Abstract
The administration of ascorbic acid (vitamin C) alone or in combination with thiamine (vitamin B1) and corticosteroids (VCTS) has recently been hypothesized to improve hemodynamics, end-organ function, and may even increase survival in critically ill patients. There are several clinical studies that have investigated the use of vitamin C alone or VCTS in patients with sepsis and septic shock or are ongoing. Some of these studies have demonstrated its safety and potential benefit in septic patients. However, many questions remain regarding the optimal dosing regimens and plasma concentrations, timing of administration, and adverse effects of vitamin C and thiamine. These questions exist because the bulk of research regarding the efficacy of vitamin C alone or in combination with thiamine and corticosteroids in sepsis is limited to a few randomized controlled trials, retrospective before-and-after studies, and case reports. Thus, although the underlying rationale and mechanistic pathways of vitamin C and thiamine in sepsis have been well described, the clinical impact of the VCTS regimen is complex and remains to be determined. This review aims to explore the current evidence and potential benefits and adverse effects of the VCTS regimen for the treatment of sepsis.
Keywords: Vitamin C, thiamine, sepsis, septic shock, levels, laboratory testing, critical illness
Introduction
Ascorbic acid (Vitamin C) and thiamine (Vitamin B1) are naturally occurring water-soluble micronutrients that may hold potential as adjuvant therapies in critically ill patients.1 The early physiology, pathology and laboratory measurements associated with these two vitamins and their deficiencies are described primarily through old diseases such as scurvy, beriberi and Korsakoff syndrome and Wernicke’s encephalopathy.1–3 Nevertheless, there is a large and current body of basic science, preclinical and clinical studies that has evaluated the role of both vitamins in patients with sepsis, acute respiratory distress syndrome (ARDS), and other inflammatory states. Both vitamin C and thiamine have broad physiological and immunological functions. Similarly, deficiencies of both vitamins have been treated empirically, with or without measuring levels, and with variable dosing regimens. Moreover, deficiencies of vitamin C and thiamine occur rapidly in critical illness, and since these deficiencies are not so prevalent, their diagnosis requires a high level of suspicion.
Recent studies have generated keen interest in the use of vitamin C, thiamine, and corticosteroids (VCTS) to treat sepsis.4,5 The context of this new approach is the decades of costly failures of new targeted agents to mitigate sepsis. One of the most exciting advantages of the VCTS regimen is the generic availability of these agents, the paucity of reported side-effects and the low cost of both the intravenous (IV) and oral forms of vitamin C and thiamine and the corticosteroids.
In this review, we address vitamin C and thiamine as relates to the knowledge base required of bedside ICU clinicians, and ICU associated pharmacists and laboratorians. We include a brief overview of biochemical properties and then dwell upon the practical aspects of vitamin C and thiamine with a focus on historical perspectives, physiological mechanisms of action, metabolism, route of administration, laboratory testing, and the current evidence supporting these agents alone or in combination, as well as their potential limitations. Considering the abundant literature on the use of corticosteroids during critical illness in general, and sepsis in particular, our focus on corticosteroids will only be addressed within the context of its role within the VCTS regimen for sepsis.
How did vitamin C come about?
The history of vitamin C spans across the centuries beginning with James Lind’s discovery of scurvy treatment in the 1700’s to the isolation of vitamin C in 1928 by Albert Szent-Gyorgyi.6 Vitamin C was called by the letter “C” because it was the third “vital mineral” isolated. Vitamin C was also named ascorbic acid because of its antiscorbutic (anti-scurvy) properties.
Why do humans require vitamin C supplementation?
Humans, unlike most animals, are unable to synthesize vitamin C due to the absence of functional L-gulonolactone oxidase, an enzyme required for the final conversion of glucose to ascorbic acid.7 Hence, humans depend on dietary sources of vitamin C. Failure to generate vitamin C makes humans very susceptible to dysfunction in various vitamin C dependent biochemical pathways necessary for surviving a critical illness.8
How is vitamin C obtained and stored in the human body?
Ascorbic acid and dehydroascorbic acid (DHAA or oxidized vitamin C), components of fruits and vegetables or marketed as vitamin supplements, are the primary dietary sources of vitamin C for humans. Both ascorbic acid and DHAA are absorbed from the lumen of the intestine and renal tubules by enterocytes and renal epithelial cells, respectively, and then circulate in the blood and enter all body cells (Figure 1A).9 Vitamin C is also secreted into the gastric juice, cerebrospinal fluid, and aqueous humor, all of which have higher concentrations than those in plasma. Additionally, vitamin C is rapidly secreted by the adrenal gland in humans in response to adrenocorticotropic hormone (ACTH). The latter would imply that adrenal vitamin C secretion is an integral part of the stress response (like cortisol) and acts to reduce the oxidants released during steroidogenesis, facilitate nitric oxide synthesis to promote cortisol release, modify ACTH receptor sensitivity, and allow synthesis of norepinephrine.10
Figure 1.
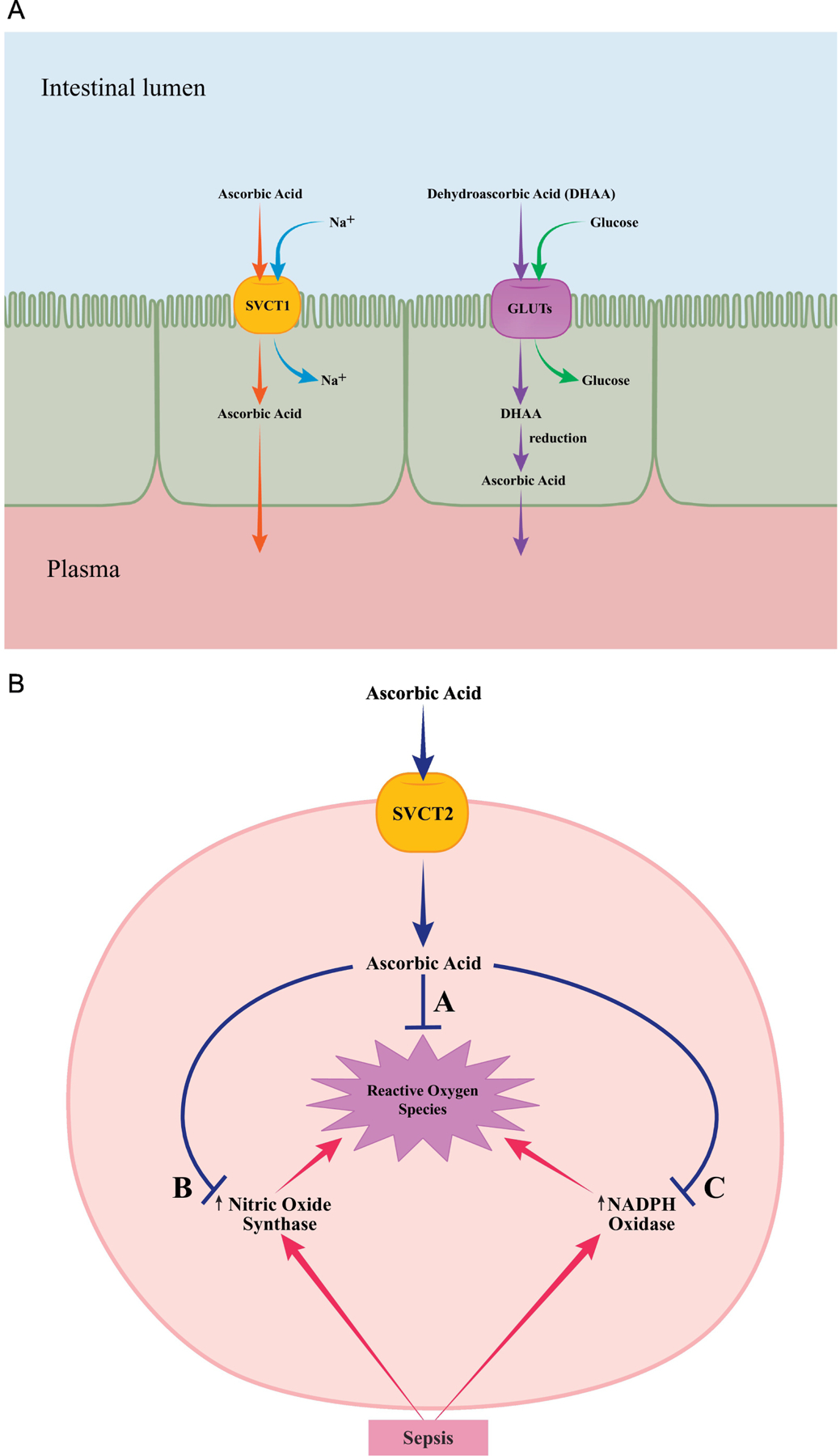
A: Transport of vitamin C
Ingested ascorbic acid is actively transported from the intestinal lumen into cells through sodium-dependent vitamin C transporter 1 (SVCT1) found in small bowel epithelial cells. Dehydroascorbic acid (DHAA) uptake is carried out by facilitated diffusion through glucose transporters (GLUTs). DHAA is then reduced to ascorbic acid intracellularly. Ascorbic acid is ultimately transported into the plasma from the epithelial cells by diffusion.
B: Vitamin C as an antioxidant
Sodium-dependent vitamin C transporter 2 (SVCT2) is responsible for non-epithelial cell uptake and delivery of ascorbic acid in tissues of the brain, eye, bone, heart, lung, adrenal gland and skeletal muscle. Ascorbic acid scavenges reactive oxygen species (A) and diminishes free radical formation by nitric oxide synthase (B) and NADPH oxidase (C) that are activated in response to sepsis.
The maintenance of whole-body vitamin C levels and distribution to different compartments is mediated by two families of transport proteins: sodium-dependent vitamin C transporters 1 (SVCT1) and 2 (SVCT2) and glucose transporters (GLUT1, GLUT3, and GLUT 4).9, 11, 12 Vitamin C is excreted by the kidneys.
What are the physiological functions of vitamin C?
The numerous physiological roles of vitamin C are all dependent on its ability to be an electron donor, or reducing agent. In this capacity, Vitamin C serves as a potent antioxidant directly scavenging oxygen free radicals and also prevents the generation of new free radicals via its suppressive effects on the NADPH oxidase (NOX) pathway (Figure 1B). 4, 13 Vitamin C is a co-substrate for the biosynthesis of endogenous vasopressors (e.g., norepinephrine), cortisol, and vasopressin.12 Endothelial function and microcirculatory flow are preserved through vitamin C’s promotion of collagen synthesis and its effect on tightening the junctions between endothelial and epithelial cells.14 Vitamin C also has immune effects including regulation of macrophage bactericidal activity and inhibition of the activation of the transcription protein nuclear factor-κB, thereby downregulating the production of proinflammatory mediators (e.g., tumor necrosis factor alpha).15, 16 Finally, vitamin C prevents sepsis-induced immunosuppression likely by reducing apoptosis of lymphocytes and monocytes.4, 12, 17
What are the clinical manifestations of chronic vitamin C deficiency?
Scurvy, the most well-known disease caused by severe and long-term vitamin C deficiency is manifested by swollen and bleeding gums, poor wound closure, easy bruising, hair and tooth loss, joint pain and swelling.15 These symptoms appear to be related to impaired collagen biosynthesis leading to the weakening of blood vessels, connective tissue, and bone.
What happens to vitamin C in critical illness and what acute deficiencies result?
Vitamin C levels become abnormally low within 24 hours of acute injury, critical illness, multiple organ failure and sepsis, and is related to the patient’s severity of illness.1, 4, 12, 18 Animal studies have demonstrated that the decrease in vitamin C levels is associated with a fall in intracellular levels. In humans, the rapid decline in vitamin C levels is likely secondary to a combination of decreased vitamin C intake and absorption, increased metabolism and distribution, increased urinary losses, and the oxidation of ascorbic acid by excess oxygen free radicals that occur during critical illness.18, 19 An estimated 40% of critically ill patients with septic shock have vitamin C serum levels suggestive of scurvy (<11.3 µmol/L).8 Since vitamin C is an essential element in the generation of endogenous vasopressors and also a potential mediator in the maintenance of vascular vasopressor responsiveness, an acute deficiency may contribute to hypotension, exaggerated inflammation, capillary leakage, and microcirculatory compromise.19
How did thiamine come about?
Thiamine was first discovered in 1910 by Umetaro Suzuki in Japan when researching how rice bran cured patients afflicted with beriberi.20 It was first crystallized by Jansen and Donath in 1926.21 Thiamine was the first B vitamin discovered hence its name carries the number 1. To date, much of what is known about thiamine originates from studies of alcohol abuse and nutritional deficiency disorders.
How is thiamine obtained and stored in the human body?
Humans cannot synthesize thiamine and obtain thiamine through dietary intake of cereal grains, beans, nuts, and meat.22 Thiamine is also commercially available in combination with other B vitamins (vitamin B complex). Thiamine is largely absorbed in the upper jejunum and to a lesser extent in the duodenum and ileum. Absorption is highly influenced by overall nutritional status and alcohol consumption.
Thiamine exists in four forms due to the addition of one or more phosphate groups: non-phosphorylated or free thiamine, thiamine monophosphate, thiamine diphosphate (TPP), and thiamine triphosphate.22 Approximately 30 mg of thiamine can be stored by the human body primarily in skeletal muscles and the rest in the liver, kidney and heart. The cellular transport of thiamine is mediated by two thiamine carrier transporters: human thiamine transporter (hTHTR-1) and hTHTR-2. Like vitamin C, thiamine is renally excreted.23
What are the functions of thiamine?
Thiamine, specifically the TPP form, is an important cofactor for enzymes utilized in multiple biochemical reactions for carbohydrate metabolism and energy production. These include pyruvate dehydrogenase, which catalyzes the conversion of pyruvate to acetyl coenzyme A, α-ketoglutarate dehydrogenase, which converts α-ketoglutarate to succinyl coenzyme A in the Krebs cycle, and transketolase of the pentose phosphate pathway that generates NADPH for reductive biosynthesis (Figure 2).24 Thiamine also is involved in optimal function of the central nervous system and repair of myelin nerve sheaths.25
Figure 2. Carbohydrate metabolism pathways that rely on thiamine.
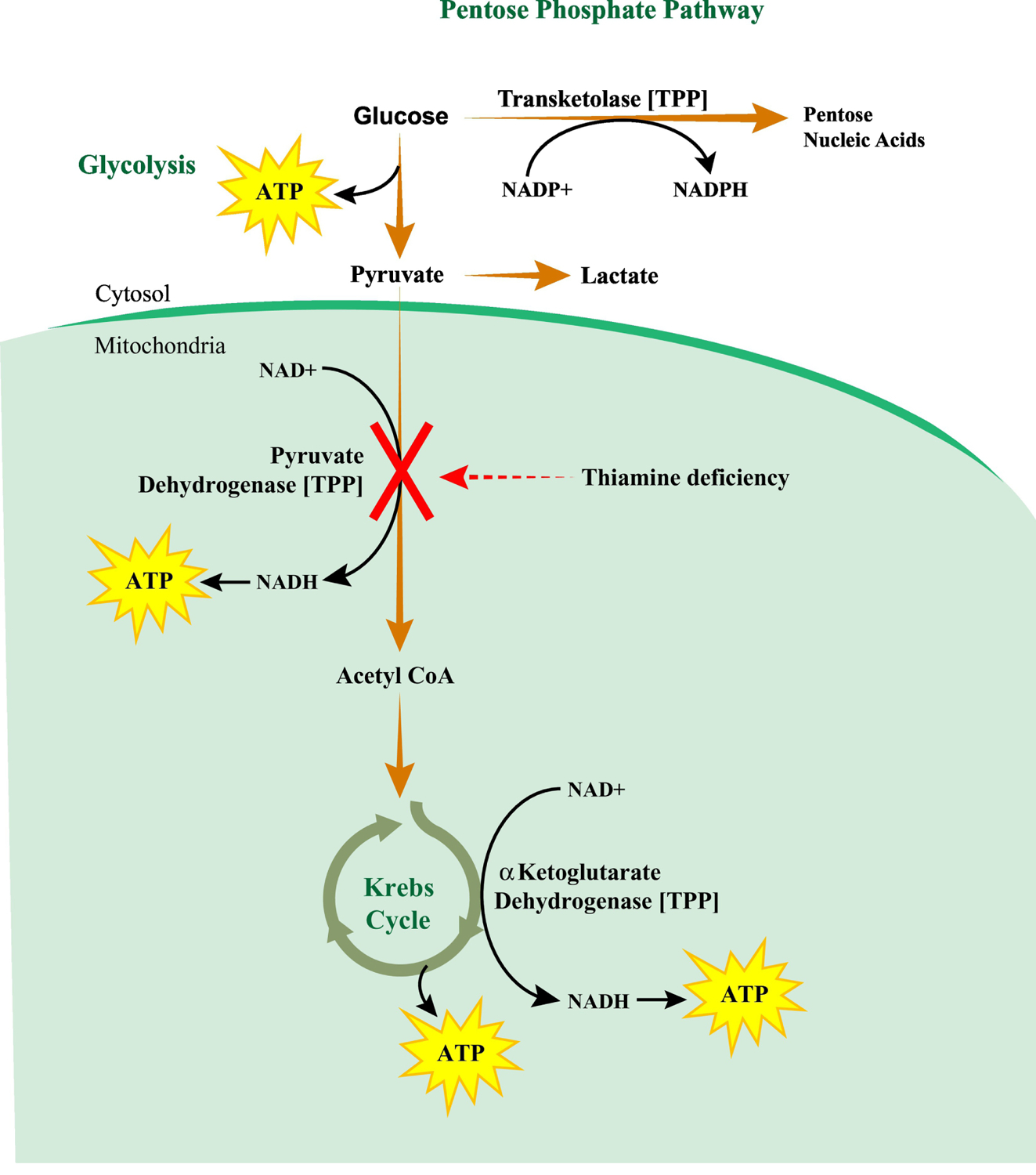
Thiamine in the form of thiamine diphosphate (TPP) is an important cofactor for three key enzymes of the carbohydrate metabolic pathways. The first is transketolase of the pentose phosphate pathway, which converts glucose metabolites to pentose nucleic acids for nucleotide synthesis and generates NADPH for reductive biosynthesis including that of fatty acids, cholesterol, and steroid hormones. The second is pyruvate dehydrogenase in the glycolysis pathway that converts pyruvate to acetyl coenzyme A (acetyl CoA) with subsequent entry into the Krebs cycle. During thiamine deficiency, conversion of pyruvate to acetyl CoA is blocked and pyruvate is converted to lactate. This may result in lactic acidosis that can be reversed with repletion of thiamine. The third enzyme is α-ketoglutarate dehydrogenase, which catalyzes the conversion of α-ketoglutarate to succinyl coenzyme A in the Krebs cycle and produces NADH utilized for ATP synthesis. Thiamine deficiency compromises NADPH, NADH and ATP production as well as reductive biosynthetic reactions, which together ultimately leads to organ dysfunction.
NADP+, oxidized nicotinamide adenine dinucleotide-phosphate; NADPH, reduced nicotinamide adenine dinucleotide-phosphate; NAD+, oxidized nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide
What are the clinical manifestations of chronic thiamine deficiency?
Thiamine deficiency occurs through a largely carbohydrate-based diet with limited vitamin and mineral intake, as well as in patients with history of alcohol abuse, dialysis, diabetic ketoacidosis, chronic diarrhea, and consumption of high doses of diuretics.26, 27 Prolonged thiamine deficiency results in beriberi with cardiovascular symptoms (wet), or neurologic symptoms (dry), or a combination of the two variants. Neurologic deficiencies may present as Wernicke’s encephalopathy with ophthalmologic manifestations and Wernicke-Korsakoff syndrome with the addition of psychiatric symptoms.24, 28, 29
What happens to thiamine in critical illness and what deficiencies acutely result?
Similar to vitamin C, the hypermetabolic state that occurs during critical illness in the adult patient may be associated with the rapid depletion of thiamine stores.1, 3, 4, 24, 26, 29 Thiamine deficiency may also occur due to sudden or aggressive nutrition delivery to malnourished patients (refeeding syndrome) or excessive removal (during prolonged continuous renal replacement therapy [CRRT]).3 Inadequate thiamine levels can lead to cellular metabolic and bioenergetic failure with the inability to generate adenosine triphosphate (ATP) (Figure 2).
The critically ill patient with thiamine deficiencies may not present with the classic cardiac and neurologic syndromes observed in beriberi. Instead, thiamine depletion may contribute to or worsen symptoms of common ICU disorders and lead to blunted responses to standard supportive ICU treatments. Patients may manifest with broad non-specific symptomatology including delirium, hallucinations, encephalopathy, polyneuropathy, high output cardiac failure, and gastrointestinal system dysfunction (i.e. nausea, vomiting, and abdominal pain).24, 29
How are vitamin C and thiamine levels measured?
Vitamin C
The invigorated interest in vitamin C has highlighted the fact that clear measurement guidelines and cut-offs for vitamin C in critically ill patients are absent. This is partly due to the technical challenges involved with the laboratory measurement of vitamin C.15 Vitamin C can be measured in plasma (or serum), leukocytes and urine. Plasma or serum vitamin C measurements reflect metabolic turnover and do not correlate well with tissue vitamin C levels.30 High performance liquid chromatography (HPLC) with electrochemical detection is one of the most reliable and accurate methods for determining “plasma” vitamin C levels compared to older methods using radiolabeled vitamin C.31 A fasting specimen is preferred. The adult fasting reference range of vitamin C is 23–114 µmol/L (0.4–2.0 mg/dl). Values of <11.3 µmol/L (0.2 mg/dl) indicate significant deficiency.32
Unfortunately, most hospital laboratories are unable to measure plasma or leukocyte vitamin C concentrations due to the lack of adequate instrumentation and technical expertise. Therefore, specimens, hopefully collected into the correct tubes, are sent to reference laboratories for analysis with results not readily available for daily practice. Moreover, there is no uniformity of practice regarding the specimen type [plasma (or serum), leukocytes and urine] analyzed by the reference laboratories. Hence, based upon laboratory challenges, it is not surprising that vitamin C therapy in the ICU setting is often based upon the assumption that vitamin C stores are depleted in critical illness.
Of note, vitamin C can potentially affect laboratory tests that rely on oxidation-reduction reactions. High plasma concentrations of vitamin C (15 and 30 mg/dl) may cause false elevations of whole blood glucose measurements with certain point-of-care glucometers (e.g., Roche Accu-Chek).33 False increases in serum sodium, potassium, calcium, and creatinine as well as false decreases in serum chloride, total bilirubin, uric acid, total cholesterol, triglyceride, ammonia, and lactate have been reported with serum vitamin C concentration of 12 mmol/L even on standard laboratory analyzers.34 False negative fecal occult blood testing may also occur with ingestion of vitamin C.35
Thiamine
Thiamine may be measured in plasma (or serum), whole blood, red blood cells and urine. Plasma thiamine contains < 10 % of blood thiamine and reflects recent intake. Whole blood samples contain thiamine diphosphate (TPP), the biologically active form and is the current specimen of choice. HPLC is the most reliable and accurate method for measuring whole blood TPP levels.29, 36, 37 In whole blood, the reference range of TPP is 70–180 nmol/L. Values of < 70 nmol/L are suggestive of thiamine deficiency.38 However, this data may not be directly applicable to critically ill patients because thiamine measurements historically were performed primarily to identify patients at risk of developing Wernicke’s encephalopathy.
Similar to vitamin C, specimens for thiamine analysis in the correct tubes, are sent to reference laboratories. Thus, thiamine results too are not available for daily practice and the decision to treat a patient prophylactically or to make a presumptive or definitive diagnosis of thiamine deficiency is commonly based on clinical judgment.
How are vitamin C and thiamine restored in critical illness?
Vitamin C
In healthy individuals, vitamin C deficiency is readily restored with vitamin C 200 mg/day IV or 500 mg/day orally for seven days. Vitamin C plasma and tissue concentrations are tightly controlled by saturable enteral absorption by SVCT-1 (Figure 1), tissue transport, and renal reabsorption and excretion. In critically ill patients, variations in timing, dose, and administration route of vitamin C plays a role because direct radical scavenging depends on plasma concentrations > 175 mg/L (1,000 mmol/L).39 Oral regimens cannot increase plasma concentrations to normal levels because transported-mediated enteral uptake is rate-limited and possibly impaired in critical illness.40 Thus, intravenous administration is the preferred route in critical illness where levels of vitamin C must be acutely raised.41, 42
It has been suggested that due to increased metabolic demands in critically ill patients with sepsis, restoring low levels of plasma vitamin C (i.e., < 23 µmol/L) may require approximately 6 g/day, a 30-fold higher total daily dose than healthy subjects.18 However, the details of the optimal dosing approach are still being studied. In a recent pharmacokinetic trial, 20 critically ill patients with multiple organ failure were randomized into four-groups to receive either 2 or 10 g/d vitamin C administered as a twice daily 15-min bolus infusion or continuous infusion for 48 h. Two grams per day resulted in normal plasma concentrations (29–50 mg/L), and 10 g/d resulted in “supranormal” concentrations as well as increased oxalate excretion, and metabolic alkalosis.40
Vitamin C should be diluted either in sodium chloride 0.9% or dextrose 5% water prior to administration and should be infused no faster than 250 mg/min. An infusion rate of 33 mg/min has been recommended43 as more rapid infusion may result in faintness, lethargy, flushing, dizziness, and headache.
Addressing vitamin C deficiency in patients receiving renal replacement therapy is complicated. Vitamin C is dialyzable with up to 50% of the vitamin C dose cleared during a 4-hour intermittent hemodialysis session.44 Significant clearance is observed with CRRT. An optimal dosing strategy in patients with renal insufficiency and those requiring CRRT is unknown and requires further study.45
Thiamine
Like vitamin C, thiamine can be administered both enterally and parenterally. IV supplementation is preferred in symptomatic patients and those with impaired absorption due to changes in gastrointestinal structure or function associated with malnutrition and alcoholism. 46, 47 Thiamine dosing recommendations vary because of an absence of consensus on dose, frequency, or duration of therapy.36 Recent literature recommends 200–500 mg IV thiamine every 8 hours on the first day of ICU admission for patients with a chronic alcohol use disorder who have symptoms that may mimic or mask Wernicke’s encephalopathy.48 In the published and ongoing clinical studies of patients with sepsis and septic shock, the dose range of thiamine was lower at 100–200 mg IV thiamine every 6 or 12 hours for 4 days.
Thiamine is renally excreted. Its elimination may be doubled when administered with loop diuretics.49 Like vitamin C, thiamine is also dialyzable with CRRT removing an estimated 4–5 mg of thiamine per day.50 While this clearance warrants supplementation, the best dosing regimen is largely based on expert opinion and requires further study.
Intravenous thiamine is overall well tolerated; however, on occasion patients may experience infusion related hypersensitivity or rarely anaphylaxis. Therefore, parenteral doses of thiamine greater than 100 mg are diluted with 100 ml of 0.9% sodium chloride or dextrose 5% and administered by slow infusion over 30 minutes to minimize the risk of a hypersensitivity reaction.51 Parenteral thiamine doses of 100 mg to 500 mg have not been found to cause toxic effects. There is no established upper limit for the total daily dose.
What do the prior studies of vitamin C and thiamine administered alone in critical illness show?
Vitamin C
Vitamin C alone has been studied in a variety of critical care conditions (sepsis and septic shock, acute respiratory distress syndrome, major burns, trauma, and cardiac surgery) with a variety of dosing regimens (2–10 g/day IV) sometimes described as “low to high” doses by various investigators. Outcome parameters have included vasopressor- and ventilator-free days, organ failure scores, biomarker levels, fluid resuscitation volumes, occurrence of postoperative atrial fibrillation, acute kidney and stroke, risk of infection, ICU and hospital length of stay and mortality.12, 52–56
Four systematic reviews and meta-analyses recently summarized the vitamin C studies in critically ill patients.57–60 A consistent finding in studies of non-cardiac surgery ICU patients with sepsis and/or respiratory failure was that the administration of IV vitamin C alone was associated with a decreased need for vasopressor support, decreased duration of mechanical ventilation and reduced length of ICU stay, but no differences in incidence of AKI, mortality or hospital LOS.
Fowler et al in a recently published multicenter study not included in the above meta-analyses, randomized 167 patients with sepsis and acute lung injury (ALI) present for less than 24 hours to receive IV vitamin C infusion (50 mg/kg) or placebo every 6 hours for 96 hours.61 Of note, 65% of the patients were on corticosteroids at study entry. The investigators did not find any significant differences between the vitamin C and placebo groups in the primary endpoints of change in mean Sequential Organ Failure Assessment (SOFA) score from baseline to 96 hours or in the levels of biomarkers of inflammation (C-reactive protein) and vascular injury (thombomodulin) at 168 hours. However, the patients in the vitamin C group had a lower mortality rate during the first 96 hours (at which time the SOFA score was calculated) compared to the placebo group (~4% vs. ~23%) and more ICU- and hospital-free days.
Overall, the quality and quantity of evidence for the use of vitamin C alone in critically ill patients especially those with sepsis remains inconclusive and warrants further investigation.
Thiamine
Thiamine has commonly been administered empirically in a variety of conditions including sepsis, lactic acidosis, cardiac failure, neurological and psychiatric disorders, delirium, alcoholic withdrawal and refeeding syndrome.24, 27, 29 To our knowledge, there are a few observational studies focused on treating lactic acidosis and reversing shock with inconsistent results62–64 and one RCT that evaluated the treatment of septic shock with thiamine as a single agent. This RCT involved 88 patients with septic shock, who received either thiamine 200 mg IV twice daily or placebo for 7 days or until hospital discharge and showed no difference in lactate levels, shock reversal, or mortality.65 A post-hoc analysis of this study found that patients who received thiamine had decreased requirement for CRRT suggesting thiamine may decrease the risk of AKI in sepsis.66 Overall, the evidence for use of thiamine supplementation to improve outcomes in sepsis is inconclusive.
How did the vitamin C and thiamine cocktail with corticosteroids for sepsis come about?
Experimental studies have shown that both vitamin C and hydrocortisone have multiple and overlapping beneficial pathophysiologic effects in sepsis.5 Vitamin C helps to reverse the oxidation of the glucocorticoid receptor and restore its function.4, 12 Hydrocortisone increases the expression of the sodium-vitamin C transporter-2 (SVCT2) (Figure 1), which actively transports vitamin C into the tissue cells, but is downregulated in sepsis.67 In addition, hydrocortisone works synergistically with vitamin C to inhibit nuclear factor-κB , downregulating the production of proinflammatory mediators; increase tight junctions between endothelial and epithelial cells; preserve endothelial function; increase catecholamine synthesis; and increase vasopressor sensitivity.4, 5, 18, 68 Thiamine is thought to act synergistically with vitamin C and hydrocortisone to promote aerobic metabolism and reverse the mitochondrial dysfunction and oxidative stress observed in sepsis.19, 24, 26 The VCTS regimen is thus hypothesized to provide a multipronged approach to reduce organ dysfunction and improve outcomes in sepsis.
Based on the experimental and emerging clinical data,49, 51 vitamin C pharmacokinetic modeling and the package insert40, and dramatic recovery of three patients with fulminant sepsis at a university medical center, Marik et al described their experience with the vitamin C, thiamine and hydrocortisone (VCTS) regimen in 47 patients with severe sepsis and septic shock in 2017.5 Vitamin C was administered at 1500 mg IV every 6 h (6 g/day), thiamine 200 mg IV every 12 h and hydrocortisone 50 mg IV every 6 h). This combination was found to be effective in preventing progressive organ dysfunction, including AKI, and in reducing mortality. However, this study had several limitations including the small study size, before-and-after study design, single center, and lack of blinding. Nevertheless, the positive outcomes noted by Marik et al were supported by another study of VCTS therapy in 53 critically ill patients with severe pneumonia.69 Although there were baseline imbalances in the control and treatment groups in the latter study, the mortality benefit persisted after propensity-adjustment and propensity-matching. However, in a recent retrospective before-and-after cohort study of 229 septic shock patients who received vitamin C (3 g every 12 h or 1500 mg every 6 h) and thiamine 200 mg every 12 h) within 6 hours of shock recognition and for the initial 24 h resuscitation period, Shin et al failed to show any survival benefit.70
Are there safety concerns with using the vitamin C and thiamine cocktail in combination with corticosteroids?
While vitamin C is largely considered a non-toxic vitamin, safety data is limited for the 6 g/day IV vitamin C doing that is administered as part of the VCTS regimen in the ongoing or completed clinical trials. Known side effects of vitamin C from past studies include gastrointestinal disturbances, hypersensitivity reactions, oxaluria, kidney stones, and glycosuria.71 There were no unexpected adverse events reported in the most recent trial of vitamin C infusions in patients with sepsis and ALI.61 However, large doses of vitamin C should be used with caution in patients with renal impairment and those prone to kidney stones.71 Thiamine minimizes the risk of vitamin C associated calcium oxalate kidney stone formation and oxalate nephropathy.4 Another safety concern pertains to patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency; vitamin C may cause hemolysis in these patients.4, 72
There have been no reported adverse events directly attributed to the use of thiamine in the VCTS studies published to date.5, 69 Corticosteroid use is well known to be associated with hyperglycemia, but this has never been translated to worse or adverse outcomes.
What does the future hold for the VCTS cocktail?
The VCTS cocktail appears to be a promising adjuvant therapy for sepsis. Vitamin C and thiamine have favorable side effect and cost profiles. These vitamins are usually readily available; however, recent occasional shortages of the IV formulations have been reported. As of November 2019, there are 14 completed and/or ongoing clinical trials with the VCTS regimen for the treatment of adult patients with sepsis and septic shock listed at Clinical Trials.gov (Table 1).73 Five of these RCTs involving a total of 1137 patients have completed patient accrual including 3 in the US, 1 in Australia, New Zealand and Brazil, and 1 in China. The primary outcome parameters have included vasopressor- and ventilator-free days, change in organ failure scores, and hospital mortality. A recent fiscal analysis comparing standard sepsis care to the early adoption of the VCTS regimen showed that the VCTS regimen, if proven safe and effective in ongoing high-quality RCTs, has the potential to save billions of dollars and millions of life-years in the US.74
Table 1.
Completed and Ongoing Clinical Trials of Vitamin C, Thiamine, and Corticosteroids in Sepsis and Septic Shock (adult only >18 yrs)*
| Trial name | Centers | Location | #of Subjects | Study design | Entry diagnosis | Primary outcome | Trial identifier |
|---|---|---|---|---|---|---|---|
| Ascorbic acid, Corticosteroids, and Thiamine in Sepsis (ACTS) Trial | Multicenter | US | 200 (C) | Randomized, double-blind, placebo-controlled trial | Sepsis/septic shock | Change in Sequential Organ Failure Assessment (SOFA) score at 72 hours | NCT03389555 |
| Vitamin C, Thiamine and Steroids in Sepsis (VICTAS) | Multicenter | US | 501 (C) | Randomized, placebo-controlled, double-blind, adaptive trial | Sepsis with acute respiratory or cardiovascular organ dysfunction | Vasopressor and ventilator-free days at 30 days after randomization | NCT03509350 |
| Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in Sepsis (ORANGES) | Two centers | NJ, US | 140 (C) | Randomized, double-blind placebo-controlled trial | Sepsis/septic shock | Time to vasopressor independence (hours) through study completion; Change in SOFA score at 4 days post-randomization | NCT03422159 |
| The Vitamin C, Hydrocortisone and Thiamine in Patients with Septic Shock Trial (VITAMINS) | Multicenter | Australia, New Zealand, Brazil | 216 (C) | Randomized, placebo-controlled trial | Sepsis/septic shock | Time alive and free of vasopressors at day 7 (168 hours) after randomization. | NCT03333278 |
| Thiamine, Vitamin C and Hydrocortisone in the Treatment of Septic Shock | Single center | Michigan, US | 60 (T) (R) | Placebo-controlled trial | Septic shock | Mortality rate compared to Marik’s 2017 study5 | NCT03540628 |
| Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Sepsis and Septic Shock (HYVCTTSSS) | Single center | Guangdong, China | 80 (C) | Randomized, placebo-controlled trial | Sepsis/septic shock | Hospital survival at 14 days | NCT03258684 |
| Pilot Study on the Use of Hydrocortisone, Vitamin C and Thiamine in Patient with Sepsis and Septic Shock | Single center | Girona, Spain | 40(T) (R) | Placebo-controlled trial | Septic shock and multiorgan failure | 28-day mortality | NCT04111822 |
| Outcomes of Septic Shock Patients Treated with a Metabolic Resuscitation Bundle Consisting of Intravenous Hydrocortisone, Ascorbic Acid and Thiamine) | Single center | Wisconsin, US | 80 (T) (NR) | Placebo-controlled trial | Sepsis/septic shock | Duration of vasopressors at study conclusion, up to 3 months | NCT03913468 |
| Vitamin C, Thiamine and Hydrocortisone for the Treatment of Septic Shock | Single center | Yangzhou, China | 406 (T) (R) | Placebo-controlled trial | Sepsis/septic shock | 90-day mortality | NCT03872011 |
| Effect of IV Vitamin C, Thiamine, and Steroids on Mortality of Septic Shock | Single center | NY, US | 130(T) (R) | Placebo-controlled trial | Sepsis/septic shock | 30-day mortality | NCT03828929 |
| Evaluation of Hydrocortisone, Vitamin C and Thiamine for the Treatment of Septic Shock (HYVITS) | Single center | Doha, Qatar | 216 (T) (R) | Prospective, randomized, two-arm parallel-group trial | Sepsis/septic shock | 60-day mortality | NCT03380507 |
| Vitamin C, Hydrocortisone and Thiamine for Septic Shock (CORVICTES) | Single center | Athens, Greece | 400 (T) (R) | Placebo-controlled trial | Septic shock | Cerebral autoregulation 24–78 hours after randomization; Cerebral blood flow 24–78 hours after randomization | NCT03592693 |
| Vitamin C, Steroids, and Thiamine, and Cerebral Autoregulation and Functional Outcome in Septic Shock (CORVICTES-ϒM) | Single center | Athens, Greece | 100 (T) (R) | Placebo-controlled trial | Septic shock | Cerebral autoregulation 24–78 hours after randomization; Cerebral blood flow 24–78 hours after randomization | NCT03649633 |
| Therapy With Hydrocortisone, Ascorbic Acid, Thiamine in Patients With Sepsis | Single center | Tanta, Egypt | 80 (T) (NR) | Placebo-controlled trial | Sepsis/septic shock | Change in SOFA score at 28 days and procalcitonin level | NCT04160676 |
C-Completed, T-Target, R-Recruiting, NR-Not Recruiting
Even if the preliminary positive results of the VCTS trials are not replicated, the new RCTs may help provide a better understanding of the possible role of vitamin C and thiamine in critical illness. For instance, one of the trials (Vitamin C, Thiamine, and Steroids in Sepsis, VICTAS), that was completed in October 2019 with 501 patients, will create a biorepository which will be used to characterize vitamin C pharmacokinetics and to measure standard and emerging markers of sepsis severity in general and in the setting of high concentrations of vitamin C.75
However, these trials have potential limitations and may lead to further perplexity because the optimal dosing regimen for vitamin C is not known. Thus, a negative study could be consistent with an ineffective therapy or dosing regimen. It is also unclear how the results of the recently published (Vitamin C Infusion for Treatment in Sepsis Induced Acute Lung Injury, CITRIS-ALI) trial of vitamin C infusion alone in patients with sepsis and acute lung injury61 will impact the continuation of the currently ongoing VCTS studies or their interpretation. The clinical benefit of the VCTS cocktail in sepsis will likely depend on several factors including the patient’s underlying severity of illness, comorbidities and attributable mortality from sepsis being a highly heterogenous clinical syndrome, and the heterogeneity of sepsis treatment (e.g., fluid management, antimicrobial selection, source control, dose and timing of intervention with VCTS) and certainly the dosing ranges and timing of administration of the drugs.
In conclusion, the potential of the VCTS regimen for sepsis has generated a fair amount of interest and hope. However, the question of acceptable effective dosing and measurement techniques for vitamin C and thiamine may become a significant issue if ongoing clinical trials testing vitamin C alone or in combination with thiamine and hydrocortisone in patients with sepsis and septic shock confirm the promising findings of the published studies to date.
Highlights.
Recent studies have generated keen interest in the use of vitamin C, thiamine, and corticosteroids (VCTS) to treat sepsis.
Many questions surround the mechanisms of action, laboratory measurements, monitoring, dosing regimens and side effect profiles of vitamin C and thiamine.
This review aims to explore the current evidence and potential benefits and adverse effects of the VCTS regimen for the treatment of sepsis.
Acknowledgments
We acknowledge the assistance of Susan Weil-Kazzaz, CMI, Manager, Design and Creative Services, Department of Communications, Memorial Sloan Kettering Cancer Center, New York, NY for preparation of the figures.
Financial Support: This study was supported, in part, by the Core Grant (P30 CA008748) and the Department of Anesthesiology and Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Drs. Pastores and Halpern were clinical investigators in the recently concluded Vitamin C, Thiamine and Steroid (VICTAS) sepsis trial. The remaining authors have no conflicts to disclose.
References
- 1.Amrein K, Oudemans-van Straaten HM, Berger MM. Vitamin therapy in critically ill patients: focus on thiamine, vitamin C, and vitamin D. Intensive Care Med 2018;44:1940–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langlois PL, Manzanares W, Adhikari NKJ, et al. et al. Vitamin C administration to the critically ill: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr 2019;43(3):335–46. [DOI] [PubMed] [Google Scholar]
- 3.Mallat J, Lemyze M, Thevenin D. Do not forget to give thiamine to your septic shock patient! J Thorac Dis 2016;8(6):1062–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moskowitz A, Andersen LW, Huang DT, et al. Ascorbic acid, corticosteroids, and thiamine in sepsis: a review of the biologic rationale and the present state of clinical evaluation. Crit Care 2018;22(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest 2017;151(6):1229–38. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter KJ. The discovery of vitamin C. Ann Nutr Metab 2012;61(3):259–64. [DOI] [PubMed] [Google Scholar]
- 7.Nishikimi M, Yagi K. Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am J Clin Nutr 1991;54(6 Suppl):1203S–1028S. [DOI] [PubMed] [Google Scholar]
- 8.Marik PE, Hooper MH. Doctor-your septic patients have scurvy! Crit Care 2018;22(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr 2005;25:105–25. [DOI] [PubMed] [Google Scholar]
- 10.Padayatty SJ, Doppman JL, Chang R, et al. Human adrenal glands secrete vitamin C in response to adrenocorticotrophic hormone. Am J Clin Nutr 2007;86(1):145–9. [DOI] [PubMed] [Google Scholar]
- 11.KC S, Carcamo JM, Golde DW. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J 2005;19(12):1657–67. [DOI] [PubMed] [Google Scholar]
- 12.Oudemans-van Straaten HM, Spoelstra-de Man A, de Waard MC. Vitamin C revisited. Crit Care 2014;18(4):460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu F, Tyml K, Wilson JX. iNOS expression requires NADPH oxidase-dependent redox signaling in microvascular endothelial cells. J Cell Physiol 2008;217(1):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal 2013;19(17):2068–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padayatty SJ, Levine M. Vitamin C physiology: the known and the unknown and Goldilocks. Oral Dis 2016;22(6):463–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey S, Bishayi B. Killing of S. aureus in murine peritoneal macrophages by Ascorbic acid along with antibiotics Chloramphenicol or Ofloxacin: Correlation with inflammation. Microbial Pathogenesis 2018; 115:239–50. [DOI] [PubMed] [Google Scholar]
- 17.Gao YL, Lu B, Zhai JH, et al. The parenteral vitamin C improves sepsis and sepsis-induced multiple organ dysfunction syndrome via preventing cellular immunosuppression. Mediators Inflamm 2017; 2017:4024672. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Carr AC, Rosengrave PC, Bayer S, et al. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care 2017;21:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marik PE. Hydrocortisone, ascorbic acid and thiamine (HAT Therapy) for the treatment of sepsis. Focus on ascorbic acid. Nutrients 2018;10(11):1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki U, Shimamura T. Active constituent of rice grits preventing bird polyneuritis. Tokyo Kagaku Kaishi 1911; 32: 4–7, 144–146, 335–358. [Google Scholar]
- 21.Jansen BCT, Donath WF. On the isolation of the antiberiberi vitamin. Proceedings of the Koninklijke Nederlandse Academie van Wetenschappen 1926;29:1390–1400. [Google Scholar]
- 22.Lonsdale D. Thiamin. Adv Food Nutr Res 2018;83:1–56. [DOI] [PubMed] [Google Scholar]
- 23.Rindi G, Laforenza U. Thiamine intestinal transport and related issues: recent aspects. Proc Soc Exp Biol Med 2000; 224: 246–55. [DOI] [PubMed] [Google Scholar]
- 24.Manzanares W, Hardy G. Thiamine supplementation in the critically ill. Curr Opin Clin Nutr Metab Care 2011;14:610–17. [DOI] [PubMed] [Google Scholar]
- 25.Calderon-Ospina CA, Nava-Mesa MO. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci Ther 2019;00:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Counts JP, Rivera VF, Kimmons LA, Jones GM. Thiamine Use in Sepsis: B1 for Everyone? Crit Care Nurs Q 2019. Jul/Sep;42(3):292–303 [DOI] [PubMed] [Google Scholar]
- 27.Moskowitz A, Graver A, Giberson T, et al. The relationship between lactate and thiamine levels in patients with diabetic ketoacidosis. J Crit Care 2014;29:182.e5–182.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha S, Kataria A, Kolla BP, et al. Wernicke encephalopathy – clinical pearls. Mayo Clin Proc 2019;94(6):1065–72. [DOI] [PubMed] [Google Scholar]
- 29.Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci 2018;356(4):382–90. [DOI] [PubMed] [Google Scholar]
- 30.Lykkesfeldt J. Determination of ascorbic acid and dehydroascorbic acid in biological samples by high-performance liquid chromatography using subtraction methods: reliable reduction with tris[2-carboxyethyl] phosphine hydrochloride. Anal. Biochem 2000;282:89–93. [DOI] [PubMed] [Google Scholar]
- 31.Behrens WA, Madere RA. Highly sensitive high-performance liquid-chromatography method for the estimation of ascorbic and dehydroascorbic acid in tissues, biological fluids and foods. Anal. Biochem 1987; 165: 102–107. [DOI] [PubMed] [Google Scholar]
- 32.Mayo Medical Laboratories: Ascorbic acid (Vitamin C), Plasma Available at: https://www.mayomedicallaboratories.com/test-catalog/Overview/42362. Accessed October 1, 2019
- 33.Cho J, Ahn S, Yim J, et al. Influence of vitamin C and maltose on the accuracy of three models of glucose meters. Ann Lab Med 2016; 36(3): 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng QH, Irwin WC, Fesser J, Massey KL. Interference of ascorbic acid with chemical analytes. Ann Clin Biochem 2005. November;42(Pt 6):475–7. [DOI] [PubMed] [Google Scholar]
- 35.Jaffe RM, Kasten B, Young DS, MacLowry JD. False-negative stool occult blood tests caused by ingestion of ascorbic acid (vitamin C). Ann Intern Med 1975;83(6):824–826. [DOI] [PubMed] [Google Scholar]
- 36.Collie JTB, Greaves RF, Jones OAH, et al. Vitamin B1 in critically ill patients: needs and challenges. Clin Chem Lab Med 2017;55(11):1652–68. [DOI] [PubMed] [Google Scholar]
- 37.Ihara H, Matsumoto T, Shino Y, Hashizume N. Assay values for thiamine or thiamine phosphate esters in whole blood do not depend on the anticoagulant used. J Clin Lab Anal 2005;19:205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayo Medical Laboratories: Thiamin (Vitamin B1), Whole Blood Available at: http://www.mayomedicallaboratories.com/test-catalog/print/81019. Accessed October 1, 2019.
- 39.Jackson TS, Xu A, Vita JA, Keaney JF Jr. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res 1998;83(9):916–22. [DOI] [PubMed] [Google Scholar]
- 40.de Grooth HJ, Manubulu-Choo WP, Zandvliet AS, et al. Vitamin C pharmacokinetics in critically ill patients: a randomized trial of four IV regimens. Chest 2018;153(6):1368–1377. [DOI] [PubMed] [Google Scholar]
- 41.Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr 2011;2(2):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padayatty SJ, Sun H, Wang Y, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 2004;140(7):533–7. [DOI] [PubMed] [Google Scholar]
- 43.Ascor (ascorbic acid injection) for intravenous use Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209112s000lbl.pdf Accessed October 18, 2019.
- 44.Fehrman-Ekholm I, Lotsander A, Logan K, et al. Concentrations of vitamin C, vitamin B12 and folic acid in patients treated with hemodialysis and on-line hemodiafiltration or hemofiltration. Scand J Urol Nephrol 2008;42:74–80. [DOI] [PubMed] [Google Scholar]
- 45.Honore PM, De Bels D, Kugener L, et al. Dosing adjuvant vitamin C in critically ill patients undergoing continuous renal replacement therapy: We are not there yet! Crit Care 2019;23(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank LL. Thiamin in Clinical Practice. JPEN J Parenter Enteral Nutr 2015;39(5):503–20. [DOI] [PubMed] [Google Scholar]
- 47.Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke–Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev 2013;(7):CD004033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the Banana Bag: Evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med 2016; 44:1545–52. [DOI] [PubMed] [Google Scholar]
- 49.Rieck J, Halkin H, Almog S, et al. Urinary loss of thiamine is increased by low doses of furosemide in healthy volunteers. J Lab Clin Med 1999;134(3):238–43. [DOI] [PubMed] [Google Scholar]
- 50.Honoré PM, De Waele E, Jacobs R, et al. Nutritional and metabolic alterations during continuous renal replacement therapy. Blood Purif 2013;35(4):279–84. [DOI] [PubMed] [Google Scholar]
- 51.Thiamine. In: IBM Micromedex® DRUGDEX® (electronic version) IBM Watson Health, Greenwood Village, Colorado, USA. Available at: https://www.micromedexsolutions.com Accessed October 1, 2019. [Google Scholar]
- 52.Tanaka H, Matsuda T, Miyagantani Y, et al. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg 2000;135(3):326–331. [DOI] [PubMed] [Google Scholar]
- 53.Nathens AB, Neff MJ, Jurkovich GJ, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg 2002;236(6):814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fowler AA 3rd, Syed AA, Knowlson S, et al. ; Medical Respiratory Intensive Care Unit Nursing: Phase 1 safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med 2014;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zabet MH, Mohammadi M, Ramezani M, Khalili H. Effect of high-dose ascorbic acid on vasopressor’s requirement in septic shock. J Res Pharm Pract 2016;5(2):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahn JH, Oh DK, Huh JW, et al. Vitamin C alone does not improve treatment outcomes in mechanically ventilated patients with severe sepsis or septic shock: a retrospective cohort study. J Thorac Dis 2019;11(4):1562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang M, Jativa DF. Vitamin C supplementation in the critically ill: a systematic review and meta-analysis. SAGE Open Med 2018;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hemilä H, Chalker E. Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients 2019;11:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Putzu A, Daems AM, Lopez-Delgado JC, et al. The effect of vitamin C on clinical outcome in critically ill patients: a systematic review with meta-analysis of randomized controlled trials. Crit Care Med 2019;47(6):774–83. [DOI] [PubMed] [Google Scholar]
- 60.Langlois PL, Manzanares W, Adhikari NKJ, et al. Vitamin C administration to the critically ill: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr 2019;43(3):335–346. [DOI] [PubMed] [Google Scholar]
- 61.Fowler AA, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure. JAMA 2019;322(13):1261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donnino MW, Carney E, Cocchi MN, et al. Thiamine deficiency in critically ill patients with sepsis. J Crit Care 2010;25(4):576–81. [DOI] [PubMed] [Google Scholar]
- 63.Woolum JA, Abner EL, Kelly A, et al. Effect of thiamine administration on lactate clearance and mortality in patients with septic shock. Crit Care Med 2018;46(11):1747–52. [DOI] [PubMed] [Google Scholar]
- 64.Corcoran T, O’ Neill MP, Webb SA, Ho KM. Prevalence of vitamin deficiencies on admission: relationship to hospital mortality in critically ill patients. Anaesth Intensive Care 2009;37(5):740–7. [DOI] [PubMed] [Google Scholar]
- 65.Donnino MW, Andersen LW, Chase M, et al. Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Crit Care Med 2016;44(2):360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moskowitz A, Andersen LW, Cocchi MN, et al. Thiamine as a renal protective agent in septic shock. a secondary analysis of a randomized, double-blind, placebo-controlled trial. Ann Am Thorac Soc 2017;14(5):737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burzle M, Hediger MA. Functional and physiological role of vitamin C transporters. Curr Top Membr 2012;70:357–5. [DOI] [PubMed] [Google Scholar]
- 68.Annane D, Pastores SM, Arlt W, et al. Critical Illness-Related Corticosteroid Insufficiency (CIRCI): A Narrative Review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Crit Care Med 2017;45(12):2089–98. [DOI] [PubMed] [Google Scholar]
- 69.Kim WY, Jo EJ, Eom JS, et al. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: propensity score-based analysis of a before-after cohort study. J Crit Care 2018;47:211–18. [DOI] [PubMed] [Google Scholar]
- 70.Shin TG, Kim YJ, Ryoo SM, et al. Early Vitamin C and thiamine administration to patients with septic shock in emergency departments: propensity score-based analysis of a before-and-after cohort study. J Clin Med 2019;8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khoshnam-Rad N, Khalili H. Safety of vitamin C in sepsis: a neglected topic. Curr Opin Crit Care 2019;25(4):329–33. [DOI] [PubMed] [Google Scholar]
- 72.Mehta JB, Singhal SB, Mehta BC. Ascorbic acid-induced haemolysis in G-6-PD deficiency. Lancet 1990;336:944. [DOI] [PubMed] [Google Scholar]
- 73.Vitamin C studies in sepsis Available at: https://clinicaltrials.gov/ct2/results?cond=sepsis&term=vitamin+C&cntry=&state=&city=&dist= Accessed November 27, 2019.
- 74.Blythe R, Cook D, Graves N. Scepticaemia: The impact on the health system and patients of delaying new treatments with uncertain evidence; a case study of the sepsis bundle. F1000Res 2018;7:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hager DN, Hooper MH, Bernard GR, et al. The Vitamin C, Thiamine and Steroids in Sepsis (VICTAS) Protocol: a prospective, multi-center, double-blind, adaptive sample size, randomized, placebo-controlled, clinical trial. Trials 2019;20(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]


