Abstract
Background
This study aimed to investigate the epidemiology, microbiology, and risk factors associated with mortality and multi-drug resistance bacterial bloodstream infections (BSIs) among adult cancer patients in Shiraz, Iran. We also report a four-year trend of antimicrobial resistance patterns of BSIs.
Methods
We conducted a retrospective study at a referral oncology hospital from July 2015 to August 2019, which included all adults with confirmed BSI.
Results
2393 blood cultures tested during the four-year study period; 414 positive cultures were included. The mean age of our patients was 47.57 ± 17.46 years old. Central Line-Associated BSI (CLABSI) was more common in solid tumors than patients with hematological malignancies. Gram-negative (GN) bacteria were more detected (63.3%, 262) than gram-positive bacteria (36.7%, 152). Escherichia coli was the most common gram-negative organism (123/262, 47%), followed by Pseudomonas spp. (82/262, 31%) and Klebsiella pneumoniae (38/262, 14.5%). Coagulase-negative staphylococci (CoNS) was the most frequently isolated pathogen among gram-positive bacteria (83/152, 54.6%). Acinetobacter spp., Pseudomonas spp., E. coli, and K. pneumoniae were the most common Extended-Spectrum Beta-Lactamase (ESBL) producers (100, 96.2, 66.7%, and 60.7, respectively). Acinetobacter spp., Pseudomonas spp., Enterobacter spp., E. coli, and K. pneumoniae were the most common carbapenem-resistant (CR) isolates (77.8, 70.7, 33.3, 24.4, and 13.2%, respectively). Out of 257 Enterobacterales and non-fermenter gram-negative BSIs, 39.3% (101/257) were carbapenem-resistant. Although the incidence of multi-drug resistance (MDR) gram-negative BSI increased annually during 2015–2018, the mortality rate of gram-negative BSI remains unchanged at about 20% (p-value = 0.55); however, the mortality rate was significantly greater (35.4%) in those with resistant gram-positive BSI (p-value = 0.001). The overall mortality rate was 21.5%. Early (7-day mortality) and late mortality rate (30-day mortality) were 10 and 3.4%, respectively.
Conclusions
The emergence of MDR gram-negative BSI is a significant healthcare problem in oncology centers. The high proportion of the most frequently isolated pathogens were CR and ESBL-producing Enterobacterales and Pseudomonas spp. We have few effective choices against MDRGN BSI, especially in high-risk cancer patients, which necessitate newer treatment options.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-021-06243-z.
Keywords: Bloodstream infection, Carbapenem-resistant isolates, Extended-Spectrum Beta-lactamase producing pathogens, Multidrug-resistant gram-negative infection, Mortality, Cancer
Background
Bacterial bloodstream infection (BSI) is one of the most common complications of chemotherapy-induced neutropenia in patients with hematologic malignancies and solid organ tumors [1–3], which is associated with high mortality and morbidity [4–8]. Bacterial BSIs account for the etiologic cause of approximately 20 to 30% of all febrile neutropenic episodes in adult patients with malignancy [9, 10]. While proper diagnosis and treatment are essential to decrease BSI-associated complications, inappropriate empiric antimicrobial therapy increases mortality [11]. BSI’s reported crude mortality rates to reach as high as 34 to 50%, especially in MDR gram-negative BSI [9, 12]. The antimicrobial stewardship program (ASP) helps to reduce the overuse of antibiotics and control the increased antimicrobial resistance. Surveillance of antimicrobial resistance is one of ASP’s critical aspects and guides clinicians for appropriate empiric antimicrobial therapy [13, 14].
This study aimed to determine the current epidemiology of bacterial BSI and its changes during the different study years in a large cohort of patients with solid organ and hematological malignancy. We also assessed BSI attributed mortality risk factors and MDR gram-negative BSI predictors.
Methods
Setting and data collection
This study performed at Amir oncology hospital, an educational 100-bed inpatient center. The adult units consist of four inpatient wards and an autologous hematopoietic stem cell transplantation ward. Since 2015 our institution has carried out a blood culture surveillance program using an automated blood culture system (BD BACTEC™). Hospital Information System (HIS) and microbiology department records used for data collection. Patients followed up 30 days after BSI by infectious disease specialist consultant (AA) and chief infection control unit staff (MS).
Study population and design
In this retrospective single-center study, we analyzed all consecutive episodes of BSI occurring in adult patients with hematological malignancies and solid organ tumors from July 2015 to August 2019. Each patient ≥18 years of age with positive blood culture was considered to BSI when he/she had clinical signs and symptoms of bacteremia. Patients with a fungal infection, contaminant result, or a mix of more than two organisms, excluded from this study. Finally, 414 patients recruited, and data analyzed for age, gender, underlying diseases, presence of central venous catheters, leucocyte count, neutropenia, etiologic microorganisms, susceptibility testing, and outcome.
Definitions
Patient with a recognized bacterial pathogen, which not included on the commensal list, identified from one or more blood specimens obtained by culture and at least one of the following signs or symptoms: fever (> 38 °C), chills, or hypotension and not be related to an infection at another site considered as true BSI [15].
Febrile neutropenia defined as temperature > 38.5 °C or two consecutive temperature > 38 °C for 2 h and an absolute neutrophil count< 0.5 X 109 cell/L or expected to fall below < 0.5 X 109 cell/L. Imipenem, meropenem, cefepime, piperacillin-tazobactam, and colistin are available on our hospital formulary. We use piperacillin-tazobactam and carbapenem as the first-line agents for patients with febrile neutropenia.
Methicillin-resistant coagulase-negative staphylococci (MRCoNS) defined as cefoxitin-resistant strains. Enterobacteriaceae family, P. aeruginosa, and A. baumannii isolates resistant to ceftazidime or cefotaxime are considered extended-spectrum beta-lactamase (ESBL) producers [16]. Phenotypic confirmation of ESBL production carried out by using the double-disk synergy test [17]. Carbapenem-resistant Enterobacterales (CREs), carbapenem-resistant Pseudomonas spp., and carbapenem-resistant Acinetobacter spp. isolates defined as Enterobacterales that test intermediate or resistant to one or more carbapenems using the CLSI current breakpoints. However, not all isolates tested against all carbapenems [16]. MDR defined as the strain non-susceptible to at least one agent in ≥3 classes of antibiotics, including carbapenems, combinations of beta-lactams plus beta-lactamase inhibitors, cephalosporins, aminoglycosides, and fluoroquinolones [18].
Microbiological methods
Blood cultures obtained by physicians based on clinical suspicion of bacteremia. BACTEC™ FX Automated Blood Culture Systems used for detection of BSI. Time-to-Detection (TTD) defined as the time between the placement of each blood culture bottle in the incubation cabinet and the detection of growth. Based on the result of the gram-stain, the subculture routinely was performed, and differentiation tests such as catalase, oxidase, coagulase, bacitracin, optochin, and CAMP test were applied. In our center, the susceptibility testing is done based on the disc diffusion method (Kirby-Bauer method) according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [17] using commercial antibiotic discs (MAST Group Ltd.; UK).
Statistical analysis
In this study, we used a univariate logistic regression model to examine the critical factors that may be influencing the patient’s survival status and possible predictors of MDR gram-negative BSI. All variables in the univariate analysis (P ≤ 0.25) and variables with clinical significance entered into a multivariable model. The independent variables of sex, age, malignancy, ESR, CRP, leukopenia, neutropenia, febrile neutropenia, year, and Enterobacterales separately entered a logistic regression model. We reported odds ratio values and also the confidence interval of the odds ratio for each variable. A p-value < 0.05 was considered statistically significant. The analysis was done by SPSS version 25 (IBM Corp., Armonk, NY, USA).
Results
Demographics and epidemiology
In this study, four-hundred, fourteen positive blood cultures were analyzed (Fig. 1). Two hundred twenty-three patients were male (53.9%). The mean age was 47.57 ± 17.46 years old. Hematologic malignancies (212, 51.4%), solid tumors (167, 40.3%), and non-malignant disorders (20, 4.8%), including aplastic anemia, were the most common underlying diseases, respectively.
Fig. 1.
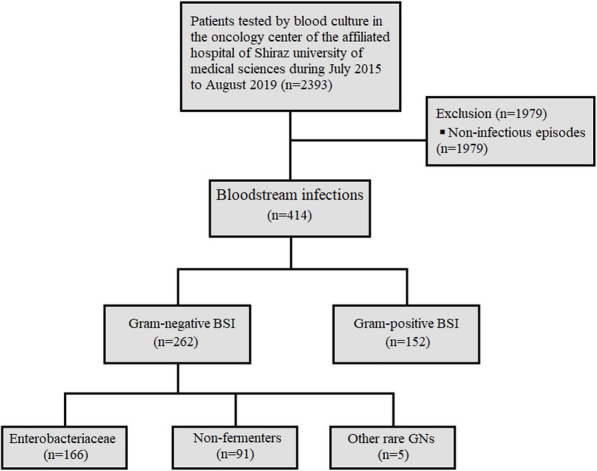
Flow chart of study population screened and enrolled associated with bloodstream infections caused by gram-negative and gram-positive bacteria in patients treated at the Amir oncology hospital between July 2015 to August 2019
Clinical and laboratory features
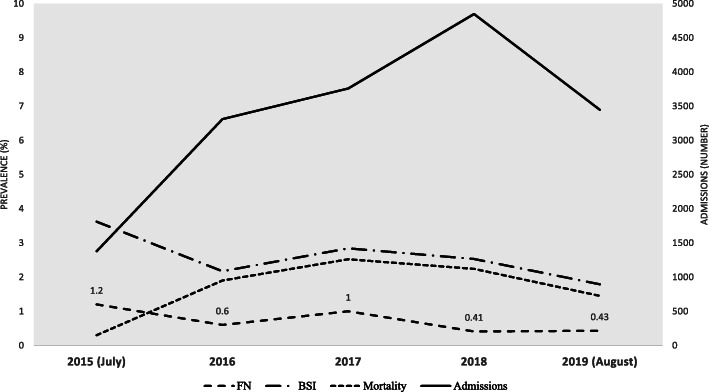
The mean of white blood cell (WBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were (6.94 ± 11.28) X 109 /L, (71.68 ± 37.26) mm/hour, (82.59 ± 41.44) mg/dl, respectively. ESR and CRP were not statistically different between two malignancy types (P = 0.6646; 95% CI: − 7.07 to 11.07 and P = 0.0663, 95% CI: − 19.64 to 0.64, respectively). Neutropenia was more prominent in those with hematologic malignancies (117, 69.6%) compare with patients with solid organ tumors (38, 22.6%); P < 0.001. Febrile neutropenia also was significantly higher in those with hematologic malignancies (83, 74.8%; P < 0.001). Overall, 63.5% of positive culture was primary BSI, and 36.5% was CLABSI. CLABSI was more common in those with solid tumors; the difference was not significant p = 0.165. The most reported TTD was less than 12 h (188/414, 50%), while 30.3% detected during 12–24 h, 11.7% between 24 and 36 h, and only 8% diagnosed in more than 36 h by BACTEC™ Systems. The rate of admission, BSI, febrile neutropenia, and mortality during the study years are shown in Fig. 2. The summary characteristics of the patients with solid tumors and hematological malignancies could be found in Table S1 in the supplement file.
Fig. 2.
Incidence of febrile neutropenia, bloodstream infections, mortality rate/10000 cases, and hospitalized adults with cancer during 2015–2019. FN: febrile neutropenia episodes; BSI: bloodstream infection
Microbiology
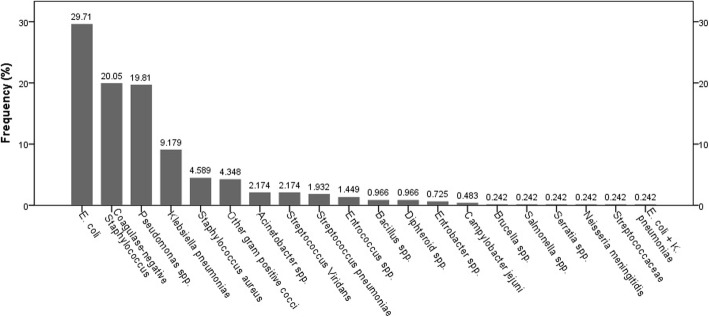
262 (63.3%) and 152 (36.7%) gram-negative and gram-positive pathogens organisms isolated from blood cultures. Escherichia coli was the most common gram-negative organism (123/262, 47%), followed by Pseudomonas spp. (82/262, 31%) and K. pneumoniae (38/262, 14.5%). Coagulase-negative staphylococci (CoNS) was the most frequently isolated pathogen among gram-positive bacteria (83/152, 54.6%). The incidence of gram-positive and gram-negative bacteria isolated from blood culture is shown in Fig. 3. Acinetobacter spp., Pseudomonas spp., E. coli, and K. pneumoniae were the most common ESBL producers (100, 96.2, 66.7%, and 60.7, respectively).
Fig. 3.
The percentage frequency distribution of different gram-negative and gram-positive bacteria isolated from blood cultures
Acinetobacter spp., Pseudomonas spp., Enterobacter spp., E. coli, and K. pneumoniae were the most common carbapenem-resistant isolates (77.8, 70.7, 33.3, 24.4, and 13.2%, respectively).
Out of 257 Enterobacterales and non-fermenter gram-negative BSIs, 39.3% (101/257) were carbapenem-resistant. The incidence of CRE and carbapenem-resistant non-fermenter BSIs increased annually between 2015 and 2018 (p < 0.001).
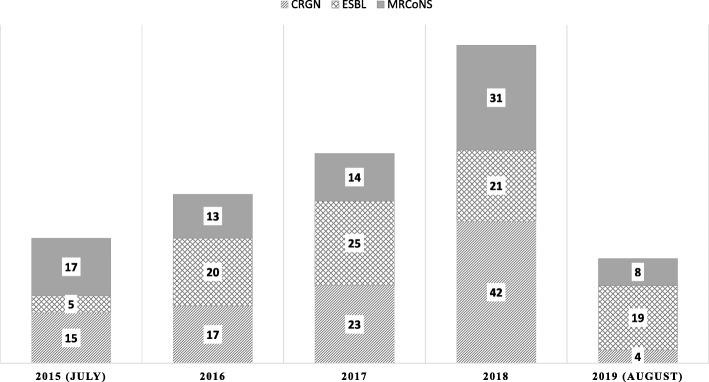
The frequency of isolated organisms, MRCoNS, ESBL, and CRGN associated BSI, is shown in Fig. 4. As shown, there are emerging ESBL and CRGN BSIs during different study years.
Fig. 4.
The annual frequency of ESBL and CRE-associated BSI, in addition to MRCoNS, associated BSI (labels represent case numbers). CRGN includes carbapenem resistance Enterobacterales and non-fermenter spp.
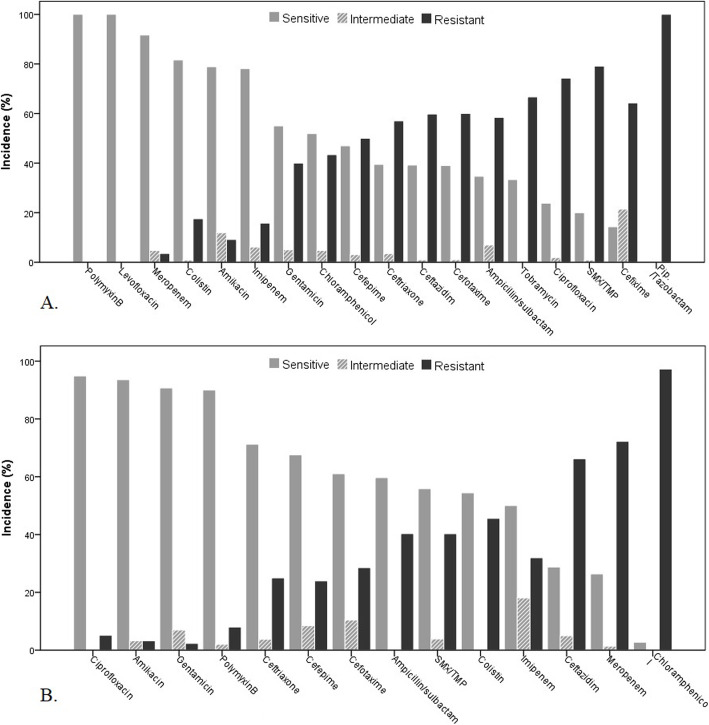
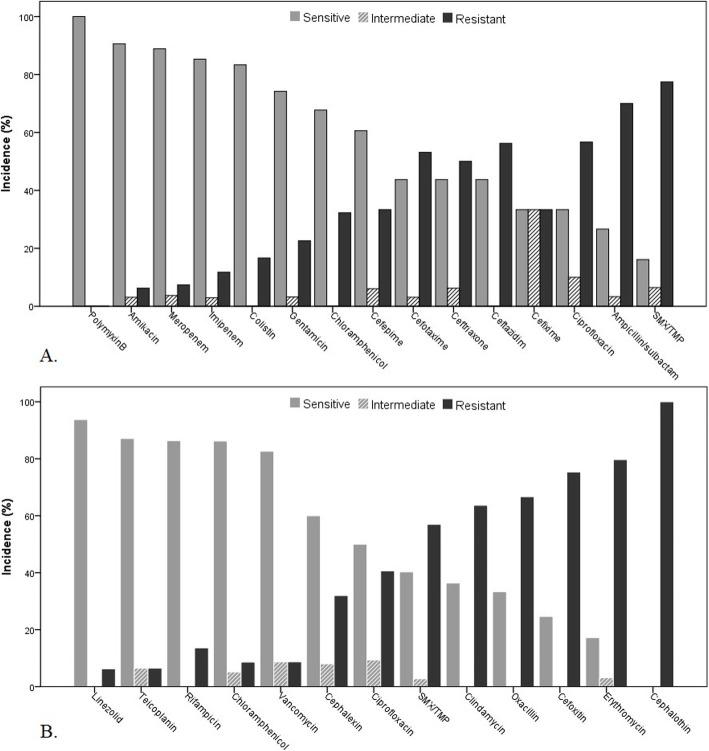
Antibiotic susceptibility results for the most common isolated bacteria are shown in Figs. 5 and 6. All E. coli were sensitive to polymyxin b. The sensitivity of E. coli for meropenem, colistin, amikacin, and imipenem were 92, 82.79, and 78%, respectively. All E. coli was resistant to piperacillin-tazobactam. More than 90% of Pseudomonas spp. were sensitive to ciprofloxacin, amikacin, gentamicin, and polymyxin-b, while 97% were chloramphenicol- resistant. All K. pneumoniae was sensitive to polymyxin-b, and 91% were susceptible to amikacin. More than 80% of K. pneumoniae was sensitive to meropenem, imipenem, and colistin. Trimethoprim/sulfamethoxazole and ampicillin-sulbactam found less sensitive agents. Most coagulase-negative staphylococci (94%) were susceptible to linezolid, and more than 80% of them were susceptible to teicoplanin, rifampicin, chloramphenicol, and vancomycin.
Fig. 5.
Antimicrobial susceptibility results of 123 E. coli (A) and 81 Pseudomonas spp. (B) isolates recovered from blood cultures during 2015–2019
Fig. 6.
Antimicrobial susceptibility results of 38 K. pneumonia (A) and of 80 coagulase-negative staphylococci (CoNS) isolates (B) recovered from blood cultures during 2015–2019
Among the Enterobacteriaceae family, 91 (64.1%) isolates were ESBL-producer, and 36 (28.8%) were CRE. CR detected in 71.6% P. aeruginosa and 87.5% of Acinetobacter spp.
Overall, in those with gram-negative BSI, 49.3% found to be non-susceptible to at least one agent in ≥3 classes of antibiotics, including carbapenems (imipenem or meropenem), combinations of beta-lactams plus beta-lactamase inhibitors (piperacillin-tazobactam or ampicillin-sulbactam), cephalosporins (3rd or 4th generation cephalosporins), aminoglycosides (amikacin or gentamicin), or fluoroquinolones (ciprofloxacin or levofloxacin). Although the MDR gram-negative (MDRGN) BSI cases increased from July 2015 to 2018, the difference was not significant (p = 0.588). Table 1 represents the incidence of MRCoNS, ESBL producing organisms, CRE and Acinetobacter spp. and Pseudomonas spp. in different study years.
Table 1.
MDRGN, ESBL, and CRGN associated BSIs frequency among most common gram-negative bacteria as well as MRSA and MRCoNS among gram-positive associated BSIs during 2015–2019
| 2015 n (%) |
2016 n (%) |
2017 n (%) |
2018 n (%) |
2019 n (%) |
p-value | ||
|---|---|---|---|---|---|---|---|
| MDR | 20 (40) | 36 (50) | 53 (49.5) | 66 (53.75) | 29 (46.8) | 0.588 | |
| E. coli | 12 (60) | 22 (61.1) | 26 (49.1) | 13 (19.7) | 18 (62.1) | ||
| K. pneumoniae | 3 (15) | 3 (8.3) | 4 (7.5) | 9 (13.6) | 5 (17.2) | ||
| Acinetobacter spp. | 2 (10) | 3 (8.3) | 2 (3.8) | 0 (0) | 0 (0) | ||
| Pseudomonas spp. | 1 (5) | 6 (16.7) | 18 (34) | 43 (65.2) | 6 (20.7) | ||
| ESBL | 5 (15.2) | 31 (46.3) * | 43 (43) * | 66 (55.5) | 28 (45.9) | 0.002 | |
| E. coli | 5 (100) | 18 (75) | 20 (60.6) | 14 (82.4) | 13 (50) | ||
| K. pneumoniae | 0 (0) | 3 (15) | 4 (16) | 7 (33.3) | 6 (31.6) | ||
| Acinetobacter spp. | 0 (0) | 2 (100) | 3 (100) | 0 (0) | 0 (0) | ||
| Pseudomonas spp. | 0 (0) | 6 (100) | 15 (88.2) | 45 (97.8) | 9 (100) | ||
| CRGN | 15 (14.9) | 17 (16.8) | 23 (22.8) | 42 (41.6) | 4 (4) | < 0.0001 | |
| E. coli | 11 (84.6) | 11 (40.7) | 5 (13.9) | 3 (14.3) | 0 (0) | ||
| K. pneumoniae | 2 (66.7) | 2 (40) | 0 (0) | 1 (7.1) | 0 (0) | ||
| Acinetobacter spp. | 1 (33.3) | 3 (100) | 3 (100) | 0 (0) | 0 (0) | ||
| Pseudomonas spp. | 0 (0) | 1 (16.7) | 15 (75) | 38 (82.6) | 4 (44.4) | ||
| MRSA | 0 (0) | 3 (50) | 1 (25) | 0 (0) | 0 (0) | 0.038 | |
| MR-CONS | 13 (86.7) | 7 (53.8) | 13 (92.9) | 22 (78.6) | 5 (62.5) | 0.112 |
MDRGN: multidrug-resistant gram-negative bacteria; ESBL: extended-spectrum beta-lactamases (ESBL)-producing gram-negative bacteria; CRE: carbapenem-resistant Enterobacterales; CRGN: carbapenem-resistant gram-negative bacteria; MRCONS: methicillin-resistant coagulase-negative Staphylococci; MRSA: methicillin-resistant Staphylococcus aureus. P-values marked with bold indicate statistically significant p-values
Among tested ESBL-producer gram-negative isolates, susceptibility to polymyxin-b, amikacin, colistin, imipenem, and meropenem, were 95, 87, 70, 65.4, and 54.5%, respectively. Polymyxin-b, amikacin, and colistin found the most active agent against CRE clinical isolates (87.5, 75, and 43.2%, respectively).
Sixty cases detected as MRCoNS (76.9%). Among MRCoNS, 94% were susceptible to linezolid, and more than 80% were susceptible to teicoplanin, chloramphenicol, and rifampin, while vancomycin was less sensitive (74%). All of the isolated enterococci were VRE.
Clinical influence of drug-resistance BSI and predictors of mortality
Based on the obtained results, third-generation cephalosporin-resistant E. Coli and P. aeruginosa, carbapenem-resistant P. aeruginosa, and polymyxin-resistant K. pneumoniae BSIs associated with increased mortality; however, the difference was not statistically significant (p-value: 0.719, > 0.999, 0.521, and 0.467; respectively).
Based on results of univariate logistic regression analysis of variables investigated for survival in cancer patients with BSI, none of them associated with a greater risk of mortality (Table 2). Our results revealed that the odds of mortality decreased annually during 2015–2018 (Table 2).
Table 2.
Univariate logistic regression analysis of variables investigated for prediction of mortality in cancer patients with bloodstream infection
| Alive (n = 324, 78.5%) | Dead (n = 89, 21.5%) | OR (95% CI) | p-value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 136 (45.8) | 37 (45.7) | 1.005 (0.613,1.645) | 0.986 |
| Male* | 161 (54.2) | 44 (54.3) | ||
| Age | ||||
| < 60 | 209 (70.4) | 52(64.2) | 1.325 (0.789,2.224) | 0.288 |
| > 60* | 88 (29.6) | 29(35.8) | ||
| Malignancy type | ||||
| Solid organ tumor* | 131 (44.1) | 36 (44.4) | 1.014 (0.618,1.662) | 0.957 |
| Hematologic malignancy | 166 (55.9) | 45 (55.6) | ||
| ESR | 73.58 (36.10) | 63.77 (36.88) | 1.008 (0.999,1.017) | 0.091 |
| CRP | 82.12 (43.12) | 88.36 (28.62) | 0.996 (0.989,1.004) | 0.332 |
| WBC count | ||||
| < 4000/μl* | 169 (57.3) | 49 (60.5) | 1.142 (0.691,1.885) | 0.605 |
| > 4000/μl | 126 (42.7) | 32 (39.5) | ||
| Neutropenia | ||||
| Yes (< 1500/μl) | 121 (41.3) | 33 (41.3) | 1.002 (0.606,1.656) | 0.994 |
| No (> 1500/μl) * | 172 (58.7) | 47 (58.8) | ||
| Febrile neutropenia | ||||
| No | 212 (71.4) | 60 (74.1) | 0.873 (0.500,1.524) | 0.633 |
| Yes* | 85 (28.6) | 21 (25.9) | ||
| Year | ||||
| 2015 | 36 (12.1) | 6 (7.4) | 1.773 (0.612,5.132) | 0.291 |
| 2016 | 52 (17.5) | 11 (13.6) | 1.397 (0.569,3.427) | 0.466 |
| 2017 | 81 (27.3) | 22 (27.2) | 1.088 (0.500,2.368) | 0.832 |
| 2018 | 84 (28.3) | 29 (35.8) | 0.856 (0.405,1.810) | 0.684 |
| 2019* | 44 (14.8) | 13 (16) | ||
| Enterobacterales | ||||
| No* | 70 (36.1) | 18 (38.3) | 1.100 (0.570,2.121) | 0.777 |
| Yes | 124 (63.9) | 29 (61.7) | ||
* Reference
Besides, univariate logistic regression analysis showed that only WBC count < 4000/μl (OR: 1.753; 1.159–2.651) and non-fermenter gram-negative BSI (OR: 3.120; 1.481–6.575) were associated with greater risk for MDR gram-negative BSI (p-value 0.008 and 0.003, respectively). Furthermore, we found that the odds of MDR gram-negative BSI increased annually during 2016–2018 (Table 3).
Table 3.
Univariate logistic regression analysis of variables investigated for prediction of MDR gram-negative infection in cancer patients with bloodstream infection
| Non-MDR (n = 19,50.4%) | MDR (n = 188, 49.6%) | OR (95% CI) | p-value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 78 (40.8) | 95 (50.5) | 1.48 (0.986, 2.221) | 0.059 |
| Male* | 113 (59.2) | 93 (49.5) | ||
| Age | ||||
| < 60 | 125 (65.4) | 136 (72.3) | 1.381 (0.892,2.138) | 0.148 |
| > 60* | 66 (34.6) | 52 (27.7) | ||
| Malignancy | ||||
| Solid organ tumor | 84 (44) | 83 (44.1) | 1.007 (0.671,1.511) | 0.973 |
| Hematologic malignancy* | 107 (56) | 105 (55.9) | ||
| ESR | 67.96 (34.76) | 75.40 (37.68) | 1.006 (0.999,1.013) | 0.101 |
| CRP | 78.60 (37.28) | 88.05 (43.48) | 1.006 (1.000,1.012) | 0.065 |
| WBC count | ||||
| < 4000/μl | 97 (51.3) | 122 (64.9) | 1.753 (1.159,2.651) | 0.008 |
| > 4000/μl* | 92 (48.7) | 66 (35.1) | ||
| Neutropenia | ||||
| Yes (< 1500/μl) | 69 (36.7) | 86 (46.2) | 1.483 (0.981,2.243) | 0.062 |
| No (> 1500/μl) * | 119 (63.3) | 100 (53.8) | ||
| Febrile neutropenia | ||||
| No* | 47 (24.6) | 59 (31.4) | 1.401 (0.893,2.200) | 0.142 |
| Yes | 144 (75.4) | 129 (68.6) | ||
| Year | ||||
| 2015 | 24 (12.6) | 18 (9.6) | 0.960 (0.429,2.146) | 0.921 |
| 2016 | 32 (16.8) | 31 (16.5) | 1.240 (0.604,2.546) | 0.558 |
| 2017 | 51 (26.7) | 52 (27.7) | 1.305 (0.681,2.501) | 0.422 |
| 2018 | 52 (27.2) | 62 (33) | 1.526 (0.805,2.894) | 0.195 |
| 2019* | 32 (16.8) | 25 (13.3) | ||
| Enterobacterales | ||||
| No ** | 44 (81.5) | 110 (58.8) | 3.120 (1.481,6.575) | 0.003 |
| Yes* | 10 (18.5) | 78 (41.5) | ||
* Reference
** non-fermenter gram-negative BSI. P-values marked with bold indicate statistically significant p-values
Given the emergence of CRGN bacteria isolated in blood culture of patients with BSI during 2015–2018 (as we shown in Fig. 4) and the high proportion of CR isolates in non-fermenters (more than 70%) and also in Enterobacterales (about 25%), we investigated the odds of different possible risk factors for carbapenem-resistant BSI by logistic regression model (Table 4). Accordingly, we found that age < 60-year-old, solid organ neoplasms, non-fermenter gram-negative BSI, third-generation cephalosporine resistant gram-negative isolates, and polymyxin-resistance infections were significantly associated with carbapenem-resistant BSI based on univariate logistic regression analysis.
Table 4.
Logistic regression analysis of factors associated with carbapenem-resistant gram-negative bacterial bloodstream infection in cancer patients
| n (%) | n (%) | Univariate | p-value | |
|---|---|---|---|---|
| Age | ||||
| < 60 | 78 (83) | 54(56.8) | 3.701 (1.887,7.262) | < 0.0001 |
| > 60* | 16 (17) | 41(43.2) | ||
| Malignancy type | ||||
| Solid organ | 55 (58.5) | 35 (36.8) | 2.418 (1.347,4.339) | 0.003 |
| Hematologic malignancy* | 39 (41.5) | 60 (63.2) | ||
| Enterobacterales | ||||
| Yes* | 33 (35.1) | 81 (85.3) | 10.695 (5.269,21.709) | < 0.0001 |
| No | 61 (64.9) | 14 (14.7) | ||
| 4th GCRGN | ||||
| Resistance | 42 (44.7) | 31 (32.6) | 1.667 (0.923,3.011) | 0.090 |
| Sensitive* | 52 (55.3) | 64 (67.4) | ||
| 3rd GCRGN | ||||
| Resistance | 88 (93.6) | 57 (60) | 9.778 (3.884,24.615) | < 0.0001 |
| Sensitive* | 6 (6.4) | 38 (40) | ||
| Aminoglycoside-resistant | ||||
| Resistance | 31 (33) | 21 (22.1) | 1.734 (0.907,3.314) | 0.096 |
| Sensitive* | 63 (67) | 74 (77.9) | ||
| Polymyxin-resistance | ||||
| Resistance | 50 (53.2) | 6 (6.3) | 16.856 (6.713,42.323) | < 0.0001 |
| Sensitive* | 44 (46.8) | 89 (93.7) | ||
* Reference. P-values marked with bold indicate statistically significant p-values
As illustrated in Fig. 7, based on both univariate and multivariate logistic regression analysis, Pseudomonas-associated BSI and polymyxin-resistance BSI were significantly associated with carbapenem-resistant BSI. Among tested antibiotics, only fluoroquinolones (including ciprofloxacin and levofloxacin) and aminoglycosides have acceptable sensitivity (resistance rate lesser than 10%) for Pseudomonas associated BSI. For E. coli-associated BSIs, polymyxins were the most active drug, while polymyxins and carbapenems were the best choices for K. pneumoniae-associated BSI (resistance rate < 15%).
Fig. 7.
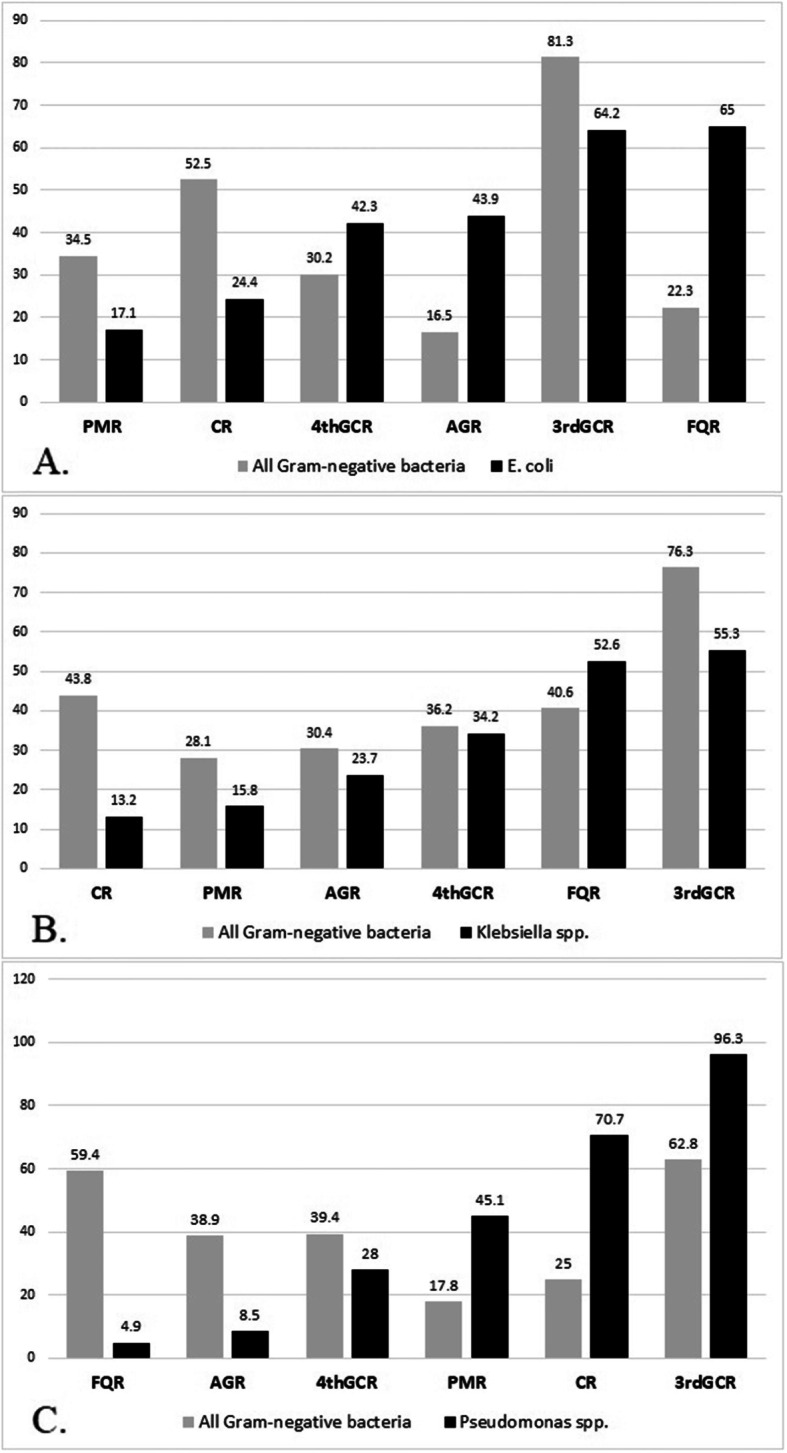
Comparative analysis for antibiotic resistance rate between E. coli, K. pneumoniae, and Pseudomonas spp. with all gram-negative isolates (A, B, and C, respectively)
PMR: polymyxin-resistant; CR: carbapenem-resistant; 3rdGCR: third-generation cephalosporine-resistant gram-negative isolates; 4thGCR: fourth-generation cephalosporine-resistant gram-negative isolates; AGR: aminoglycoside- resistant; FQR: fluoroquinolone-resistant.
Detail information regarding susceptibility profile of MDR, CR, and ESBL-producer E. coli, Pseudomonas spp., and K. pneumoniae isolates against different antimicrobial classes summarized in Table 5.
Table 5.
The susceptibility profile of carbapenem-resistant and ESBL-producer E. coli, Pseudomonas spp., and K. pneumoniae isolates against different antimicrobial classes
| Sensitivity rate (n, %) | 4th GC | Ciprofloxacin | AGs | BL/BLI | Polymyxins | Carbapenems |
|---|---|---|---|---|---|---|
| Carbapenem-resistant | ||||||
| E. coli | 16 (53.3%) | 8 (26.7%) | 9 (30%) | 11 (36.7%) | 18 (69.2%) | – |
| Pseudomonas spp. | 35 (60.3%) | 54 (93.1%) | 51 (78.9%) | 47 (81%) | 6 (66.6%) | – |
| K. pneumoniae | 2 (40%) | 1 (20%) | 2 (25%) | 2 (40%) | 5 (62.5%) | – |
| ESBL | ||||||
| E. coli | 20 (28.6%) | 17 (24.3%) | 38 (54.3%) | 21 (30%) | 59 (84.3%) | 51 (72.9%) |
| Pseudomonas spp. | 52 (69.3%) | 71 (94.7%) | 70 (93.3%) | 53 (70.7%) | 41 (54.7%) | 20 (26.7%) |
| K. pneumoniae | 7 (35%) | 6 (30%) | 16 (80%) | 1 (5%) | 6 (75%) | 17 (85%) |
| MDR | ||||||
| E. coli | 42 (46.2%) | 19 (20.9%) | 40 (44%) | 27 (29.7%) | 71 (78%) | 62 (68.1%) |
| Pseudomonas spp. | 51 (68.9%) | 70 (94.6%) | 67 (90.5%) | 49 (66.2%) | 37 (50%) | 18 (24.3%) |
| K. pneumoniae | 11 (45.8%) | 6 (25%) | 15 (62.5%) | 3 (12.5%) | 19 (79.2%) | 19 (79.2%) |
4th GC: fourth-generation cephalosporins (cefepime); AGs: aminoglycosides (amikacin and gentamicin); BL/BLI: beta-lactamase/beta-lactamase inhibitors combination (piperacillin-tazobactam)
Discussion
Patients with malignancy are predisposed to developing BSI during their chemotherapy courses. Lots of evidence showed that the epidemiology of nosocomial infections in cancer patients changed over the past decades, with the reemergence of GNB as the predominant causative pathogens. The current study, therefore, conducted to describe the antibiotic-resistant patterns and outcomes of nosocomial infections caused by GNB in adult cancer patients.
Overall, despite the annual increase in the admission rate, no significant increase in the BSI incidence rate and attributed death occurred during the five-year study period (Fig. 2). Like some other reports, our study showed that CLABSI was more common in solid tumors than hematologic malignancies [19]; however, a higher prevalence of CLABSI detected in patients with hematological malignancies in other studies [20]. We found that gram-negative BSI was the most common etiology of BSI in cancer patients (63.3%), which agrees with other reports [21, 22]. Like recent reports, we observed a gradual increase in the incidence of MDRGN and CRGN associated BSIs annually during our surveillance [23, 24].
E. coli, Pseudomonas spp., and K. pneumoniae were the most common recovered gram-negative isolates in our study, which is in line with previous studies conducted in cancer patients [12, 21, 22, 25]. We found a high proportion of ESBL producers and carbapenem-resistant isolates, predominantly in non-fermenters (96.4 and 82.3%) and the Enterobacteriaceae family (64.1 and 28.8%). Although there are scarce reports on the incidence of carbapenem-resistant GNs in our region, obtained results are comparable to available reports [26]; however, compared to Europe, North America, Latin America global surveillance studies, and Asia-Pacific regional surveillance studies, our results showed significant higher-resistance rate [27]. We observed that the incidence of MDR BSI increased from 2015 to 2018.
The overall mortality rate of GNB BSIs among cancer patients in our study was about 20%, which was more significant compared with studies conducted in our region, for example, Calik Basaran et al. (17.0%) [28] and Garcia-Vidal et al. (14.8%) [12]; however, is much lower than some other studies, for example, Al-Otaibi et al. (32.1%) [5], and Yawei Zhang et al. (33.5%) [29].
The mortality rate of carbapenem-resistant Enterobacterales, ESBL producing Enterobacterales, MDR Acinetobacter spp., and MDR P. aeruginosa is around 6–7% in the US, and Europe reports [24, 30]. However, in this study, the BSI attributed mortality rate among MDRGN, CRGN, ESBL producer, Pseudomonas spp., E. coli, and K. pneumoniae were 20.2, 18.8, 19.1, 22, 22.1, and 21.5%, respectively.
We did not observe an association between investigated factors and survival; however, in a study by Chien-Yuan Chen et al. age ≥ 60 years, prior allogeneic transplantation and BSI due to VRE found as independent predictors for mortality [31]. CRGN BSI [26, 32–34], unresolved neutropenia, monotherapy, septic shock [32, 35], and polymicrobial BSI [32] are other risk factors associated with increased mortality in cancer patients with GN BSI.
We identified MDR gram-negative infections more likely to occur in patients with WBC count< 4000 and non-fermenter gram-negative BSI (Table 3). Other studies found that male sex, age ≥ 60, previous antimicrobial use, liver disease, and bacteremia caused by K. pneumoniae are associated with increased risk of MDRGN BSI [36].
In agreement with current concerns regarding the efficacy of colistin against carbapenem-resistant pathogens, including CRE [37–40], we found that CR E. coli and K. pneumoniae isolates did not show acceptable sensitivity to our available antimicrobial choices, including colistin. However, ESBL producer K. pneumoniae and ESBL E. coli isolates had acceptable sensitivity to carbapenems and aminoglycosides (> 80%) and polymyxins (84.3%), respectively. Like the previous reports, our study reemphasized that carbapenems could still act as the drugs of choice in ESBL associated BSIs in cancer patients [41]. Besides, MDR E. coli and K. pneumoniae showed a high resistance rate to available antimicrobial agents. Our findings are consistent with previous studies concerning the global emergence of MDRGN pathogens as a significant healthcare burden that could be attributed to the overuse of antibiotics and necessitates the development of new antibiotics for treating CRE, CR P. aeruginosa, and CR Acinetobacter baumannii [42–44].
We also found that MDR, CR, and ESBL Pseudomonas spp. isolates still are sensitive to ciprofloxacin in our setting and could be considered a good treatment choice as recommended by current guidelines [45]. Remarkably, piperacillin-tazobactam, which is frequently preferred as one of the initial antibiotic therapies in our febrile neutropenic patients, may not be an excellent empiric choice against CR E. coli and K. pneumoniae. Our study showed that carbapenems might be less active against ESBL-producer Pseudomonas spp. and to some extent against ESBL-producer E. coli. In MDRGN BSI, none of our empiric treatments (carbapenems and piperacillin-tazobactam) could overcome GN bacteremia and need other choices such as colistin (Table 5). Although febrile neutropenia should be considered a medical emergency and a prompt administration of empirical antibiotic therapy is mandatory, increased mortality could be seen due to inappropriate empiric antibiotic therapy in setting with high rates of resistant pathogens [2, 12, 19, 46]. Accordingly, regular epidemiological and microbiological surveillance of BSI should be encouraged strongly in oncology centers.
Limitation
Our study has some limitations. First, given the retrospective nature of this study, it was difficult to collect some variables (chemotherapeutic protocols, antibiotics treatment before admission, and some clinical and laboratory examination results) in this retrospective study. So, there might be hidden biases in the analysis of the relationship. Second, carbapenemase type and enzyme were not investigated in this study. Finally, our research was conducted with data collected from a single center. Therefore, a prospective multicenter study is needed for further validation.
Conclusions
In conclusion, the overall case-fatality rate of BSI caused by GNB among cancer patients was 20%. The high proportion of the most frequently isolated pathogens were CR and ESBL-producing Enterobacterales and Pseudomonas spp. Although fluoroquinolones remain a good choice for CR P. aeruginosa, we have few effective choices against CR E. coli and K. pneumoniae. The new agents such as ceftazidime/avibactam could be helpful in such cases.
Supplementary Information
Additional file 1: Table S1. The summary characteristics of the patients with solid tumors and hematological malignancies.
Acknowledgments
Our thanks go to Infection Control Unit staffs in Amir Medical Oncology Hospital, Shiraz University of Medical Sciences, for their technical support and assistance.
Abbreviations
- BSI
bloodstream infections
- CLABSI
Central Line-Associated BSI
- ASP
Antimicrobial stewardship program
- GN
gram-negative
- CR
Carbapenem-resistant
- MRCoNS
Methicillin-resistant coagulase-negative staphylococci
- ESBL
extended-spectrum beta-lactamase
- CRE
Carbapenem-resistant Enterobacterales
- CRGN
Carbapenem-resistant gram-negative
- MDRGN
multi-drug resistance gram-negative
Authors’ contributions
Study concept and design: AA; Acquisition of data: GS, KS, SZ, and MS; Statistical Analysis: AA and SZ, Analysis and interpretation of data: AA and NS; Drafting of the manuscript: AA, SS, and SZ, Critical revision of the manuscript for important intellectual content: AA; Study supervision: AA and SZ. All individuals listed as (co)-authors have met the authorship criteria, and nobody who qualifies for authorship omitted from the list. The final manuscript was corrected and approved by all authors.
Funding
Not applicable.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics and consent to participate
The project was found to be in accordance with the ethical principles and the national norms and standards for conducting Medical Research in Iran with approval ID IR.SUMS.MED.REC.1399.082 on 2020-05-18 [47].
Consent for publication
Not applicable.
Competing interests
The authors do not have any financial or other relationships, which could regard as a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Samane Nematolahi and Zahra Shahhosein contributed equally to this work.
Contributor Information
Samane Nematolahi, Email: samane.nematolahi@yahoo.com.
Zahra Shahhosein, Email: nshahhosein@yahoo.com.
References
- 1.El-Mahallawy HA, Hassan SS, El-Wakil M, Moneer MM, Shalaby L. Increasing antimicrobial resistance monitored in surveillance analysis of blood stream infections in febrile neutropenic pediatric oncology patients. Asian Pac J Cancer Prev. 2015;16(14):5691–5695. doi: 10.7314/APJCP.2015.16.14.5691. [DOI] [PubMed] [Google Scholar]
- 2.Kang C-I, Kim S-H, Park WB, Lee K-D, Kim H-B, Kim E-C. Oh M-d, Choe K-W: bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49(2):760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van de Louw A, Lewis AM, Yang Z. Autopsy findings in patients with acute myeloid leukemia and non-Hodgkin lymphoma in the modern era: a focus on lung pathology and acute respiratory failure. Ann Hematol. 2019;98(1):119–129. doi: 10.1007/s00277-018-3494-3. [DOI] [PubMed] [Google Scholar]
- 4.Easow JM, Joseph NM, Dhungel BA, Chapagain B, Shivananda PG. Blood stream infections among febrile patients attending a teaching hospital in Western region of Nepal. Australasian Medical Journal. 2010;3(10):633–637. doi: 10.4066/AMJ.2010.422. [DOI] [Google Scholar]
- 5.Al-Otaibi FE, Bukhari EE, Badr M, Alrabiaa AA. Prevalence and risk factors of gram-negative bacilli causing blood stream infection in patients with malignancy. Saudi Medical Journal. 2016;37(9):979–984. doi: 10.15537/smj.2016.9.14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokkayil P, Agarwal R, Mohapatra S, Bakhshi S, Das B, Sood S, Dhawan B, Kapil A. Bacterial profile and antibiogram of blood stream infections in febrile neutropenic patients with haematological malignancies. Journal of Infection in Developing Countries. 2018;12(6):442–447. doi: 10.3855/jidc.9725. [DOI] [PubMed] [Google Scholar]
- 7.Prabhash K, Medhekar A, Ghadyalpatil N, Noronha V, Biswas S, Kurkure P, Nair R, Kelkar R. Blood stream infections in cancer patients: A single center experience of isolates and sensitivity pattern. Indian J Cancer. 2010;47(2):184–188. doi: 10.4103/0019-509X.63019. [DOI] [PubMed] [Google Scholar]
- 8.McNamara JF, Righi E, Wright H, Hartel GF, Harris PNA, Paterson DL. Long-term morbidity and mortality following bloodstream infection: A systematic literature review. J Inf Secur. 2018;77(1):1–8. doi: 10.1016/j.jinf.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–2266. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 10.Feld R. Bloodstream infections in cancer patients with febrile neutropenia. Int J Antimicrob Agents. 2008;32:S30–S33. doi: 10.1016/j.ijantimicag.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Tang Y, Wu X, Cheng Q, Li X. Inappropriate initial antimicrobial therapy for hematological malignancies patients with gram-negative bloodstream infections. Infection. 2020;48(1):109–116. doi: 10.1007/s15010-019-01370-x. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Vidal C, Cardozo-Espinola C, Puerta-Alcalde P, Marco F, Tellez A, Agüero D, Romero-Santana F, Díaz-Beyá M, Giné E, Morata L. Risk factors for mortality in patients with acute leukemia and bloodstream infections in the era of multiresistance. PLoS One. 2018;13(6):e0199531. doi: 10.1371/journal.pone.0199531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doron S, Davidson LE. Antimicrobial stewardship. Mayo Clin Proc. 2011;86(11):1113–1123. doi: 10.4065/mcp.2011.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diekema DJ, Hsueh P-R, Mendes RE, Pfaller MA, Rolston KV, Sader HS, Jones RN. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 2019;63(7):e00355–e00319. doi: 10.1128/AAC.00355-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Organization WH: Antimicrobial resistance – global report on surveillance. https://apps.who.int/iris/bitstream/handle/10665/112642/97892415 64748_eng.pdf;jsessionid=519244EE0EEF520027CE4098504150B4?sequence=1. 2014.
- 16.Clinical and Laboratory Standards Institute (CLSI): Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100. In. 950 West Valley Road. Suite 2500, Wayne, Pennsylvania 19087 USA: Clinical and Laboratory Standards Institute; 2020: 108.
- 17.Clinical and Laboratory Standards Institute (CLSI): Performance standards for antimicrobial susceptibility testing: approved 28th ed. In.: CLSI Wayne, PA; 2018.
- 18.Magiorakos A-P, Srinivasan A, Carey R, Carmeli Y, Falagas M, Giske C, Harbarth S, Hindler J, Kahlmeter G, Olsson-Liljequist B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 19.Islas-Muñoz B, Volkow-Fernández P, Ibanes-Gutiérrez C, Villamar-Ramírez A, Vilar-Compte D, Cornejo-Juárez P. Bloodstream infections in cancer patients. Risk factors associated with mortality. Int J Infect Dis. 2018;71:59–64. doi: 10.1016/j.ijid.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Moell J, Svenningsson A, Af Sandeberg M, Larsson M, Heyman M, Harila-Saari A, Nilsson A. Early central line-associated blood stream infections in children with cancer pose a risk for premature catheter removal. Acta Paediatr. 2019;108(2):361–366. doi: 10.1111/apa.14432. [DOI] [PubMed] [Google Scholar]
- 21.Vahedian-Ardakani HA, Moghimi M, Shayestehpour M, Doosti M, Amid N. Bacterial Spectrum and antimicrobial resistance pattern in Cancer patients with febrile neutropenia. Asian Pac J Cancer Prev. 2019;20(5):1471–1474. doi: 10.31557/APJCP.2019.20.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamal A, Fatima N, Shaikh S, Kaleem B, Rizvi QA, Zaidi U, Borhany M, Shamsi T. Pattern of antimicrobial sensitivity in microbiologically documented infections in neutropenic patients with Haematological malignancies: A single center study. Indian J Microbiol. 2019;59(2):188–192. doi: 10.1007/s12088-019-00789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litwin A, Fedorowicz O, Duszynska W. Characteristics of microbial factors of healthcare-associated infections including multidrug-resistant pathogens and antibiotic consumption at the university intensive care unit in Poland in the years 2011–2018. Int J Environ Res Public Health. 2020;17(19):6943. doi: 10.3390/ijerph17196943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) [https://ecdc.europa.eu/sites/porta/files/documents/EARS-Net-report-2017-update-jan-2019.pdf.]
- 25.Andersen MA, Moser CE, Lundgren J, Niemann CU. Epidemiology of bloodstream infections in patients with chronic lymphocytic leukemia: a longitudinal nation-wide cohort study. Leukemia. 2019;33(3):662–670. doi: 10.1038/s41375-018-0316-5. [DOI] [PubMed] [Google Scholar]
- 26.Righi E, Peri AM, Harris PN, Wailan AM, Liborio M, Lane SW, Paterson DL. Global prevalence of carbapenem resistance in neutropenic patients and association with mortality and carbapenem use: systematic review and meta-analysis. J Antimicrob Chemother. 2017;72(3):668–677. doi: 10.1093/jac/dkw459. [DOI] [PubMed] [Google Scholar]
- 27.Nordmann P, Poirel L: Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clinical Infectious Diseases 2019, 69(Supplement_7):S521-S528. [DOI] [PMC free article] [PubMed]
- 28.Başaran NÇ, Karaağaoğlu E, Hasçelik G, Tanrıöver MD, Akova M. Prospective evaluation of infection episodes in cancer patients in a tertiary care academic center: microbiological features and risk factors for mortality. Turkish Journal of Hematology. 2016;33(4):311–319. doi: 10.4274/tjh.2015.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B, Xie L, Yang C, Ma X, Li H: Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE Network. Antimicrob Agents Chemother 2018, 62(2). [DOI] [PMC free article] [PubMed]
- 30.Mehrad B, Clark NM, Zhanel GG, Lynch JP., III Antimicrobial resistance in hospital-acquired gram-negative bacterial infections. Chest. 2015;147(5):1413–1421. doi: 10.1378/chest.14-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C-Y, Tien F-M, Sheng W-H, Huang S-Y, Yao M, Tang J-L, Tsay W, Tien H-F, Hsueh P-R. Clinical and microbiological characteristics of bloodstream infections among patients with haematological malignancies with and without neutropenia at a medical Centre in northern Taiwan, 2008–2013. Int J Antimicrob Agents. 2017;49(3):272–281. doi: 10.1016/j.ijantimicag.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Andria N, Henig O, Kotler O, Domchenko A, Oren I, Zuckerman T, Ofran Y, Fraser D, Paul M. Mortality burden related to infection with carbapenem-resistant gram-negative bacteria among haematological cancer patients: a retrospective cohort study. J Antimicrob Chemother. 2015;70(11):3146–3153. doi: 10.1093/jac/dkv218. [DOI] [PubMed] [Google Scholar]
- 33.Moghnieh R, Estaitieh N, Mugharbil A, Jisr T, Abdallah DI, Ziade F, Sinno L, Ibrahim A. Third generation cephalosporin resistant Enterobacteriaceae and multi-drug resistant gram-negative bacteria causing bacteremia in febrile neutropenia adult cancer patients in Lebanon, broad spectrum antibiotics use as a major risk factor, and correlation with poor prognosis. Front Cell Infect Microbiol. 2015;5:11. doi: 10.3389/fcimb.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y-a, Yong choi J, Ki kim C, Oh kim C, Soo kim M, Hoon choi S, Sik chin B, Hoon han S, Sung lee H, Kyoung choi H : Risk factors and outcomes of bloodstream infections with metallo-β-lactamase-producing Acinetobacter. Scand J Infect Dis 2008, 40(3):234–240. [DOI] [PubMed]
- 35.Tofas P, Skiada A, Angelopoulou M, Sipsas N, Pavlopoulou I, Tsaousi S, Pagoni M, Kotsopoulou M, Perlorentzou S, Antoniadou A. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections in neutropenic patients with haematological malignancies or aplastic anaemia: analysis of 50 cases. Int J Antimicrob Agents. 2016;47(4):335–339. doi: 10.1016/j.ijantimicag.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Leal HF, Azevedo J, Silva GEO, Amorim AML, de Roma LRC, Arraes ACP, Gouveia EL, Reis MG, Mendes AV, de Oliveira SM. Bloodstream infections caused by multidrug-resistant gram-negative bacteria: epidemiological, clinical and microbiological features. BMC Infect Dis. 2019;19(1):609. doi: 10.1186/s12879-019-4265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansour W, Haenni M, Saras E, Grami R, Mani Y, Khalifa ABH, El Atrouss S, Kheder M, Hassen MF, Boujâafar N. Outbreak of colistin-resistant carbapenemase-producing Klebsiella pneumoniae in Tunisia. Journal of global antimicrobial resistance. 2017;10:88–94. doi: 10.1016/j.jgar.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 38.El-Mokhtar MA, Daef E, Mohamed Hussein AA, Hashem MK, Hassan HM. Emergence of nosocomial pneumonia caused by Colistin-resistant Escherichia coli in patients admitted to chest intensive care unit. Antibiotics. 2021;10(3):226. doi: 10.3390/antibiotics10030226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paterson DL, Harris PN. Colistin resistance: a major breach in our last line of defence. Lancet Infect Dis. 2016;16(2):132–133. doi: 10.1016/S1473-3099(15)00463-6. [DOI] [PubMed] [Google Scholar]
- 40.Hussein NH, Al-Kadmy IM, Taha BM, Hussein JD. Mobilized colistin resistance (mcr) genes from 1 to 10: a comprehensive review. Mol Biol Rep. 2021:1–11. [DOI] [PubMed]
- 41.Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I. Pascual A: Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev. 2018:31(2). [DOI] [PMC free article] [PubMed]
- 42.Shrivastava SR, Shrivastava PS, Ramasamy J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Journal of Medical Society. 2018;32(1):76. doi: 10.4103/jms.jms_25_17. [DOI] [Google Scholar]
- 43.Abadi ATB, Rizvanov AA, Haertlé T, Blatt NL. World Health Organization report: current crisis of antibiotic resistance. BioNanoScience. 2019;9(4):778–788. doi: 10.1007/s12668-019-00658-4. [DOI] [Google Scholar]
- 44.Zhou C, Jin L, Wang Q, Wang X, Chen F, Gao Y, Zhao C, Chen H, Cao B, Wang H. Bloodstream infections caused by Carbapenem-resistant Enterobacterales: risk factors for mortality, antimicrobial therapy and treatment outcomes from a prospective multicenter study. Infection and Drug Resistance. 2021;14:731. doi: 10.2147/IDR.S294282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of extended-Spectrum β-lactamase producing Enterobacterales (ESBL-E), Carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa) Clin Infect Dis. 2021;72(7):e169–e183. doi: 10.1093/cid/ciaa1478. [DOI] [PubMed] [Google Scholar]
- 46.Lodise TP, Jr, PN, Kwa A, Graves J, Furuno JP, Graffunder E, Lomaestro B, McGregor JC. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother. 2007;51(10):3510–3515. doi: 10.1128/AAC.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Research Ethics Certificate [https://ethics.research.ac.ir/ProposalCertificateEn.php?id=133949&Print=true&NoPrintHeader=true&NoPrintFooter=true&NoPrintPageBorder=true&LetterPrint=true].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The summary characteristics of the patients with solid tumors and hematological malignancies.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.