Abstract
Purpose
Extracellular matrix remodeling is essential for extravillous trophoblast (EVT) cell migration and invasion during placental development and regulated by matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteases (TIMPs). Sphingosine kinases (SPHK1 and SPHK2) synthesize sphingosine‐1‐phosphate (S1P), which works either intracellularly or extracellularly via its receptors S1PR1‐5 in an autocrine or paracrine manner. The role of SPHKs/S1P in regulating the expression of MMPs and TIMPs in EVT is mostly unknown and forms the primary objective of the study.
Methods
HTR‐8/SVneo cells were used as a model of EVT. To inhibit the expression of SPHKs, cells were treated with specific inhibitors, SK1‐I and SKI‐II, or gene‐specific siRNAs. The expressions of MMPs and TIMPs were estimated by qPCR.
Results
We demonstrated that SPHK1, MMP1‐3, and TIMP1‐3 were highly expressed in HTR‐8/SVneo cells. We found that treatment of cells with SK1‐I, SKI‐II, and knockdown of SPHK1 or SPHK2 increased the expression of MMP1, MMP3, and TIMP3. The addition of extracellular S1P inhibits the upregulation of MMPs and TIMPs in treated cells.
Conclusions
SPHKs negatively regulate the expression of MMP1, MMP3, and TIMP3. The level of intracellular S1P acts as a negative feedback switch for MMP1, MMP3, and TIMP3 expression in EVT cells.
Keywords: extravillous trophoblast, matrix metalloproteinases, Sphingosine 1‐phosphate, Sphingosine kinase, tissue inhibitors of metalloproteases
The levels of intracellular S1P act as a controlling switch for MMP1, MMP3, and TIMP3 expression in EVT cells suggesting a new role of intracellular S1P in ECM remodeling.
1. INTRODUCTION
Extravillous trophoblast (EVT) cell migration is crucial during placental development, spiral artery remodeling, and successful pregnancy outcome. Aberrant cell migration causes various pregnancy‐related diseases, such as preeclampsia 1 and placental abnormalities. 2 Extracellular matrix (ECM) remodeling is one of the critical processes for proper cell migration. It is tightly regulated by ECM‐degrading enzymes, such as matrix metalloproteases (MMPs) and tissue inhibitors of metalloproteases (TIMPs) family proteins. 3
MMPs are zinc‐dependent proteases that are secretory or membrane proteins and get activated during matrix remodeling in various physiological and pathophysiological conditions. 3 There are 24 MMP genes reported in humans that encode for 23 MMP proteins. 3 , 4 To avoid excessive and deleterious degradation of tissues, TIMPs inhibit activated‐MMPs and maintain tissue homeostasis. TIMP family consists of four members, TIMP1, TIMP2, TIMP3, and TIMP4. Except for TIMP3, all TIMPs are secretory proteins. 5 Co‐expression of MMPs and TIMPs has been demonstrated in trophoblast cells. 6 MMP2 and MMP7 have a significant role in endometrial tissue remodeling during decidualization. 7 , 8 Various studies showed that nearly all MMPs were expressed in decidua and cytotrophoblasts. 6 , 9 , 10 , 11
Sphingosine‐1‐phosphate (S1P), a sphingolipid, plays a vital role in various cellular processes, including cell proliferation, cell migration, secretion, and apoptosis. 12 , 13 , 14 S1P is synthesized intracellularly by the phosphorylation of sphingosine through two conserved Sphingosine kinases (SPHK1 and SPHK2). 15 S1P works either intracellularly as a secondary messenger or through its receptors S1PR1‐5 in an autocrine or paracrine manner. 16 Intracellular S1P can regulate the expression of various genes by binding to many intracellular targets, such as histone deacetylase (HDAC), 17 atypical protein kinase C (aPKC), 18 PPARγ, 19 and TNF receptor‐associated factor 2 (TRAF2). 20
S1PR1/S1P signaling is required for the expression of MMP2 in bone marrow‐derived mesenchymal stromal cells. 21 The expression of MMP2 and MMP9 was upregulated by S1P treatment in pancreatic cancer cells. 22 S1P promotes EVT cell invasion through MMP2 using S1P/S1PR1 signaling. 23 Knockdown of SPHK1 inhibited the secretion of MMP2 and MMP9 in fibroblast‐like synoviocytes. 24 Recently, Liu et al. showed that SPHK1 promotes MMP2 and MMP9 expression in the colon cancer cell line RKO. 25 A recent study showed that S1P inhibits migration of chondrosarcoma through upregulation of TIMP3 expression. 26 TIMP3 was upregulated in HDAC9‐knockdown HTR‐8/SVneo cells. 27 SPHK1 is activated via TGF‐β and mediates TIMP1 upregulation. 28
In the present study, we investigated whether SPHK activity and intracellular S1P play any role in the expression of MMPs and TIMPs in EVT cells. Indeed, we found that the expression of MMP1, MMP3, and TIMP3 genes was regulated through the intracellular level of S1P synthesized by SPHK1 and SPHK2.
2. MATERIAL AND METHODS
2.1. Materials
Primers were synthesized from Eurofins, India, and IDT, India. SPHK1 inhibitor SK1‐I was purchased from Enzo Life Sciences, USA. S1P and SKI‐II were procured from Cayman chemicals. TAMRA‐S1P was from Echelon Biosciences. All cell culture reagents were purchased from HyClone, GE Life Sciences. iScript cDNA synthesis kit was from Bio‐Rad and DyNAmo ColorFlash SYBR Green qPCR kit was from Thermo Fisher. Control, SPHK1, and SPHK2 specific siRNAs were procured from Sigma‐Aldrich.
2.2. Cell culture
HTR‐8/SVneo cell line, a first‐trimester human EVT cell line, was a kind gift from Dr Charles H Graham. 29 These cells were grown as described previously. 12 Cells were grown in factor‐reduced media (basal media + 5% charcoal‐stripped FBS [dFBS]) 24 hours prior to the experiment. Cells were kept in serum‐starved media (basal media + 0.5% dFBS) and treated with inhibitors for 30 minutes before activating with S1P for the specified time.
2.3. Real‐time PCR
Cells were lysed with TRIzol reagent (Invitrogen), and then total RNA was isolated as per the manufacturer's protocol. cDNA was synthesized with an iScript cDNA synthesis kit as per the manufacturer's protocol. Primers were designed using the NCBI Primer‐BLAST tool, and real‐time PCR was performed on Roche LightCycler 96 machine, as described previously. 30 The list of primers used in this study are shown in Table S1. The cycling program followed for the reaction included 7 minutes of initial denaturation at 95°C and then 40 cycles with 10 seconds at 95°C and 20 seconds at 60°C. The specificity of the amplicons was analyzed by thermal dissociation curve and agarose gel electrophoresis. Data were normalized against the house‐keeping gene, β‐actin.
2.4. Gene silencing
Fifty thousand cells/well were grown in 24‐well plates. Transfection of SPHK1 and SPHK2 specific siRNAs was performed in duplicate wells using Lipofectamine RNAiMAX transfection reagent as per manufacturer's protocol. siRNA (10 pmol) was added to each well, and the plates were incubated for 48‐72 hours with 5% CO2 in a humidified incubator at 37°C. Scrambled siRNA was used as a negative control.
2.5. Immunofluorescent staining and fluorescence microscopy
HTR‐8/SVneo cells were grown in a 24‐well plate and incubated with TAMRA‐S1P (1 μM) for 1 hour. For nuclear staining, Hoechst 33258 was added to the cell culture media for 20 minutes before capturing the images. After incubation, cells were washed with PBS, and images were taken with Zeiss Axio Observer fluorescence microscope with ZEN software.
2.6. Gelatin zymography
Expression of MMP1 and MMP3 in the HTR‐8/SVneo cells was analyzed using gelatin zymography as described previously with modifications. 31 Cells were grown in a 12‐well plate and were treated with S1P, SK1‐I, and SKI‐II as described earlier. Control and treated cells were lysed in zymogen sample buffer (62.5 mM Tris pH 6.8, 10% v/v glycerol, 2% SDS, 0.01% w/v bromophenol blue). The cell lysates were subjected to 10% SDS‐PAGE (without β‐mercaptoethanol) containing 1 mg/mL gelatin. The gel was briefly washed with distilled water and kept in a renaturation buffer (2.5% Triton X‐100) for 1 hour at room temperature. The gel was then incubated in development buffer (50 mM Tris‐HCl pH 7.5, 200 mM NaCl, 5 mM CaCl2 and 0.02% Tween‐20) overnight at 37°C. The gel was stained with 0.1% (w/v) coomassie brilliant blue R‐250 and destained with deionized water.
2.7. Statistical analysis
Every experiment was performed three or more times independently with different passages of cells. Statistical analysis was performed using GraphPad Prism 7 software (GraphPad, La Jolla, CA, USA) using one‐way ANOVA or two‐tailed unpaired t test. The results are calculated as mean ± SD and data are shown as mean + SD. P‐values of <.05 were considered statistically significant.
3. RESULTS
3.1. Expression profiling of SPHK, MMP, and TIMP genes in HTR‐8/SVneo cells
To examine SPHK1 and SPHK2 genes' expression in HTR‐8/SVneo cells, we measured the abundance of mRNA by real‐time PCR. We found that SPHK1 is highly expressed (~45‐fold) as compared to SPHK2 in these cells (Figure 1A). These data suggest a functional role of SPHK1 in EVT cells.
FIGURE 1.
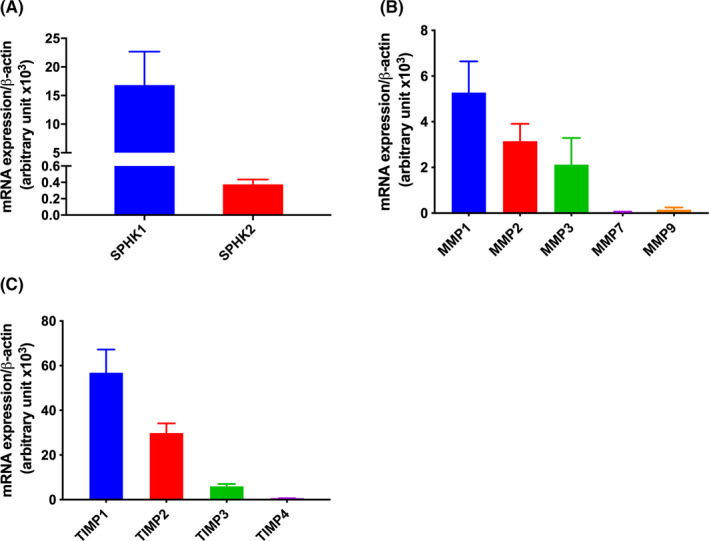
Expression analysis of SPHKs, MMPs, and TIMPs in HTR‐8/SVneo cells. The expressions of different genes were measured by real‐time PCR. Bar diagrams show the expression of A) SPHK1 and SPHK2; B) matrix metalloproteinases MMP1, MMP2, MMP3, MMP7, and MMP9; C) tissue inhibitors of matrix metalloproteinases TIMP1, TIMP2, and TIMP3. All the genes except MMP7 and MMP9 were expressed in HTR‐8/SVneo cells. The data are means + SD of three independent experiments with different passages of HTR‐8/SVneo cells
Matrix remodeling proteins are crucial for cell migration and placental development. We first analyzed the basal expression of these genes in an EVT cell line model, HTR‐8/SVneo cells. We could find the expression of MMP1, MMP2, and MMP3 genes in HTR‐8/SVneo cells, but MMP7 and MMP9 were not in the detectable range (Figure 1B). Further, we checked the expression of TIMPs and found that all the TIMP genes (TIMP1‐3) except TIMP4 were expressed in EVT. Expression of TIMP3 was ~10‐fold lower than that of TIMP1 (Figure 1C). The differential expression of MMPs and TIMPs suggested a cell‐specific role of these genes in EVT.
3.2. Extracellular S1P gets transported in HTR‐8/SVneo cells
Previous studies showed that a high concentration of extracellular S1P significantly increases the intracellular level by 7‐fold. 20 To confirm that exogenous S1P can enter the cells, we incubated the cells with fluorescently tagged S1P (TAMRA‐S1P, 1 µM). In agreement with previous studies, 20 , 32 we could observe that TAMRA‐S1P was transported into the cells (Figure 2). The data show that S1P is transported into the cells and it might have a role independent of receptor activation.
FIGURE 2.
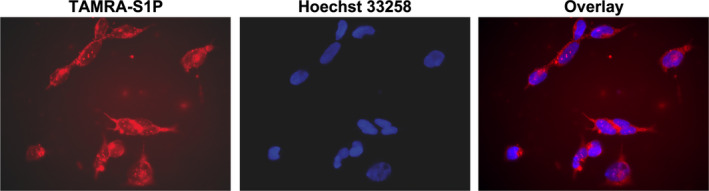
Extracellular S1P transport in the cytoplasm of HTR‐8/SVneo cells. A, Cells were treated with TAMRA‐S1P (1 µM, red, left panel) for 1 h, washed with PBS, and then nuclei were stained with Hoechst 33258 (blue, middle panel). The overlay is shown in the right panel. TAMRA‐S1P was able to enter the cells within 30 minutes
3.3. SPHKs‐dependent S1P regulates the expression of MMP1 and MMP3 in HTR‐8/SVneo cells
To examine whether SPHKs regulate the gene expression of MMPs, we used the specific SPHK1 inhibitor SK1‐I, 33 , 34 and SKI‐II (inhibitor of both isoforms). 35 , 36 We performed concentration and time course for SPHKs inhibitors and S1P and found that SK1‐I and SKI‐II showed maximum effect at 10 µM concentration after 24 hours of treatment (Figure [Link], [Link], [Link]). Cells were, therefore, treated with S1P (10 µM), SK1‐I (10µM), and SKI‐II (10µM) for 24 hours in subsequent experiments. We found that the expression of MMP1 (~5‐ fold; P < .001; Figure 3A left panel) and MMP3 (~20‐fold; P < .01; Figure 3A right panel) was significantly upregulated after the treatment of cells with SK1‐I for 24 hours. Similarly, the expression of MMP1 (~6.6‐fold; P < .01; Figure 3B left panel) and MMP3 (~70‐fold; P < .001; Figure 3B right panel) was significantly upregulated after the treatment of cells with SKI‐II inhibitor. The effect of SK1‐I and SKI‐II inhibitors on MMP2 gene expression was not found to be significant (Figure 3A,B, middle panels). The expression of MMPs was confirmed by gelatin zymography. The activity of MMPs could not be observed in control cells and cells treated with S1P for 24 hours and significantly enhanced in cells treated with SK1‐I and SKI‐II (Figure 3E). The enhanced activity was reversed upon treatment with S1P (Figure 3E). These data suggest that SPHK1 or both the isoforms play a crucial role in regulating MMP1 and MMP3 expression.
FIGURE 3.
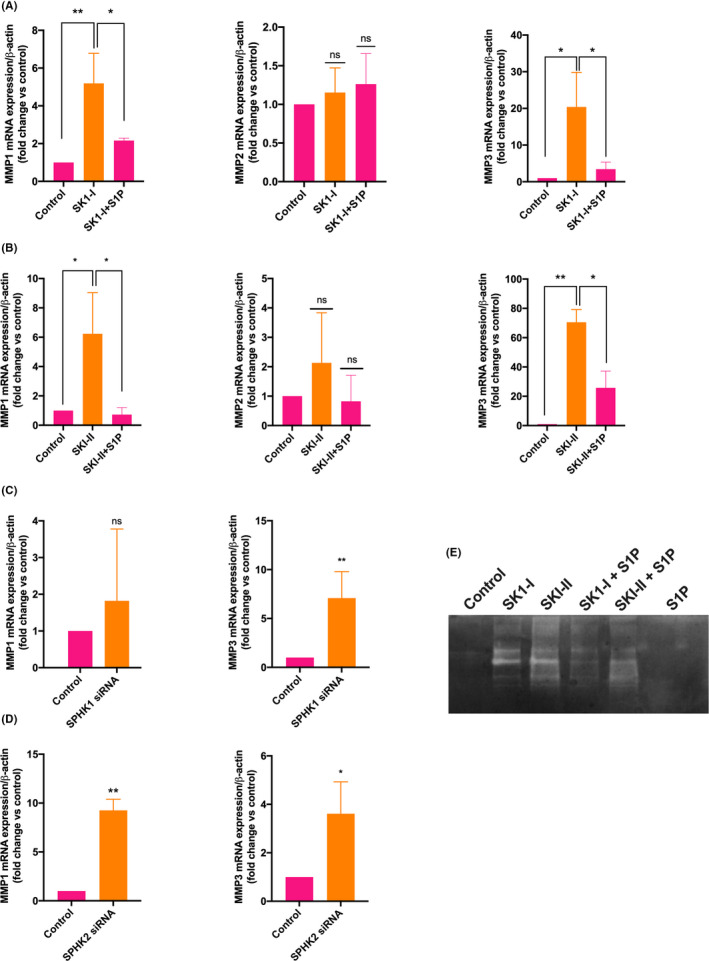
SPHKs regulate the expression of MMP1 and MMP3 genes. HTR‐8/SVneo cells were treated with solvent (control), specific SPHK1 inhibitor SK1‐I (10 µM), SPHK 1 and 2 inhibitor, SKI‐II (10 µM), and then the expressions of different genes were measured by real‐time PCR. A & B, Bar diagrams show the effect of A) SK1‐I and B) SKI‐II on the expression of MMP1, MMP2, and MMP3 genes. The expression of MMP1 (left panel) and MMP3 (right panel) but not MMP2 (middle panel) was significantly upregulated after the treatment of SK1‐I and SKI‐II. The upregulation of these genes was reduced by treating the cells with S1P (10 µM). C) SPHK1 and D) SPHK2 genes were knocked down in HTR‐8/SVneo cells using SPHK1 and SPHK2 specific siRNA treatment. The bar diagram shows the effect of gene knockdown on the expression of MMP1 and MMP3 genes. E) Gelatin zymography gel shows the activity of MMPs in control and cells treated with S1P, SK1‐I, and SKI‐II. The activity of MMPs are visible as clear areas (bands) on the gel, indicating where the gelatin is digested. The data are means + SD of three independent experiments with different passages of HTR‐8/SVneo cells. *P < .05; **P < .01; ***P < .001
We asked whether the formation of intracellular S1P by SPHKs has a role in regulating MMPs gene expression. To examine the effect of S1P on the expression of MMPs, cells treated with SK1‐I and SKI‐II were co‐incubated with 10 µM S1P, as extracellular S1P can be internalized by the cells. We found that the upregulation of MMP1, MMP3 was significantly reduced in SK1‐I or SKI‐II‐treated cells (Figure 3A,B). These data suggest that SPHKs‐dependent intracellular S1P regulates the gene expression of MMP1 and MMP3.
To identify the specific role of SPHK1 and SPHK2 in regulating the expression of MMPs, we knocked down SPHK1 and SPHK2 genes in HTR‐8/SVneo cells using specific SPHK1 and SPHK2 siRNAs. A set of three different siRNAs were used for each SPHK isoform. Only one siRNA against the SPHK1 gene could inhibit the expression by ~65% (Figure S1A). All three siRNAs against SPHK2 could knock down the SPHK2 gene by ~92% (Figure S1B). In contrast to the effect of SKI‐1, MMP3 (~7‐fold; P < .01) but not MMP1 gene was significantly upregulated after the knockdown of SPHK1 (Figure 3C). This observation might be due to the partial knockdown of SPHK1 by siRNA. Additionally, MMP1 (~9‐fold; P < .01) and MMP3 (~3.6‐fold; P < .05) were significantly upregulated after the knockdown of the SPHK2 gene (Figure 3D). These data indicate that both SPHK1 and SPHK2 regulate the expression of MMP1 and MMP3.
3.4. SPHKs regulate the expression of the TIMP3 in HTR‐8/SVneo cells
Co‐expression of MMPs and TIMPs has been demonstrated in trophoblast cells. 6 We asked whether SPHK/S1P axis also regulates the expression of TIMP s . When the cells were treated with SK1‐I for 24 hours, the expression of the TIMP3 (~6.8‐fold; P < .01; Figure 4A right panel) was highly upregulated while TIMP1 and TIMP2 were not affected by SPHK1 inhibition (Figure 4A left and middle panels). Additionally, treatment of cells with SKI‐II could significantly induce the expression of TIMP3 gene expression (~9.4‐fold; P < .0001; Figure 4B right panel) was significantly upregulated within 24 hours, while other two genes were not affected (Figure 4B left and middle panel). To further validate our data, we knocked down the SPHK1 and SPHK2 in HTR‐8/SVneo cells. The expression of the TIMP3 was enhanced by ~2‐fold (P < .001) and by ~2.6‐fold (P < .0001) by knockdown of SPHK1 and SPHK2, respectively (Figure 4C). These data indicate that SPHK1 and SPHK2 regulate the expression of TIMP3.
FIGURE 4.
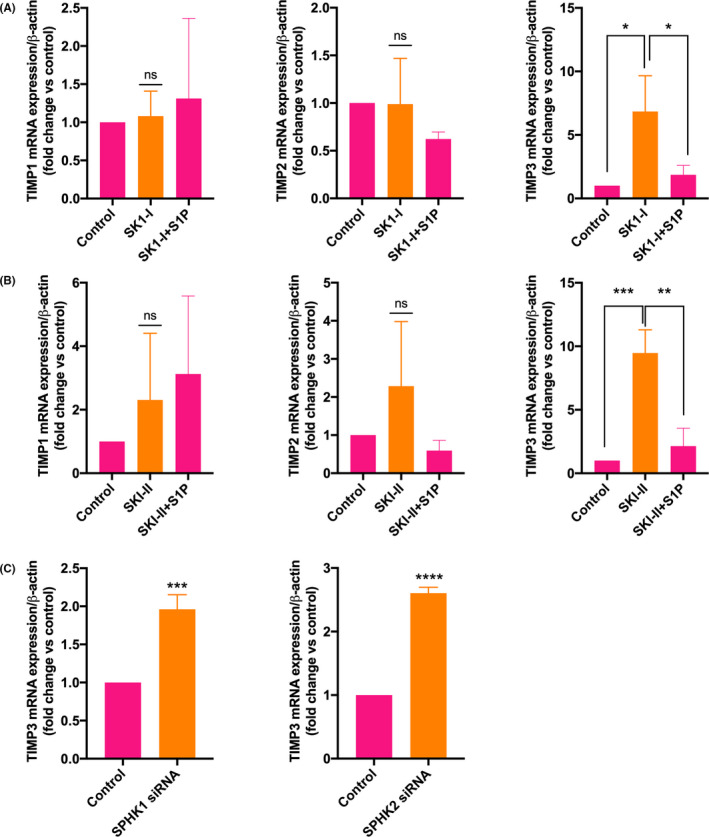
SPHKs regulate the expression of the TIMP3 gene. HTR‐8/SVneo cells were treated with specific SPHK1 inhibitor SK1‐I (10 µM), SPHK 1 and 2 inhibitor SKI‐II (10 µM), and the expressions of TIMPs were measured by real‐time PCR. A) and B) Bar diagrams show the effect of A) SK1‐I and B) SKI‐II on the expression of TIMP1, TIMP2, and TIMP3. The expression of TIMP3 (right panel) but not that of TIMP1 and TIMP2 (left and middle panels, respectively) was significantly upregulated with SK1‐I and SKI‐II treatment that was reduced after S1P (10 µM) treatment. C) Bar diagrams show the effect of SPHK1 and SPHK2 knockdown on the expression of TIMP3. The data are means + SD of three independent experiments with different passages of HTR‐8/SVneo cells. *P < .05; **P < .01; ***P < .001; ****P< .0001
To determine the role of intracellular S1P on the expression of TIMPs, cells treated with SK1‐I and SKI‐II were incubated with 10 µM S1P. We found that the upregulation of TIMP3 was significantly reduced in SK1‐I or SKI‐II‐treated cells (Figure 4A,B). These data suggest that SPHKs ‐dependent intracellular S1P regulates the gene expression of TIMP3.
3.5. S1P alone doesn't regulate the basal expression of MMPs and TIMPs in HTR‐8/SVneo cells
To examine whether extracellular S1P regulates the gene expression of MMPs and TIMPs, we treated the cells with S1P (10 µM). We found that S1P did not significantly affect the expression of MMPs and TIMPs after treating the cells with S1P for 24 hours (Figure 5A,B). These data suggest that extracellular S1P did not control the basal expression of these genes. If the concentration of intracellular S1P is lower than the threshold, the expression of these genes is upregulated.
FIGURE 5.
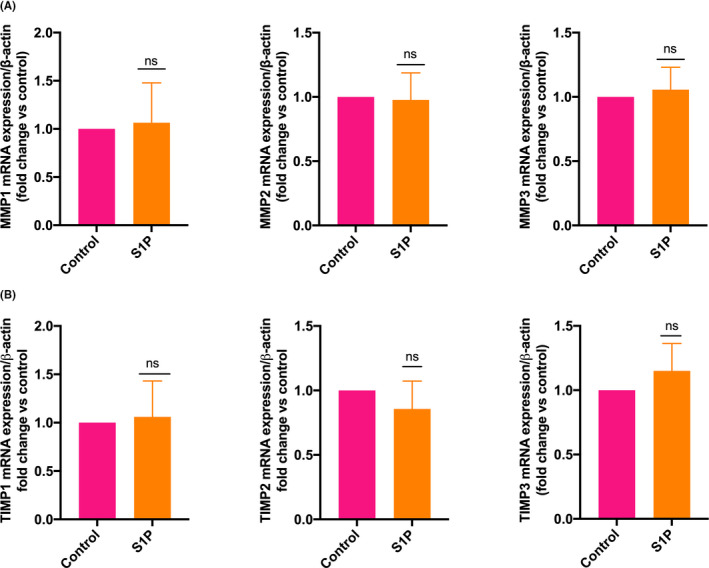
Extracellular S1P does not control the expression of MMPs and TIMPs expression. HTR‐8/SVneo cells were treated with solvent (control), S1P (10 µM), and the expressions of MMPs and TIMPs were measured by real‐time PCR. A) Bar diagrams show that extracellular S1P did not regulate the expression of MMP1, MMP2, and MMP3. B) Bar diagrams show that extracellular S1P did not regulate the expression of TIMP1, TIMP2, and TIMP3. The data are means + SD of three independent experiments with different passages of HTR‐8/SVneo cells. ns, not significant
4. DISCUSSION
Trophoblast cells regulate the matrix remodeling via the secretion of various MMPs and TIMPs spatially and temporally. In this study, we evaluated the role of SPHKs and S1P in regulating MMPs/TIMPs expression in EVT cells. We could show that MMP1, MMP2, and MMP3 genes were highly expressed in HTR‐8/SVneo cells. Expression of MMP7 and MMP9 was lesser as compared to the other genes in EVT cells. Our data are consistent with a previous report in which MMP2 expression was observed in EVT cells and MMP9 mainly in villous cytotrophoblast. 37 Another study showed that MMP2 is highly expressed in early trophoblast (6‐8 weeks) while MMP9 is highly expressed in late first‐trimester trophoblasts (9‐12 weeks). 38 MMP1, MMP3, and MMP7 were differentially expressed in trophoblasts throughout the pregnancy. 10 It suggests that the expression of a specific MMP depends on the trophoblasts' type and pregnancy stage. TIMP1‐3 but not TIMP4 was expressed in EVT cells.
In contrast, a study showed that all four TIMPs are expressed in cytotrophoblast cells. 9 It was shown that TIMP2 was highly expressed in trophoblasts. 39 Contrary to this, TIMP1 expression was increased from week 6 to 9 in cytotrophoblast while TIMP2 was undetectable. 11 These studies suggest that differential regulation of TIMPs might regulate the migration and invasion of EVTs.
We showed at gene expression level for the first time that both the isoforms, SPHK1 (~45‐fold of SPHK2) and SPHK2, were expressed in EVT cells. SPHK1 was downregulated in term placentae and term chorionic villous explants from patients with preeclampsia, suggesting a role of SPHK1 in trophoblast cell functions and preeclampsia. 40 S1P induces the expression of MMP2 in endothelial cells, 41 MMP7 in hepatocellular carcinoma cells, 42 and MMP9 in breast cancer cells. 43 In contrast, we could not find any change in the expression of MMPs and TIMPs after the treatment of HTR‐8/SVneo cells with S1P. Brocklyn et al. showed that intracellular S1P regulates apoptosis independent of S1P receptor 1. 32 These data suggest that extracellular S1P does not control the expression of MMPs and TIMPs in EVT, and intracellular S1P might regulate the expression of these genes.
Various studies showed that SPHK1 promotes MMP2 and MMP9 expression in fibroblast‐like synoviocytes and colon carcinoma RKO cells. 24 , 25 Interestingly, we found that SPHK1 and SPHK2 negatively regulate the gene expression of MMP1, MMP3, and TIMP3 in HTR‐8/SVneo cells. The expression of MMP2, TIMP1, and TIMP2 was not affected significantly after the inhibition of SPHK1 and SPHK2. Together with our finding, we suggest that SPHKs differentially regulate the expression of MMPs and TIMPs family proteins in a cell type‐specific manner. The primary role of SPHKs is to phosphorylate sphingosine and produce intracellular S1P. 15 These data suggest that SPHKs regulate the expression of these genes either independent or dependent on intracellular S1P. A study showed that activation of SPHK1 mediates the upregulation of TIMP1 in human fibroblast cells, and the intracellular level of S1P was increased in SPHK1 overexpressed cells. 28 Previously, it was shown that the addition of exogenous S1P enhanced its intracellular level. 20 , 32 In agreement with the previous data, we could show that S1P could enter the cells by adding fluorescently labeled S1P to the cell culture medium. Intracellular S1P binds with PPARγ and PG1β complex and regulates the expression of various genes in endothelial cells. 19 Intracellular S1P synthesized by SPHK2 acts as HDAC inhibitor and enhances the expression of c‐fos and cyclin‐dependent kinase inhibitor p21. 17 Another study showed that SPHK1 and S1P bind with TRAF2 and regulate NF‐κB activation. 20 Surprisingly, in this study, we showed that the addition of exogenous S1P inhibits the upregulation of MMP1, MMP3, and TIMP3 , indicating a new role of intracellular S1P. These data suggest that SPHKs‐dependent intracellular S1P levels might act as a negative feedback switch and regulate the expression of MMP1, MMP3, and TIMP3, specifically in EVTs.
S1P and SPHK1 play an essential role in various cellular processes, including cell migration and invasion in different cell types. 44 S1P inhibits cell migration in C2C12 myoblasts via the S1PR2 receptor. 45 S1P promotes EVT cell invasion through MMP2 using S1P/S1PR1 signaling. 23 In this study, we could not observe the effect of S1P on EVT cell migration (Figure S2). A study showed that S1P attenuated the migration and thus outgrowth of EVT from the first‐trimester placental explant. 46 These contrasting results might be due to the cell type‐specific signaling. In preeclampsia, HDAC9 was downregulated, and knockdown of HDAC9 upregulated the expression of TIMP3 in HTR‐8/SVneo cells. 27 Poor EVT cell migration and invasion were observed in preeclampsia. 47 Overall, we propose that SPHKs‐dependent regulation of TIMP3 might play an essential role in preeclampsia.
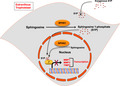
In conclusion, we found that the level of intracellular S1P acts as a controlling switch for MMP1, MMP3, and TIMP3 expression in EVT cells, suggesting a new role of intracellular S1P in ECM remodeling (Figure 6). Together with previous studies, we propose that downregulation of SPHKs in pregnancy disorders, such as preeclampsia 40 decreases the intracellular S1P level leading to the activation of TIMP3 27 and MMP1, and MMP3.
FIGURE 6.
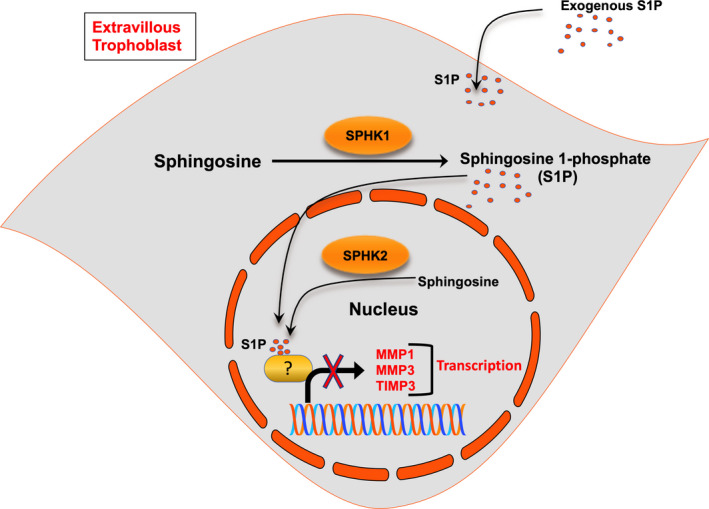
Proposed model of the role of intracellular S1P. The cartoon shows the role of intracellular S1P in MMP1, MMP3, and TIMP3 expression and regulation
DISCLOSURES
Conflict of interest: The authors declare that they have no conflict of interest. Human/Animal rights: This article does not contain any studies with human and animal subjects performed by any authors.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1
ACKNOWLEDGEMENTS
The work was supported by grants from the DBT (BT/PR22450/MED/97/351/2016), SERB (PDF/2016/000292 to IS, PDF/2016/003711 to VK), CSIR (CSIR‐SRF 09/1131(0002)/2015EMR‐I to KRC), UGC (JRF‐20/12/2015 (ii) EU‐V/2121530832 to PS, and JRF‐21/06/2015 (i) EU‐V/2061530804 to VK), Government of India.
Chahar KR, Kumar V, Sharma PK, et al. Sphingosine kinases negatively regulate the expression of matrix metalloproteases (MMP1 and MMP3) and their inhibitor TIMP3 genes via sphingosine 1‐phosphate in extravillous trophoblasts. Reprod Med Biol.2021;20:267–276. 10.1002/rmb2.12379
REFERENCES
- 1. Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre‐eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silva JF, Serakides R. Intrauterine trophoblast migration: a comparative view of humans and rodents. Cell Adh Migr. 2016;10(1–2):88‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol. 2013;13(9):649‐665. [DOI] [PubMed] [Google Scholar]
- 5. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803(1):55‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006;27(8):783‐793. [DOI] [PubMed] [Google Scholar]
- 7. Cockle JV, Gopichandran N, Walker JJ, Levene MI, Orsi NM. Matrix metalloproteinases and their tissue inhibitors in preterm perinatal complications. Reprod Sci. 2007;14(7):629‐645. [DOI] [PubMed] [Google Scholar]
- 8. Chen J, Khalil RA. Chapter Four ‐ Matrix Metalloproteinases in Normal Pregnancy and Preeclampsia. In: Khalil RA, ed. Progress in Molecular Biology and Translational Science. vol 148. Academic Press; 2017:87‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anacker J, Segerer SE, Hagemann C, et al. Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases. Mol Human Reprod. 2011;17(10):637‐652. [DOI] [PubMed] [Google Scholar]
- 10. Vettraino I, Roby J, Tolley T, Parks W. Collagenase‐I, stromelysin‐I, and matrilysin are expressed within the placenta during multiple stages of human pregnancy. Placenta. 1996;17(8):557‐563. [DOI] [PubMed] [Google Scholar]
- 11. Xu P, Wang YL, Zhu SJ, Luo SY, Piao YS, Zhuang LZ. Expression of matrix metalloproteinase‐2, ‐9, and ‐14, tissue inhibitors of metalloproteinase‐1, and matrix proteins in human placenta during the first trimester. Biol Reprod. 2000;62(4):988‐994. [DOI] [PubMed] [Google Scholar]
- 12. Goyal P, Brunnert D, Ehrhardt J, Bredow M, Piccenini S, Zygmunt M. Cytokine IL‐6 secretion by trophoblasts regulated via sphingosine‐1‐phosphate receptor 2 involving Rho/Rho‐kinase and Rac1 signaling pathways. Mol Human Reprod. 2013;19(8):528‐538. [DOI] [PubMed] [Google Scholar]
- 13. Brünnert D, Piccenini S, Ehrhardt J, et al. Sphingosine 1‐phosphate regulates IL‐8 expression and secretion via S1PR1 and S1PR2 receptors‐mediated signaling in extravillous trophoblast derived HTR‐8/SVneo cells. Placenta. 2015;36(10):1115‐1121. [DOI] [PubMed] [Google Scholar]
- 14. Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine‐1‐phosphate signaling and its role in disease. Trends Cell Biol. 2012;22(1):50‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pitson SM. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem Sci. 2011;36(2):97‐107. [DOI] [PubMed] [Google Scholar]
- 16. Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 2018;18(1):33‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hait NC, Allegood J, Maceyka M, et al. Regulation of histone acetylation in the nucleus by sphingosine‐1‐phosphate. Science. 2009;325(5945):1254‐1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kajimoto T, Caliman AD, Tobias IS, et al. Activation of atypical protein kinase C by sphingosine 1‐phosphate revealed by an aPKC‐specific activity reporter. Sci Signal. 2019;12(562):eaat6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parham KA, Zebol JR, Tooley KL, et al. Sphingosine 1‐phosphate is a ligand for peroxisome proliferator‐activated receptor‐gamma that regulates neoangiogenesis. FASEB J. 2015;29(9):3638‐3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alvarez SE, Harikumar KB, Hait NC, et al. Sphingosine‐1‐phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465(7301):1084‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sassoli C, Pierucci F, Tani A, et al. Sphingosine 1‐phosphate receptor 1 is required for MMP‐2 function in bone marrow mesenchymal stromal cells: implications for cytoskeleton assembly and proliferation. Stem Cells Int. 2018;2018:5034679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo YX, Ma YJ, Han L, Wang YJ, Han JA, Zhu Y. Role of sphingosine 1‐phosphate in human pancreatic cancer cells proliferation and migration. Int J Clin Exp Med. 2015;8(11):20349‐20354. [PMC free article] [PubMed] [Google Scholar]
- 23. Yang W, Li Q, Pan Z. Sphingosine‐1‐phosphate promotes extravillous trophoblast cell invasion by activating MEK/ERK/MMP‐2 signaling pathways via S1P/S1PR1 axis activation. PLoS ONE. 2014;9(9):e106725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuan H, Yang P, Zhou D, et al. Knockdown of sphingosine kinase 1 inhibits the migration and invasion of human rheumatoid arthritis fibroblast‐like synoviocytes by down‐regulating the PI3K/AKT activation and MMP‐2/9 production in vitro. Mol Biol Rep. 2014;41(8):5157‐5165. [DOI] [PubMed] [Google Scholar]
- 25. Liu SQ, Xu CY, Wu WH, et al. Sphingosine kinase 1 promotes the metastasis of colorectal cancer by inducing the epithelialmesenchymal transition mediated by the FAK/AKT/MMPs axis. Int J Oncol. 2019;54(1):41‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsai CH, Yang DY, Lin CY, Chen TM, Tang CH, Huang YL. Sphingosine‐1‐phosphate suppresses chondrosarcoma metastasis by upregulation of tissue inhibitor of metalloproteinase 3 through suppressing miR‐101 expression. Mol Oncol. 2017;11(10):1380‐1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xie D, Zhu J, Liu Q, et al. Dysregulation of HDAC9 represses trophoblast cell migration and invasion through TIMP3 activation in preeclampsia. Am J Hypertens. 2019;32(5):515‐523. [DOI] [PubMed] [Google Scholar]
- 28. Yamanaka M, Shegogue D, Pei H, et al. Sphingosine kinase 1 (SPHK1) is induced by transforming growth factor‐β and mediates TIMP‐1 up‐regulation. J Biol Chem. 2004;279(52):53994‐54001. [DOI] [PubMed] [Google Scholar]
- 29. Graham CH, Hawley TS, Hawley RG, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206(2):204‐211. [DOI] [PubMed] [Google Scholar]
- 30. Brünnert D, Shekhawat I, Chahar KR, et al. Thrombin stimulates gene expression and secretion of IL‐11 via protease‐activated receptor‐1 and regulates extravillous trophoblast cell migration. J Reprod Immunol. 2019;132:35‐41. [DOI] [PubMed] [Google Scholar]
- 31. Schropfer A, Kammerer U, Kapp M, Dietl J, Feix S, Anacker J. Expression pattern of matrix metalloproteinases in human gynecological cancer cell lines. BMC Cancer. 2010;10:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Brocklyn JR, Lee MJ, Menzeleev R, et al. Dual actions of sphingosine‐1‐phosphate: Extracellular through the G(i)‐ coupled receptor Edg‐1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998;142(1):229‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harikumar KB, Yester JW, Surace MJ, et al. K63‐linked polyubiquitination of transcription factor IRF1 is essential for IL‐1‐induced production of chemokines CXCL10 and CCL5. Nat Immunol. 2014;15(3):231‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maczis MA, Maceyka M, Waters MR, et al. Sphingosine kinase 1 activation by estrogen receptor alpha36 contributes to tamoxifen resistance in breast cancer. J Lipid Res. 2018;59(12):2297‐2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gao P, Peterson YK, Smith RA, Smith CD. Characterization of isoenzyme‐selective inhibitors of human sphingosine kinases. PLoS ONE. 2012;7(9):e44543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang Y, Torta F, Arai K, et al. Sphingosine kinase inhibition ameliorates chronic hypoperfusion‐induced white matter lesions. Neurochem Int. 2016;94:90‐97. [DOI] [PubMed] [Google Scholar]
- 37. Isaka K, Usuda S, Ito H, et al. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta. 2003;24(1):53‐64. [DOI] [PubMed] [Google Scholar]
- 38. Staun‐Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP‐2 and ‐9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004;2(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dimo B, Ioannidis I, Karameris A, et al. Comparative study of the immunohistochemical expression of tissue inhibitors of metalloproteinases 1 and 2 between clearly invasive carcinomas and "in situ" trophoblast invasion. Med Oncol. 2012;29(3):2270‐2275. [DOI] [PubMed] [Google Scholar]
- 40. Dobierzewska A, Palominos M, Sanchez M, et al. Impairment of angiogenic sphingosine kinase‐1/sphingosine‐1‐phosphate receptors pathway in preeclampsia. PLoS ONE. 2016;11(6):e0157221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun HY, Wei SP, Xu RC, Xu PX, Zhang WC. Sphingosine‐1‐phosphate induces human endothelial VEGF and MMP‐2 production via transcription factor ZNF580: Novel insights into angiogenesis. Biochem Biophys Res Commun. 2010;395(3):361‐366. [DOI] [PubMed] [Google Scholar]
- 42. Zeng Y, Yao X, Chen L, et al. Sphingosine‐1‐phosphate induced epithelial‐mesenchymal transition of hepatocellular carcinoma via an MMP‐7/ syndecan‐1/TGF‐beta autocrine loop. Oncotarget. 2016;7(39):63324‐63337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim ES, Kim JS, Kim SG, Hwang S, Lee CH, Moon A. Sphingosine 1‐phosphate regulates matrix metalloproteinase‐9 expression and breast cell invasion through S1P3‐Galphaq coupling. J Cell Sci. 2011;124:2220‐2230. [DOI] [PubMed] [Google Scholar]
- 44. Zheng X, Li W, Ren L, et al. The sphingosine kinase‐1/sphingosine‐1‐phosphate axis in cancer: potential target for anticancer therapy. Pharmacol Ther. 2019;195:85‐99. [DOI] [PubMed] [Google Scholar]
- 45. Becciolini L, Meacci E, Donati C, Cencetti F, Rapizzi E, Bruni P. Sphingosine 1‐phosphate inhibits cell migration in C2C12 myoblasts. Biochim Biophys Acta. 2006;1761(1):43‐51. [DOI] [PubMed] [Google Scholar]
- 46. Al‐Saghir K, Adlam D, Westwood M, Johnstone E. Sphingosine‐1‐phosphate (S1P) inhibits extravillous trophoblast migration via S1P receptor 2. Placenta. 2013;34(9):A97‐A98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lala PK, Nandi P. Mechanisms of trophoblast migration, endometrial angiogenesis in preeclampsia: the role of decorin. Cell Adh Migr. 2016;10(1–2):111‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1


