Abstract
Purpose
The purpose of this study was to develop and validate a nomogram to better assess the 2-year risk of non-alcoholic fatty liver disease (NAFLD) in non-obese population with normal blood lipid levels.
Patients and Methods
This study was a secondary analysis of a prospective study. We included 3659 non-obese adults with normal blood lipid levels without NAFLD at baseline. A total of 2744 participants were included in the development cohort and 915 participants were included in the validation cohort. The least absolute contraction selection operator (LASSO) regression model was used to identify the best risk factors. Multivariate Cox regression analysis was used to construct the prediction model. The performance of the prediction model was assessed using Harrell’s consistency index (C-index), area under the receiver operating characteristic (AUROC) curve and calibration curve. Decision curve analysis was applied to evaluate the clinical usefulness of the prediction model.
Results
After LASSO regression analysis and multivariate Cox regression analysis on the development cohort, BMI, TG, DBIL, ALT and GGT were found to be risk predictors and were integrated into the nomogram. The C-index of development cohort and validation cohort was 0.819 (95% CI, 0.798 to 0.840) and 0.815 (95% CI, 0.781 to 0.849), respectively. The AUROC of 2-year NAFLD risk in the development cohort and validation cohort was 0.831 (95% CI, 0.811 to 0.851) and 0.797 (95% CI, 0.765 to 0.829), respectively. From calibration curves, the nomogram showed a good agreement between predicted and actual probabilities. The decision curve analysis indicated that application of the nomogram is more effective than the intervention-for-all-patients scheme.
Conclusion
We developed and validated a nomogram for predicting 2-year risk of NAFLD in the non-obese population with normal blood lipid levels.
Keywords: non-alcoholic fatty liver disease, normal blood lipid levels, non-obese, nomogram
Introduction
Non-alcoholic fatty liver disease (NAFLD), also known as metabolic associated fatty liver disease (MAFLD), is the result of the accumulation of fat in the liver in the absence of large amounts of alcohol and any secondary causes.1 Although the international consensus statement on the renaming of NAFLD to MAFLD was published in authoritative journals in 2020 in the field of international gastrointestinal liver disease, the recognition of the new nomenclature of MAFLD still needs to be improved, so the widely accepted nomenclature of NAFLD is still used in this study.2,3 NAFLD is recognized as the leading cause of liver-related morbidity and mortality, with mortality being caused by cirrhosis.4 A recent meta-analysis reported that a relatively high prevalence of NAFLD was found in the Asian population (27%).5 In recent years, the prevalence of NAFLD in the general population in China has ranged from 25% to 44%.6,7 The effects of NAFLD are not confined to the liver, it has adverse effects on many organs/systems throughout the body to varying degrees.8 Several studies have highlighted that NAFLD should be considered not only as a specific disease of the liver, but also as an early mediator of multisystem disease.8,9 In addition, there is growing evidence that NAFLD is associated with an increased risk of hypertension, diabetes and cardiovascular disease, and is independent of traditional cardiovascular risk factors.10 The global epidemic of obesity is a well-known risk factor for the development of NAFLD.11 However, many non-obese patients are still diagnosed with NAFLD in clinical work, particularly in Asia. Previous epidemiological studies have shown that 8–19% of NAFLD patients in Asia are not obese.11 Moreover, dyslipidemia is a key factor in NAFLD.12 A considerable number of non-obese NAFLD patients have normal blood lipid levels.13 To date, most studies on the relationship between NAFLD and risk factors have focused on obesity and dyslipidemia, and not enough attention has been paid to the occurrence of NAFLD in the non-obese population with normal blood lipid levels.
As a debilitating chronic epidemic, a core component of NAFLD prevention strategies is the identification of people at risk for NAFLD.4 Numerous studies have shown that lifestyle changes and early pharmacological intervention can prevent or delay the onset of NAFLD in adults.14,15 Therefore, it is important to investigate the risk factors for NAFLD in the non-obese population with normal blood lipid levels, and to find a reliable, simple and accurate screening tool to accurately assess those at high risk for NAFLD in the non-obese population with normal blood lipid levels. This will facilitate the effective implementation of NAFLD prevention programs in the non-obese population with normal blood lipid levels.
The nomogram is an intuitive graphical prediction model that provides an accurate, personalized prediction of risk for each individual.16 Although several nomograms have been established for NAFLD, to our knowledge, a nomogram that predicts the 2-year risk of NAFLD in the non-obese population with normal blood lipid levels has not been reported. Therefore, in this study, we developed and validated a nomogram based on independent predictors to better assess the 2-year risk of NAFLD in the non-obese population with normal blood lipid levels.
Materials and Methods
Data Source
We have downloaded the raw data uploaded by Sun et al17 from the public database named “DATADRYAD” (https://doi.org/10.5061/dryad.1n6c4) for free. Since Sun et al have granted the Dryad website ownership of the raw data, we were able to use them for secondary analysis without infringing on the author’s rights.
Variable Collection
The following variables included in the database were extracted: age, gender, diastolic blood pressure (DBP) and systolic blood pressure (SBP), body mass index (BMI), fasting blood glucose (FPG), creatinine (Cr), uric acid (UA), blood urea nitrogen (BUN), γ-glutamyltranspeptidase (GGT), alanine aminotransferase (ALT), high-density lipoprotein-cholesterol (HDL-C), aspartate aminotransferase (AST), globulin (GLB), triglyceride (TG), low-density lipoprotein-cholesterol (LDL-C), albumin (ALB), total protein (TP), total bilirubin (TB), total cholesterol (TC), alkaline phosphatase (ALP), direct bilirubin (DBIL), follow-up duration, incident NAFLD.
Study Design & Population
In brief, this was a prospective cohort study. In the original cohort study, a total of 33,153 participants initially NAFLD-free participants who attended annual health check-ups at the Wenzhou Medical Center of Wenzhou People’s Hospital over a 5-year period (January 2010 to December 2014) were recruited. The following exclusion criteria were developed by Dan-Qin Sun and her collaborators: (1) excessive alcohol consumption (>140 g/week for male and 70 g/week for female); (2) any known cause of chronic liver disease, such as viral or autoimmune hepatitis; (3) BMI ≥ 25 kg/m2; (4) high LDL-C levels (>3.12 mmol/L); (5) those taking antihypertensive, antidiabetic or lipid-lowering medications; (6) those lost to follow-up or with missing data. More stringent exclusion criteria were developed based on the original exclusion criteria. Participants were not included in this study if any of the following conditions were met. (1) dyslipidemia [HDL-C <1. 04 mmol/L, LDL-C >3.12 mmol/L, TC >5.2 mmol/L, TG >1.7 mmol/L]; (2) missing blood pressure values, FPG, ALP, GGT ALT, AST, TP, ALB, GLB, TB, and DBIL. Ultimately, a total of 3659 non-obese people with normal blood lipid levels who were initially free of NAFLD were enrolled and completed a 5-year follow-up examination. The follow-up endpoint was the incidence of NAFLD. Subjects were assessed at least once a year during the follow-up period.
Data Acquisition
As mentioned earlier, all participants were checked by trained medical staff during the baseline check, and standardized self-filled electronic forms were used to record basic clinical data such as gender, age, height, and weight. In a quiet environment, the participants sat and measured their blood pressure with an automatic sphygmomanometer. Venous blood was drawn after an overnight fast and analysed using an automated biochemical analyser (Abbott AxSYM) operated by trained medical staff and haematological parameters were collected. BMI was calculated as the ratio of weight (kg) to height (m2).
Diagnosis of NAFLD
The ultrasound diagnostic criteria of NAFLD refer to the standards proposed by the Chinese Society of Hepatology.18 NAFLD is defined as diffusely enhanced near-field echogenicity of the liver (stronger than the renal and splenic regions) with progressively weaker far-field echogenicity and must be combined with one of the following. (1) indistinct intrahepatic striatal structures; (2) mild to moderate hepatomegaly with blunted borders; (3) indistinct or incomplete right hepatic lobe and diaphragmatic capsule; and (4) reduced blood flow signal but normal blood flow distribution. Two experienced imaging specialists, blinded to the subject’s history and study design, independently assessed the ultrasound images. Disagreements between the two imaging specialists were resolved by discussion with a third independent imaging specialist.
Ethical Approval
As the data were de-identified, the requirement for informed consent was waived. As this study is a secondary analysis of existing anonymised data, no separate ethical approval was required for this study. This study followed the Declaration of Helsinki.
Statistical Analyses
The study is consistent with the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement.19
We followed the methods of Cai et al 2021.20 To improve the robustness and reliability of our conclusions, 3659 NAFLD patients were randomly divided into a development cohort with 2744 participants and a validation cohort with 915 participants at a ratio of 7.5: 2.5 using R caret package, which met the theoretical ratio of 3: 1. The types of data for this study are divided into measured data, non-normally distributed measured data and count data. Measurement data were expressed as mean and standard deviation. For non-normally distributed measurement data, the median and quartiles [M (P25, P75)] were used. For count data, it is expressed as a percentage (%). Penalized regression models were developed for all risk variable coefficients and the best predictive risk factors were selected using the least absolute shrinkage and selection operator (LASSO) method. The risk variables selected in the LASSO regression model were then analyzed using multivariate Cox proportional risk regression to further optimize the screening of independent risk factors. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated. Finally, a prediction model is given by constructing a nomogram based on the results of multivariate Cox proportional risk regression. The Harrell consistency index (C-index) and the receiver operating characteristic (ROC) curve were used to evaluate the discrimination ability. To evaluate the agreement between the predicted probabilities and the actual observed probabilities, calibration curves were drawn based on the relationship between predicted probabilities and actual outcomes to graphically depict the calibration of the prediction model. Decision curve analysis (DCA) was used to evaluate the clinical utility of the nomogram model by quantifying the standardized net benefits under different threshold probabilities. C-index, AUROC and calibration curves were analyzed by bootstrapping (1000 bootstrap resamples) to reduce overfitting bias.
All statistical analyses were performed by R software (Version 3.6.3; https://www.R-project.org). All statistical tests were two-sided and, P values less than 0.05 were statistically significant.
Results
Baseline Characteristics of Participants
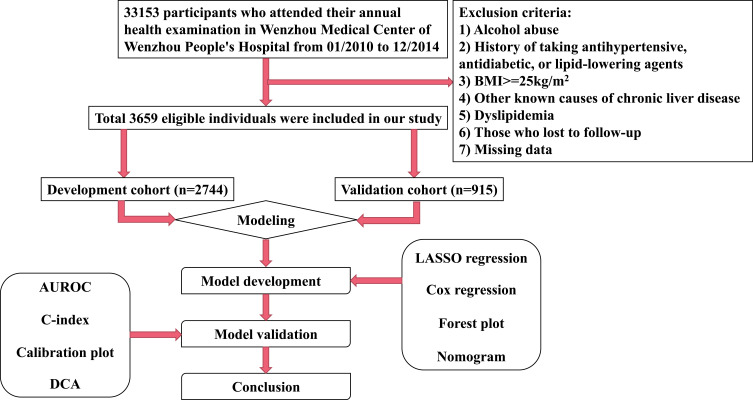
A total of 3659 participants who met the inclusion and exclusion criteria participated in the study, of which 2744 were in the development cohort and 915 in the validation cohort. The flow chart of the study design is shown in Figure 1. The overall incidence of NAFLD was 12.49% (457/3659). In the development cohort, the mean age was 44.83 years; 1519 (55.36%) male and 12.50% of participants (n=343) were diagnosed with NAFLD at the end of follow-up. In the validation cohort, the mean age was 44.57 years; 491 (53.66%) male and 12.46% of participants (n=114) were diagnosed with NAFLD at the end of follow-up. The median follow-up time for the development and validation cohorts was 721 days (quartiles: 658–1063) and 715 days (quartiles: 656–1048), respectively. There were no significant differences in baseline characteristics between the development and validation cohorts (Table 1). Baseline characteristics stratified by the incidence of NAFLD are shown in Table 2.
Figure 1.
Flow chart of the study design.
Table 1.
Characteristics of the Development Cohort and Validation Cohort
| Variables | Total | Development Cohort | Validation Cohort | P value |
|---|---|---|---|---|
| No. of participants | 3659 | 2744 | 915 | |
| Age (years) | 44.76 ± 15.55 | 44.83 ± 15.57 | 44.57 ± 15.50 | 0.671 |
| Gender (n, %) | 0.372 | |||
| Female | 1649 (45.07%) | 1225 (44.64%) | 424 (46.34%) | |
| Male | 2010 (54.93%) | 1519 (55.36%) | 491 (53.66%) | |
| DBP (mmHg) | 72.98 ± 10.28 | 73.12 ± 10.29 | 72.54 ± 10.24 | 0.137 |
| SBP (mmHg) | 121.94 ± 17.59 | 122.29 ± 17.75 | 120.90 ± 17.07 | 0.062 |
| BMI (kg/m2) | 21.35 ± 2.06 | 21.37 ± 2.03 | 21.31 ± 2.14 | 0.488 |
| LDL-c (mmol/L) | 2.14 ± 0.43 | 2.14 ± 0.43 | 2.11 ± 0.43 | 0.032 |
| HDL-c (mmol/L) | 1.52 ± 0.30 | 1.51 ± 0.29 | 1.53 ± 0.31 | 0.104 |
| TG (mmol/L) | 1.01 ± 0.30 | 1.01 ± 0.30 | 1.00 ± 0.30 | 0.425 |
| TC (mmol/L) | 4.34 ± 0.54 | 4.35 ± 0.54 | 4.32 ± 0.53 | 0.195 |
| FPG (mmol/L) | 5.23 ± 0.82 | 5.24 ± 0.83 | 5.19 ± 0.82 | 0.101 |
| UA (μmol/L) | 272.72 ± 84.69 | 274.36 ± 83.22 | 267.79 ± 88.82 | 0.042 |
| Cr (μmol/L) | 83.27 ± 30.84 | 83.43 ± 31.34 | 82.79 ± 29.30 | 0.588 |
| BUN (mmol/L) | 4.62 ± 1.54 | 4.65 ± 1.52 | 4.56 ± 1.59 | 0.129 |
| DBIL (mmol/L) | 2.33 ± 1.20 | 2.34 ± 1.20 | 2.31 ± 1.19 | 0.507 |
| TB (mmol/L) | 12.23 ± 5.00 | 12.25 ± 5.00 | 12.17 ± 4.98 | 0.699 |
| GLB (U/L) | 29.31 ± 4.15 | 29.32 ± 4.21 | 29.30 ± 3.96 | 0.916 |
| ALB (U/L) | 44.30 ± 2.75 | 44.31 ± 2.74 | 44.26 ± 2.78 | 0.588 |
| TP (U/L) | 73.61 ± 4.36 | 73.63 ± 4.37 | 73.56 ± 4.31 | 0.659 |
| AST (U/L) | 22.73 ± 9.38 | 22.68 ± 9.50 | 22.86 ± 9.02 | 0.621 |
| ALT (U/L) | 16.00 (12.00–21.00) | 16.00 (12.00–21.00) | 16.00 (12.00–22.00) | 0.605 |
| GGT (U/L) | 20.00 (15.00–27.00) | 20.00 (16.00–27.00) | 20.00 (15.00–28.00) | 0.121 |
| ALP (U/L) | 70.73 ± 22.75 | 70.86 ± 23.12 | 70.35 ± 21.60 | 0.559 |
| Follow-up (days) | 720.00 (658.00–1061.00) | 721.00 (658.00–1063.00) | 715.00 (656.00–1048.00) | 0.121 |
| Incident NAFLD (n, %) | 0.974 | |||
| No | 3202 (87.51%) | 2401 (87.50%) | 801 (87.54%) | |
| Yes | 457 (12.49%) | 343 (12.50%) | 114 (12.46%) |
Notes: Data are n (%), mean ± SD or median (interquartile range).
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ALP, alkaline phosphatase; GGT, γ-glutamyltranspeptidase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein; ALB, albumin; GLB, globulin; TB, total bilirubin; DBIL, direct bilirubin; BUN, blood urea nitrogen; Cr, creatinine; UA, uric acid; FPG, fasting plasma glucose; TC, total cholesterol; TG, triglyceride; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; NAFLD, non-alcoholic fatty liver disease.
Table 2.
Baseline Characteristics of NAFLD and Non-NAFLD Patients in Cohort
| Variables | Non-NAFLD | NAFLD | P value |
|---|---|---|---|
| No. of participants | 3202 | 457 | |
| Age (years) | 44.55 ± 15.57 | 46.25 ± 15.33 | 0.029 |
| Gender (n, %) | 0.684 | ||
| Female | 1439 (44.94%) | 210 (45.95%) | |
| Male | 1763 (55.06%) | 247 (54.05%) | |
| DBP (mmHg) | 72.38 ± 10.19 | 77.16 ± 10.00 | <0.001 |
| SBP (mmHg) | 121.04 ± 17.57 | 128.26 ± 16.40 | <0.001 |
| BMI (kg/m2) | 21.11 ± 2.02 | 23.09 ± 1.33 | <0.001 |
| LDL-c (mmol/L) | 2.11 ± 0.42 | 2.28 ± 0.44 | <0.001 |
| HDL-c (mmol/L) | 1.53 ± 0.30 | 1.38 ± 0.25 | <0.001 |
| TG (mmol/L) | 0.98 ± 0.30 | 1.18 ± 0.29 | <0.001 |
| TC (mmol/L) | 4.33 ± 0.54 | 4.43 ± 0.53 | <0.001 |
| FPG (mmol/L) | 5.18 ± 0.77 | 5.54 ± 1.06 | <0.001 |
| UA (μmol/L) | 268.21 ± 84.24 | 304.37 ± 81.12 | <0.001 |
| Cr (μmol/L) | 82.65 ± 32.11 | 87.65 ± 19.14 | 0.001 |
| BUN (mmol/L) | 4.60 ± 1.55 | 4.76 ± 1.47 | 0.037 |
| DBIL (mmol/L) | 2.36 ± 1.23 | 2.08 ± 0.92 | <0.001 |
| TB (mmol/L) | 12.23 ± 5.02 | 12.18 ± 4.81 | 0.828 |
| GLB (U/L) | 29.26 ± 4.13 | 29.66 ± 4.23 | 0.055 |
| ALB (U/L) | 44.30 ± 2.75 | 44.26 ± 2.71 | 0.759 |
| TP (U/L) | 73.57 ± 4.36 | 73.92 ± 4.37 | 0.103 |
| AST (U/L) | 22.50 ± 9.56 | 24.36 ± 7.83 | <0.001 |
| ALT (U/L) | 15.00 (12.00–20.00) | 20.00 (16.00–27.00) | <0.001 |
| GGT (U/L) | 19.00 (15.00–26.00) | 26.00 (20.00–37.00) | <0.001 |
| ALP (U/L) | 69.87 ± 22.55 | 76.73 ± 23.22 | <0.001 |
| Follow-up (days) | 722.00 (666.00–1066.00) | 696.00 (371.00–784.00) | <0.001 |
Notes: Data are n (%), mean ± SD or median (interquartile range).
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ALP, alkaline phosphatase; GGT, γ-glutamyltranspeptidase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein; ALB, albumin; GLB, globulin; TB, total bilirubin; DBIL, direct bilirubin; BUN, blood urea nitrogen; Cr, creatinine; UA, uric acid; FPG, fasting plasma glucose; TC, total cholesterol; TG, triglyceride; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; NAFLD, non-alcoholic fatty liver disease.
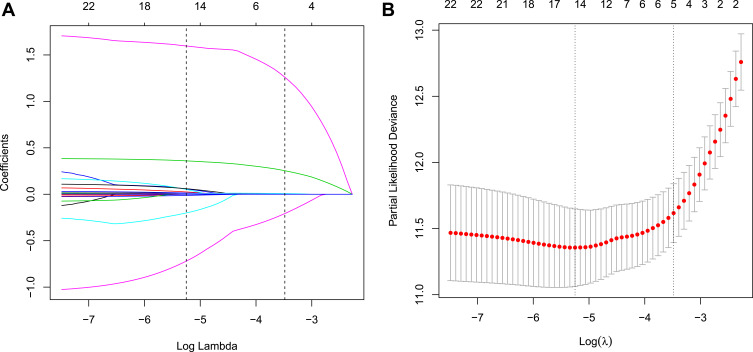
Risk Features Selection by LASSO Regression in the Development Cohort
Five non-zero coefficient characteristics were obtained from the LASSO regression analysis, indicating that we reduced the number of potential variables from 22 to 5, including BMI, TG, DBIL, ALT and GGT. The correlations between the LASSO’s lambda and regression coefficients are shown in Figure 2A and B. The detailed parameters of the characteristics in the LASSO regression model are shown in Table 3.
Figure 2.
Texture feature selection using the LASSO regression model. (A) 10-fold cross-validation via minimum criteria was applied for optimal parameter selection through LASSO model. Partial likelihood deviance curve was schemed versus log(λ). Dotted vertical lines were drawn at the optimal values by using the minimum criteria and 1 SE of the minimum criteria. (B) LASSO coefficient profiles of all the clinical features. A coefficient profile plot was produced against the log(λ) sequence.
Abbreviations: LASSO, least absolute shrinkage and selection operator; SE, standard error.
Table 3.
Coefficients and Lambda.1se Value of the LASSO Regression Model Based on the Development Cohort
| Factors | Coefficients | Lambda.1se |
|---|---|---|
| Age (years) | 0 | −3.483 |
| Gender (n, %) | 0 | |
| DBP (mmHg) | 0 | |
| SBP (mmHg) | 0 | |
| BMI (kg/m2) | 0.253 | |
| LDL-c (mmol/L) | 0 | |
| HDL-c (mmol/L) | 0 | |
| TG (mmol/L) | 1.260 | |
| TC (mmol/L) | 0 | |
| FPG (mmol/L) | 0 | |
| UA (μmol/L) | 0 | |
| Cr (μmol/L) | 0 | |
| BUN (mmol/L) | 0 | |
| DBIL (mmol/L) | −0.208 | |
| TB (mmol/L) | 0 | |
| GLB (U/L) | 0 | |
| ALB (U/L) | 0 | |
| TP (U/L) | 0 | |
| AST (U/L) | 0 | |
| ALT (U/L) | 0.007 | |
| GGT (U/L) | 0.001 | |
| ALP (U/L) | 0 |
Abbreviations: LASSO, least absolute shrinkage and selection operator; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ALP, alkaline phosphatase; GGT, γ-glutamyltranspeptidase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein; ALB, albumin; GLB, globulin; TB, total bilirubin; DBIL, direct bilirubin; BUN, blood urea nitrogen; Cr, creatinine; UA, uric acid; FPG, fasting plasma glucose; TC, total cholesterol; TG, triglyceride; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; NAFLD, non-alcoholic fatty liver disease.
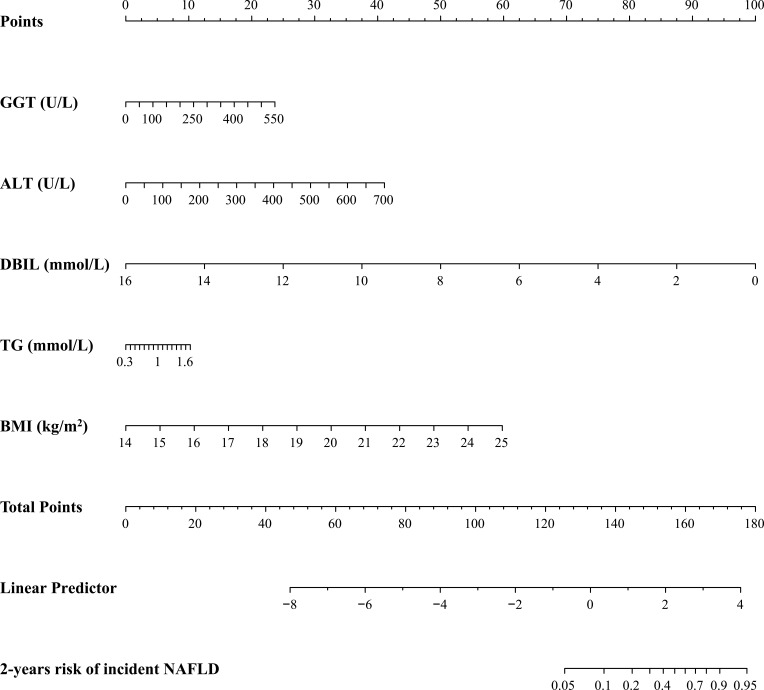
Prediction Model Development
The results of the multivariate Cox regression analysis for BMI, TG, DBIL, ALT and GGT are shown in Table 4. The results of the multivariate Cox regression analysis showed that GGT (HR 1.004; 95% CI 1.002–1.006), ALT (HR 1.006; 95% CI 1.003–1.008), DBIL (HR 0.559; 95% CI 0.490–0.636), TG (HR 1.977; 95% CI 1.394–2.806) and BMI (HR 1.659; 95% CI 1.542–1.786) were independent risk factors for the development of NAFLD in the non-obese population with normal blood lipid level. Therefore, introducing the five independent predictors mentioned above, a NAFLD risk nomogram was developed and is presented in Figure 3.
Table 4.
Multivariate Cox Regression Analysis of the Predictors for the Risk of NAFLD in the Non-Obese Chinese Population with Normal Blood Lipid Levels
| Variables | Coeff. | Std.Err | HR | Confidence Interval (2.5%) | Confidence Interval (97.5%) | P value |
|---|---|---|---|---|---|---|
| GGT | 0.004 | 0.001 | 1.004 | 1.002 | 1.006 | <0.001 |
| ALT | 0.006 | 0.001 | 1.006 | 1.003 | 1.008 | <0.001 |
| DBIL | −0.582 | 0.067 | 0.559 | 0.490 | 0.636 | <0.001 |
| TG | 0.682 | 0.179 | 1.977 | 1.394 | 2.806 | <0.001 |
| BMI | 0.507 | 0.038 | 1.659 | 1.542 | 1.786 | <0.001 |
Abbreviations: GGT, γ-glutamyltranspeptidase; ALT, alanine aminotransferase; DBIL, direct bilirubin; TG, triglyceride; BMI, body mass index; NAFLD, non-alcoholic fatty liver disease; HR, hazard ratio.
Figure 3.
Developed nomogram for predicting the 2-year risk of NAFLD in the non-obese population with normal blood lipid level.
Notes: The nomogram was developed in the cohort by integrating BMI, TG, DBIL, ALT and GGT.
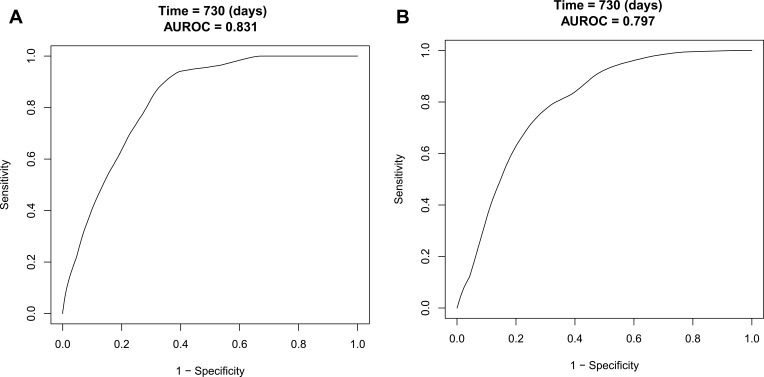
Performance of the Nomogram in the Development and Validation Cohort
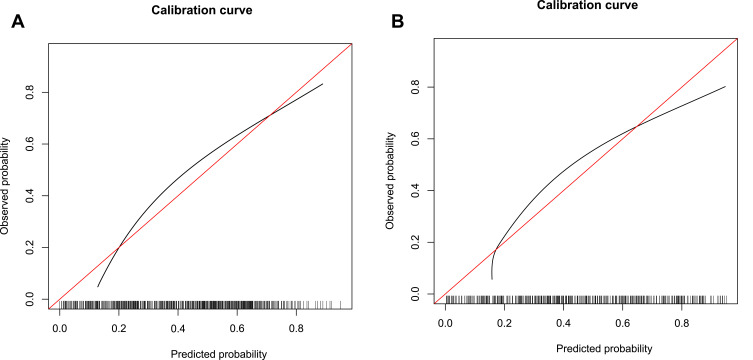
The C-index of the nomogram in the development cohort was 0.819 (95% CI, 0.798–0.840) (Table 5), and the C-index of the nomogram in the validation cohort was 0.815 (95% CI, 0.781–0.849) (Table 5), indicating the nomogram has good discrimination and prediction capabilities. The AUROC of the nomogram in the development cohort was 0.831 (95% CI, 0.811–0.851) (Figure 4A), with sensitivity and specificity of 0.910 and 0.644, respectively (Table 6). The AUROC of the nomogram in the validation cohort was 0.797 (95% CI, 0.765–0.829) (Figure 4B), and sensitivity and specificity were 0.758 and 0.714, respectively (Table 6), indicating that the nomogram performed well. From the calibration curves, the nomogram showed a good agreement between predicted and actual probabilities in both the development cohort and validation cohort (Figure 5A and B).
Table 5.
C-Index in the Nomogram Based on Development Cohort and Validation Cohort
| C-Index (95% CI) | Dxy | aDxy | Variance | Z-value | P value | n | |
|---|---|---|---|---|---|---|---|
| Development cohort | 0.819 (0.798, 0.840) | 0.638 | 0.638 | 0.021 | 27.781 | <0.001 | 2744 |
| Validation cohort | 0.815 (0.781, 0.849) | 0.630 | 0.630 | 0.034 | 19.875 | <0.001 | 915 |
Figure 4.
ROC curves of the nomogram in development cohort and validation cohort. (A) From the development cohort and (B) From the validation cohort. The black line represented the performance of the nomogram. The x-axis is the false positive rate of risk prediction, and the y-axis is the true positive rate of risk prediction.
Abbreviations: ROC, receiver operating characteristic; AUROC, area under ROC.
Table 6.
AUROC in the Nomogram Based on Development Cohort and Validation Cohort
| AUROC (95% CI) | Sensitivity | Specificity | Best Cut-off Values | Predict.Time | |
|---|---|---|---|---|---|
| Development cohort | 0.831 (0.811, 0.851) | 0.910 | 0.644 | −0.598 | 730 days |
| Validation cohort | 0.797 (0.765, 0.829) | 0.758 | 0.714 | −0.508 | 730 days |
Figure 5.
Calibration curves of the nomogram. (A) Calibration curve in the development cohort. (B) Calibration curve in the validation cohort. The horizontal coordinate axis represents the nomogram-predicted probability, and the vertical coordinate axis represents the actual probability. The red line indicates perfect prediction by an ideal model. The black curve is a calibration curve corresponding to the actual situation.
Clinical Usefulness of the Nomogram
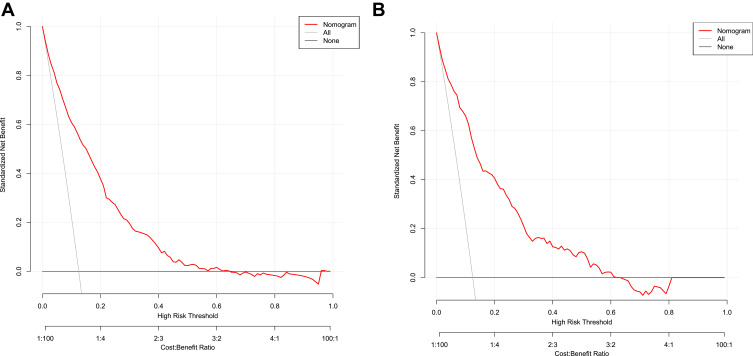
The decision curve showed that it would be more accurate to use the nomogram in the development cohort to predict the risk of NAFLD when the risk threshold probability was between 12% and 65%, and in the validation cohort, it was between 12% and 62% (Figure 6A and Figure 6B). According to the nomogram, within this range, the net benefit was equivalent to a number of overlaps. The decision curve analysis indicated that the application of the nomogram in the non-obese population with normal blood lipid levels to predict the risk of NAFLD is more effective than the intervention-for-all-patients scheme. Hereby, we applied the nomogram in 2 non-obese patients with normal lipid levels as an example. The first patient had GGT, ALT, DBIL, TG, and BMI levels of 450 U/L (19 points), 500 U/L (29 points), 4 mmol/L (75 points), 1 mmol/L (5 points), and 24 kg/m2 (54 points), respectively. The total calculated nomogram score was 182, and the 2-year risk of developing NAFLD was more than 95%. This patient had a high risk of developing NAFLD within 2 years. The second patient had GGT, ALT, DBIL, TG, and BMI levels of 100 U/L (4 points), 50 U/L (3 points), 14 mmol/L (13 points), 1 mmol/L (5 points), and 18 kg/m2 (22 points), respectively. The total calculated nomogram score was 47 and the 2-year risk of developing NAFLD was less than 5%. This patient had a very low risk of developing NAFLD within 2 years.
Figure 6.
Decision curves of the nomogram. (A) Development cohort, (B) Validation cohort. The y-axis indicates the standardized net benefit. The x-axis stands the threshold probability. The red line represents the nomogram. The thin solid line indicates the hypothesis that all patients are diagnosed with NAFLD. The thick solid line indicates the hypothesis that no patient are diagnosed as NAFLD.
Discussion
With the prosperity of the Asia-Pacific region and the rapid adoption of Western lifestyles, NAFLD has become the most common chronic liver disease in the world, affecting 25% to 30% of adults in industrialized countries.21 Although both obesity and dyslipidemia have been repeatedly discussed as independent risk factors for the development of NAFLD, NAFLD is not uncommon in the non-obese population. Due to the differences in study population selection, diagnostic methods, and lifestyle, the reported global prevalence of non-obese NAFLD ranges from 8% to 30%, and it has been further confirmed that a considerable number of non-obese NAFLD individuals present with normal blood lipid levels.22 Numerous epidemiological studies have pointed out that primary prevention and timely intervention are central to preventing or delaying the onset of NAFLD.15,23,24 Therefore, early detection of people at risk for NAFLD is crucial to reduce the morbidity, mortality, and socioeconomic burden of NAFLD, which prompted us to conduct this study.
In this physical examination population-based cohort study, we developed a nomogram to predict the 2-year risk of NAFLD in a Chinese non-obese population with normal blood lipid levels. Compared with traditional prediction models, nomograms have a visual numerical interface, higher accuracy, easier to understand risk prediction, and can be more directly applied to clinical decision making.25 After validation, the nomogram developed in this study was found to have high predictive accuracy in both the development and validation cohorts. Decision curve analysis also demonstrated the clinical value of this a nomogram using continuous values to predict the risk of NAFLD in a non-obese population with normal blood lipid levels.
Our prediction model included five parameters: BMI, TG, DBIL, ALT, and GGT. These variables identified as risk factors for NAFLD are consistent with previous studies and are widely used in NAFLD risk scoring models.23,26,27 In our prediction model, BMI is one of the main aspects of the NAFLD risk factor score. Numerous previous studies have confirmed obesity as a well-known risk factor for NAFLD.28,29 Among the metabolic disorders caused by obesity, altered lipid metabolic processes and adipose organ dysfunction play an important role in the development of NAFLD.28,29 However, the present study found that the risk of NAFLD increased with increasing BMI even in the non-obese population (BMI <25 kg/m2). According to previous studies, hypertriglyceridemia is an important risk factor for NAFLD.30,31 However, in the non-obese population with normal blood lipid levels, insulin resistance (IR) is a potential mediator, although the mechanism between TG and NAFLD has not been fully explained.32,33 On the one hand, IR promotes the secretion of TG-over-enriched very low-density lipoprotein particles, thereby contributing to the increase of TG levels. When TG levels are increased, free fatty acids increase with improved lipolysis. The increased free fatty acids can in turn lead to decreased insulin sensitivity and tissue oxidative stress, which can further lead to aggravation of tissue IR.34,35 On the other hand, IR can promote the development of NAFLD by inducing lipolysis of adipose tissue TG and de novo synthesis of hepatic TG. Therefore, IR may be an important reason for the association between TG and NAFLD.36–38
A large number of studies have shown that both ALT and GGT are independent predictors of NAFLD.39,40 ALT is a specific marker of liver inflammation and hepatocellular injury, and is most closely associated with liver fat accumulation.41,42 Therefore, ALT is often used as a surrogate marker of NAFLD in epidemiological studies.42–44 Some possible reasons may explain why higher ALT levels predispose to new-onset NAFLD. First, higher ALT levels are associated with hepatic fatty infiltration and hepatic steatosis.45 Second, in Asian populations, ALT level is considered to be a reliable marker of IR, and IR is a key pathophysiological factor of NAFLD.32 Moreover, higher ALT levels are closely associated with liver inflammation, and chronic inflammation may further induce hepatic steatosis and IR.46 In summary, higher ALT levels may lead to chronic inflammation of the liver, IR, and hepatic steatosis, which further contribute to the development of NAFLD.47 Serum GGT is a surface enzyme synthesized in intrahepatic bile duct epithelial cells that cleaves extracellular glutathione, maintains glutathione homeostasis in vivo, and plays a key role in mitigating the effects of oxidative stress.48 Several studies have shown that GGT is closely associated with hepatic steatosis and fat deposition, and is considered as a surrogate marker for NAFLD.39,48
DBIL is one of the end products of heme catabolism and has strong antioxidant and cytoprotective effects.49 At the molecular level, DBIL scavenges peroxyl radicals, hydroxyl radicals and reactive nitrogen species. In this way, it prevents the oxidation of intracellular lipids.50 Several clinical pieces of evidence strongly support the beneficial cytoprotective effects of bilirubin. Higher bilirubin levels are negatively associated with insulin levels, insulin resistance and diabetes.51,52 Elevated bilirubin levels are also associated with lowering the risk of cardiovascular disease, including coronary heart disease, stroke, and peripheral vascular disease.53,54 A number of cohort studies have found that DBIL levels are significantly associated with a reduction in the risk of NAFLD, and provide a protective biomarker for NAFLD.51,55 More importantly, the association is independent of classical risk factors including liver enzymes, diabetes, metabolic syndrome traits, coronary heart disease and other classical metabolic risk factors. The results of this study are consistent with previous studies suggesting an inhibitory effect of DBIL on the development of NAFLD.51,56 The biological mechanism of the negative association of DBIL with the risk of NAFLD has not been fully elucidated. There is increasing evidence that DBIL may reduce the risk of NAFLD by inhibiting oxidative stress, suppressing insulin resistance, and altering glucose metabolism.50,55,56
This study has several noteworthy strengths. First, it was the first study to develop an accurate, personalized predictive model for NAFLD in a non-obese population with normal blood lipid levels in China to better assess the 2-year risk of NAFLD. Second, this study used a prospective design with nomogram to visualize risk scores, and multiple new methods were used to assess the performance of the nomogram, like calibration plot. And our nomogram performed well in both internal and external validation, increasing the reliability of the research conclusions. Third, it was based on a large sample cohort study with a broad age spectrum. Therefore, there were sufficient subjects for analysis to ensure the reliability and robustness of the results. Finally, the DCA shows that the net benefit of the prediction model is significantly higher than the two extreme cases in both the development and validation cohorts. Overall, the DCA demonstrates that interventions based on the nomogram is feasible and can be effective in reducing the development of NAFLD.
Despite these strengths of this study, there are some limitations. First, all participants were from China; therefore, the results may not be applicable to other countries. Second, the biochemical parameters were measured only at the time of physical examination and the dynamic changes of these levels over time were not considered. Third, in this study, the diagnosis of NAFLD was based on ultrasonography rather than liver biopsy. Although ultrasound screening is the most widely recommended method due to its high sensitivity and specificity, it is still inaccurate compared to liver biopsy and may underestimate the true incidence of NAFLD.57 Fourth, individuals with missing data were excluded from this study, which may lead to selection bias. Therefore, a multicenter cohort study is needed to further validate our results. Fifth, this study lacked data on treatment received by participants, which may confound the prevalence of NAFLD.
Conclusion
We developed and validated a nomogram for predicting 2-year risk of NAFLD in the non-obese population with normal blood lipid levels. The application of the nomogram is helpful for clinicians, especially community medical workers, to assess the risk of NAFLD in the non-obese population with normal blood lipid levels, and formulate effective primary prevention strategies for NAFLD according to the evaluation results, to reduce the risk of NAFLD.
Acknowledgments
We appreciate Dan-Qin Sun et al for their spirit of scientific sharing.
Data Sharing Statement
All data that support the findings of this study are openly available in Dryad (http://www.datadryad.org/).
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Rinella ME, Sanyal AJ, et al. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology. 2021;73(3):1194–1198. doi: 10.1002/hep.31420 [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 5.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 6.Zhou F, Zhou J, Wang W, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta‐analysis. Hepatology. 2019;70(4):1119–1133. doi: 10.1002/hep.30702 [DOI] [PubMed] [Google Scholar]
- 7.Estes C, Anstee QM, Arias-Loste MT, et al. Modeling nafld disease burden in china, france, germany, italy, japan, spain, united kingdom, and united states for the period 2016–2030. J Hepatol. 2018;69(4):896–904. doi: 10.1016/j.jhep.2018.05.036 [DOI] [PubMed] [Google Scholar]
- 8.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1):S47–S64. doi: 10.1016/j.jhep.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 9.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330. doi: 10.1038/nrgastro.2013.41 [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM. Non-alcoholic fatty liver disease–a global public health perspective. J Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033 [DOI] [PubMed] [Google Scholar]
- 11.Fan J-G, Kim S-U, Wong VW-S. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67(4):862–873. doi: 10.1016/j.jhep.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism. 2016;65(8):1109–1123. [DOI] [PubMed] [Google Scholar]
- 13.Liu CJ. Prevalence and risk factors for non‐alcoholic fatty liver disease in Asian people who are not obese. J Gastroenterol Hepatol. 2012;27(10):1555–1560. doi: 10.1111/j.1440-1746.2012.07222.x [DOI] [PubMed] [Google Scholar]
- 14.Moore MP, Cunningham RP, Dashek RJ, Mucinski JM, Rector RS. A fad too far? Dietary strategies for the prevention and treatment of NAFLD. Obesity. 2020;28(10):1843–1852. doi: 10.1002/oby.22964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–846. doi: 10.1016/j.jhep.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 16.Tu Q, Hu C, Zhang H, et al. Establishment and validation of novel clinical prognosis nomograms for luminal a breast cancer patients with bone metastasis. Biomed Res Int. 2020;2020. doi: 10.1155/2020/1972064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun D-Q, Wu S-J, Liu W-Y, et al. Association of low-density lipoprotein cholesterol within the normal range and NAFLD in the non-obese Chinese population: a cross-sectional and longitudinal study. BMJ Open. 2016;6(12):e013781. doi: 10.1136/bmjopen-2016-013781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Zeng M, Fan JG, Lu LG, et al. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9(2):108–112. doi: 10.1111/j.1751-2980.2008.00331.x [DOI] [PubMed] [Google Scholar]
- 19.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) the TRIPOD statement. Circulation. 2015;131(2):211–219. doi: 10.1161/CIRCULATIONAHA.114.014508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai X, Ji L, Liu S, et al. Derivation and validation of a prediction model for predicting the 5-year incidence of type 2 diabetes in non-obese adults: a Population-Based Cohort Study. Diabetes Metab Syndr Obes. 2021;14:2087–2101. doi: 10.2147/DMSO.S304994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474–485. doi: 10.1016/j.cgh.2016.08.028 [DOI] [PubMed] [Google Scholar]
- 23.Cai X, Aierken X, Ahmat A, et al. A nomogram model based on noninvasive bioindicators to predict 3-year risk of nonalcoholic fatty liver in nonobese mainland chinese: a Prospective Cohort Study. Biomed Res Int. 2020;2020:1–12. doi: 10.1155/2020/8852198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71(2):371–378. doi: 10.1016/j.jhep.2019.03.033 [DOI] [PubMed] [Google Scholar]
- 25.Hu C, Yang J, Huang Z, et al. Diagnostic and prognostic nomograms for bone metastasis in hepatocellular carcinoma. BMC Cancer. 2020;20(1):1–11. doi: 10.1186/s12885-020-06995-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cen C, Wang W, Yu S, et al. Development and validation of a clinical and laboratory-based nomogram to predict nonalcoholic fatty liver disease. Hepatol Int. 2020;14(5):808–816. doi: 10.1007/s12072-020-10065-7 [DOI] [PubMed] [Google Scholar]
- 27.Xue M, Yang X, Zou Y, et al. A non-invasive prediction model for non-alcoholic fatty liver disease in adults with type 2 diabetes based on the population of Northern Urumqi, China. Diabetes Metab Syndr Obes. 2021;14:443. doi: 10.2147/DMSO.S271882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014 [DOI] [PubMed] [Google Scholar]
- 29.Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20(28):9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuda Y, Hashimoto Y, Hamaguchi M, et al. Triglycerides to high‐density lipoprotein cholesterol ratio is an independent predictor of incident fatty liver; a population‐based cohort study. Liver Int. 2016;36(5):713–720. doi: 10.1111/liv.12977 [DOI] [PubMed] [Google Scholar]
- 31.Pacifico L, Bonci E, Andreoli G, et al. Association of serum triglyceride-to-HDL cholesterol ratio with carotid artery intima-media thickness, insulin resistance and nonalcoholic fatty liver disease in children and adolescents. Nutr Metab Cardiovasc Dis. 2014;24(7):737–743. doi: 10.1016/j.numecd.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Qin H, Qiu S, Chen G, Chen Y. Correlation of triglyceride to high-density lipoprotein cholesterol ratio with nonalcoholic fatty liver disease among the non-obese Chinese population with normal blood lipid levels: a retrospective cohort research. Lipids Health Dis. 2019;18(1):1–7. doi: 10.1186/s12944-019-1104-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Zheng R, Li J, Feng S, Wang L, Huang Z. Association between triglyceride glucose-body mass index and non-alcoholic fatty liver disease in the non-obese Chinese population with normal blood lipid levels: a secondary analysis based on a prospective cohort study. Lipids Health Dis. 2020;19(1):1–11. doi: 10.1186/s12944-019-1182-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucero D, Miksztowicz V, Macri V, et al. Overproduction of altered VLDL in an insulin-resistance rat model: influence of SREBP-1c and PPAR-α. Clin Investig Arterioscler. 2015;27(4):167–174. doi: 10.1016/j.arteri.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 35.Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2012;32(9):2104–2112. doi: 10.1161/ATVBAHA.111.241463 [DOI] [PubMed] [Google Scholar]
- 36.Alves‐Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol. 2011;8(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 2013;48(4):434–441. doi: 10.1007/s00535-013-0758-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith GI, Polidori DC, Yoshino M, et al. Influence of adiposity, insulin resistance, and intrahepatic triglyceride content on insulin kinetics. J Clin Invest. 2020;130(6):3305–3314. doi: 10.1172/JCI136756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hossain IA, Shah MMR, Rahman MK, Ali L. Gamma glutamyl transferase is an independent determinant for the association of insulin resistance with nonalcoholic fatty liver disease in Bangladeshi adults: association of GGT and HOMA-IR with NAFLD. Diabetes Metab Syndr. 2016;10(1):S25–S29. doi: 10.1016/j.dsx.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 40.Zou Y, Zhong L, Hu C, Sheng G. Association between the alanine aminotransferase/aspartate aminotransferase ratio and new-onset non-alcoholic fatty liver disease in a nonobese Chinese population: a population-based longitudinal study. Lipids Health Dis. 2020;19(1):1–10. doi: 10.1186/s12944-020-01419-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan N, Peng L, Xia Z, et al. Triglycerides to high-density lipoprotein cholesterol ratio as a surrogate for nonalcoholic fatty liver disease: a cross-sectional study. Lipids Health Dis. 2019;18(1):1–6. doi: 10.1186/s12944-019-0986-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JW, Lee KJ, Yang HR, et al. Prevalence and risk factors of elevated alanine aminotransferase among Korean adolescents: 2001–2014. BMC Public Health. 2018;18(1):1–8. doi: 10.1186/s12889-018-5548-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen X, Fang Cai J, Sheng Gao J, et al. Nonalcoholic fatty liver disease and risk of diabetes: a prospective study in China. Endocr Pract. 2018;24(9):823–832. doi: 10.4158/EP-2018-0098 [DOI] [PubMed] [Google Scholar]
- 44.Zheng R, Du Z, Wang M, Mao Y, Mao W. A longitudinal epidemiological study on the triglyceride and glucose index and the incident nonalcoholic fatty liver disease. Lipids Health Dis. 2018;17(1):1–9. doi: 10.1186/s12944-018-0913-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinn DH, Gwak G-Y, Park HN, et al. Ultrasonographically detected non-alcoholic fatty liver disease is an independent predictor for identifying patients with insulin resistance in non-obese, non-diabetic middle-aged Asian adults. Am J Gastroenterol. 2012;107(4):561–567. doi: 10.1038/ajg.2011.400 [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Xu W, Zhai T, You J, Chen Y. Silibinin ameliorates hepatic lipid accumulation and oxidative stress in mice with non-alcoholic steatohepatitis by regulating CFLAR-JNK pathway. Acta Phar Sin B. 2019;9(4):745–757. doi: 10.1016/j.apsb.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang R, Lu Q, Feng J, et al. Coexistence of non-alcoholic fatty liver disease with elevated alanine aminotransferase is associated with insulin resistance in young Han males. Endocrine. 2012;41(1):70–75. doi: 10.1007/s12020-011-9511-0 [DOI] [PubMed] [Google Scholar]
- 48.Franzini M, Fornaciari I, Fierabracci V, et al. Accuracy of b‐GGT fraction for the diagnosis of non‐alcoholic fatty liver disease. Liver Int. 2012;32(4):629–634. doi: 10.1111/j.1478-3231.2011.02673.x [DOI] [PubMed] [Google Scholar]
- 49.Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Off J Am Coll Gastroenterol. 2017;112(1):18–35. doi: 10.1038/ajg.2016.517 [DOI] [PubMed] [Google Scholar]
- 50.Saoi M, Sasaki K, Sagawa H, et al. High throughput screening of serum γ-glutamyl dipeptides for risk assessment of nonalcoholic steatohepatitis with impaired glutathione salvage pathway. J Proteome Res. 2019;19(7):2689–2699. doi: 10.1021/acs.jproteome.9b00405 [DOI] [PubMed] [Google Scholar]
- 51.Kang YH, Min HK, Son SM, Kim IJ, Kim YK. The association of serum gamma glutamyltransferase with components of the metabolic syndrome in the Korean adults. Diabetes Res Clin Pract. 2007;77(2):306–313. doi: 10.1016/j.diabres.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 52.Bullón-Vela V, Abete I, Tur JA, et al. Relationship of visceral adipose tissue with surrogate insulin resistance and liver markers in individuals with metabolic syndrome chronic complications. Ther Adv Endocrinol Metab. 2020;11:2042018820958298. doi: 10.1177/2042018820958298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ndrepepa G, Kastrati A. Gamma-glutamyl transferase and cardiovascular disease. Ann Transl Med. 2016;4(24):481. doi: 10.21037/atm.2016.12.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pitisuttithum P, Chan W-K, Goh G-B-B, et al. Gamma-glutamyl transferase and cardiovascular risk in nonalcoholic fatty liver disease: the gut and obesity Asia initiative. World J Gastroenterol. 2020;26(19):2416. doi: 10.3748/wjg.v26.i19.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lallukka S, Yki-Järvinen H. Non-alcoholic fatty liver disease and risk of type 2 diabetes. Best Pract Res Clin Endocrinol Metab. 2016;30(3):385–395. doi: 10.1016/j.beem.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 56.Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Alanine aminotransferase, γ-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care. 2009;32(4):741–750. doi: 10.2337/dc08-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–1281. e4. doi: 10.1053/j.gastro.2018.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]