as potential Salmonella surrogate for thermal treatments of toasted oats cereal and peanut butter. Food Microbiol. 2019;83:187–192. doi: 10.1016/J.FM.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Baril E., Coroller L., Postollec F., Leguerinel I., Boulais C., Carlin F., Mafart P. The wet-heat resistance of Bacillus weihenstephanensis KBAB4 spores produced in a two-step sporulation process depends on sporulation temperature but not on previous cell history. Int. J. Food Microbiol. 2011;146(1):57–62. doi: 10.1016/j.ijfoodmicro.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 28.Couvert O., Gaillard S., Savy N., Mafart P., Leguérinel I. Survival curves of heated bacterial spores: effect of environmental factors on Weibull parameters. Int. J. Food Microbiol. 2005;101(1):73–81. doi: 10.1016/j.ijfoodmicro.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 29.Noman E., Norulaini Nik Ab Rahman N., Al-Gheethi A., Nagao H., Talip B.A., Ab. Kadir O. Kadir, Selection of inactivation medium for fungal spores in clinical wastes by supercritical carbon dioxide. Environ. Sci. Pollut. Res. 2018;25(22):21682–21692. doi: 10.1007/s11356-018-2335-1. [DOI] [PubMed] [Google Scholar]
- 30.Liao H., Hu X., Liao X., Chen F., Wu J. Inactivation of Escherichia coli inoculated into cloudy apple juice exposed to dense phase carbon dioxide. Int. J. Food Microbiol. 2007;118(2):126–131. doi: 10.1016/j.ijfoodmicro.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Ceni G., Fernandes Silva M., Valério C., Cansian R.L., Oliveira J.V., Dalla Rosa C., Mazutti M.A. Continuous inactivation of alkaline phosphatase and Escherichia coli in milk using compressed carbon dioxide as inactivating agent. J. CO2 Util. 2016;13:24–28. doi: 10.1016/j.jcou.2015.11.003. [DOI] [Google Scholar]
- 32.Calvo L., Muguerza B., Cienfuegos-Jovellanos E. Microbial inactivation and butter extraction in a cocoa derivative using high pressure CO2. J. Supercrit. Fluids. 2007;42(1):80–87. doi: 10.1016/j.supflu.2007.01.009. [DOI] [Google Scholar]
- 33.Garcia-Gonzalez L., Geeraerd A.H., Spilimbergo S., Elst K., Van Ginneken L., Debevere J., Van Impe J.F., Devlieghere F. High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future. Int. J. Food Microbiol. 2007;117(1):1–28. doi: 10.1016/j.ijfoodmicro.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Ortuño C., Martínez-Pastor M.T., Mulet A., Benedito J. Application of high power ultrasound in the supercritical carbon dioxide inactivation of Saccharomyces cerevisiae. Food Res. Int. 2013;51(2):474–481. doi: 10.1016/j.foodres.2013.01.041. [DOI] [Google Scholar]
- 35.Tischler B.Y., Hohl T.M. Menacing mold: recent advances in Aspergillus pathogenesis and host defense. J. Mol. Biol. 2019;431(21):4229–4246. doi: 10.1016/j.jmb.2019.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spilimbergo S., Bertucco A., Lauro F.M., Bertoloni G. Inactivation of Bacillus subtilis spores by supercritical CO2 treatment. Innov. Food Sci. Emerg. Technol. 2003;4(2):161–165. doi: 10.1016/S1466-8564(02)00089-9. [DOI] [Google Scholar]
- 37.Zhang J., Burrows S., Gleason C., Matthews M.A., Drews M.J., LaBerge M., An Y.H. Sterilizing Bacillus pumilus spores using supercritical carbon dioxide. J. Microbiol. Methods. 2006;66(3):479–485. doi: 10.1016/j.mimet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Haas G.J., Prescott H.E., Dudley E., Dik R., Hintlian C., Keane L. Inactivation of microorganisms by carbon dioxide under pressure. J. Food Saf. 1989;9(4):253–265. doi: 10.1111/jfs.1989.9.issue-410.1111/j.1745-4565.1989.tb00525.x. [DOI] [Google Scholar]
- 39.Spilimbergo S., Bertucco A. Non-thermal bacteria inactivation with dense CO2. Biotechnol. Bioeng. 2003;84:627–638. doi: 10.1002/bit.10783. [DOI] [PubMed] [Google Scholar]
- 40.Paniagua-Martínez I., Mulet A., García-Alvarado M.A., Benedito J. Orange juice processing using a continuous flow ultrasound-assisted supercritical CO2 system: Microbiota inactivation and product quality. Innov. Food Sci. Emerg. Technol. 2018;47:362–370. doi: 10.1016/J.IFSET.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 41.Evelyn, Silva F.V.M. Silva, Differences in the resistance of microbial spores to thermosonication, high pressure thermal processing and thermal treatment alone. J. Food Eng. 2018;222:292–297. doi: 10.1016/j.jfoodeng.2017.11.037. [DOI] [Google Scholar]