Abstract
Background:
Transient Receptor Potential Vanilloid 1 (TRPV1) is known to mediate itch and neurogenic inflammation, but the role of TRPV1 in psoriasiform dermal inflammation is poorly understood.
Objective:
To investigate the function of TRPV1 in imiquimod (IMQ)-induced psoriasiform dermatitis (PsD) in mice.
Methods:
Following daily treatment of topical IMQ cream for consecutive 5 days in C57BL/6 wide-type (WT) and TRPV1 gene knockout (KO) mice, we assessed the psoriasis severity index (PSI) scores, transepidermal water loss (TEWL), dermal inflammatory infiltrates, as well as gene expression levels for psoriasis related genes in mouse skin lesions.
Results:
Compared with WT mice, the clinical and TEWL scores, the extent of skin hyperplasia, the area of Munro microabscesses (MM) and angiogenesis of psoriasis were all significantly decreased in TRPV1 KO mice triggered with IMQ, suggesting a reduction in skin inflammation and barrier defects. In addition, the infiltration of CD45+ leukocytes, mast cells as well as CD3+ T cells was all reduced in the IMQ-treated skin of TRPV1 KO mice. Quantitative Real-time PCR (RT-qPCR) revealed that expression levels of IL-1β, IL-6, IL-23, S100A8 were decreased while IL-10 was increased in TRPV1 KO mice.
Conclusions:
In summary, key markers of psoriatic inflammation and epidermal hyperplasia are reduced in TRPV1 KO mice, indicating the involvement of TRPV1 in the psoriasiform inflammation and suggesting its potential as a therapeutic target.
Keywords: Psoriasis, Itch, Pain neuroinflammation
1. Introduction
Psoriasis is a chronic cutaneous inflammatory condition, which is mediated by dermal leukocytes, including dendritic cells (DCs), T cells, mast cells and neutrophils [1]. T cell activation is thought to be initiated and driven by DCs which produce various pro-inflammatory cytokines, including IL-6 and IL-23, further driving Th17 cell-mediated inflammation [2]. The following histological features of psoriasis are generally accepted: a greatly thickened epidermis with elongated rete ridges into the dermis, an increased number of prominent blood vessels in the papillary dermis, and the presence of a collection of neutrophils in the cornified layer known as a Munro’s microabscess (MM) [3]. Interestingly, there are clinical reports that psoriasis is worsened by psychosocial stress [4]. Nerve damage/denervation had resulted in localized clearance of psoriasis in the distribution of the nerve and that the psoriatic lesions recurred with recovery of cutaneous sensation [5]. TRP channels, such as Transient Receptor Potential Vanilloid 1 (TRPV1), are involved in neuropeptide release [6] and have been implicated in susceptibility to inflammation of psoriasiform dermatitis (PsD) [7]. TRPV1, a Ca2+ permeable channel, is involved in the pathogenesis of both inflammatory and neuropathic pain and itch. Pruritus induced by IL-31, a key inflammatory marker in atopic dermatitis (AD), is also mediated by TRPV1 [8]. TRPV1 antagonists may expand the dermatologist’s armamentarium against AD [9], but its role in other skin inflammatory disorders, such as psoriasis, is poorly understood.
TRPV1 played a pro-inflammatory role in some neurological disorders by amplifying the effects of IL-1β and IL-6 [10]. Immune cells in the skin, such as mononuclear cells, DCs and mast cells, not only express TRPV1, but also are directly affected by TRPV1 activation [11]. Roblin et al. reported that CT327 (a topical TrkA kinase inhibitor) inhibited nerve growth factor (NGF)-TrkA-TRPV1 pathway, thereby reducing pruritus in psoriatic patients [12]. Leigh et al. reported that overexpression of TRPV1 in lesions of patients was positively correlated to itch of psoriasis [13]. Experimentally, chemical inhibition of TRPV1+NaV1.8+ nociceptors by resiniferatoxin (RTX) reduced IL-23/IL-17 responses in imiquimod (IMQ)-induced psoriasiform inflammation [7]. RTX, however blocks TRPV1 as well as TRPA1 and thus is not specific for TRPV1 [14]. It has never been clearly addressed how TRPV1 affects the immune cells in terms of cellular types, infiltration patterns, accumulation numbers, and related skin environment changes. Therefore, our specific aims were to investigate the function of TRPV1 in skin inflammation by quantitatively and qualitatively comparing skin alterations in TRPV1 gene knockout (KO) vs. wild-type (WT) mice. Our results indeed suggest that TRPV1 mediates inflammation in the IMQ-based psoriasis model.
2. Materials and methods
2.1. Animals
Animal experiments were approved by the University of California, Davis, Animal Care and Use Committee. Female and male c57BL/6 WT and TRPV1 KO mice (purchased from JACKSON Laboratory), (8–10 weeks, 20–25 g) were used.
2.2. Induction of psoriasiform skin inflammation
The fur on the rostral back was shaved >5 days prior to treatment. PsD was induced by topically applying 50 mg of 5% IMQ cream (Aldara, 3 M Health Care Limited, UK), or control vehicle cream (Vanicream; Pharmaceutical Specialties, Rochester, MN) once daily for 5 consecutive days. Mice were euthanized on day 5. The treated skin was collected and then stored in 3 parts immersed in RNA later, 10% paraffin and optimal cutting temperature (OTC) freezing medium and stored in −80°C.
2.3. Transepidermal water loss (TEWL)
TEWL [15] was measured by applying a Vapo Meter® (Delfin Technologies Ltd., Kuopio, Finland) to the skin for 10 s.
2.4. Skin score
Assessment of local modified psoriasis severity index (PSI) of PsD inflammation: the PSI score assessment is used to monitor the progress of the psoriatic inflammation in mice on day 5. The PSI score includes PSI-E (erythema), PSI-S (scaling) and PSI-I (induration). PSI-E/PSI-S/PSI-I was evaluated and graded 0–4 (0: absent to 4: very severe) [16] by a blinded observer. Total PSI score is 0–12.
2.5. Histology and immunohistochemistry (IHC)
Skin tissue samples were formalin-fixed (10%) and embedded in paraffin for Hematoxylin and Eosin (H&E) or for Giemsa staining (Mast cells). We assessed histopathological alterations (epidermal thickness and area of MM) with computer-assisted quantitative image analysis (Image J) in lesions. Tissue sections (5 μm) were prepared for rabbit polyclonal anti-Ki67(diluted in 1:1000, Clone#Ab-4, NeoMarkers, U.S.A.), rabbit monoclonal anti-pSTAT3(diluted in 1:200,Clone#G.374.10,Thermo Scientific, U.S. A.), goat polyclonal anti-CD31(diluted in 1:1800, Clone#M-20, Santa Cruz, U.S.A.), rat polyclonal anti-CD45(diluted in 1:400, Clone#OX-1, BD Pharmingen, U.S.A.) and rabbit monoclonal anti-CD3(diluted in 1:800, Clone#Sp7, Abcam, U.S.A.) for IHC. Individual inflammatory cell types CD45 were counted in 5 microscopic fields at 400X and expressed as cells/HPF, with mean ± S.D. Ki67, pSTAT3 and CD3 positive cells and mast cells were counted in 5 microscopic fields at 200X and expressed as cells/Field (200X), with mean ± S.D.
2.6. Quantitative real-time PCR (RT-qPCR)
RNA was extracted from cryopreserved skin specimens with Mini Spin Columns to perform gene expression analyses using RT-qPCR with SYBR Green methods. Primers for IL-23 (Mm.PT.58.10594618.g), IL-6 (Mm.PT.58.10005566), IL-1β (Mm.PT.58.10005566), S100A8 (Mm.PT.58.44003402.gs), CXCL1 (Mm.PT.58.42076891), IL-10 (Mm.PT.58.13531087), IL-17 A (Mm.PT.58.6531092), IL-17 F (Mm.PT.58.9739903), IL-22(L: 5′-CTG CTT CTC ATT GCC CTG TG-3′; R: 5′-AGC ATA AAG GTG CGG TTG AC-3′) and DEFB4 (Mm. PT.58.29993789) were designed by Integrated DNA Technologies, Inc., Iowa, USA.
2.7. Statistical analysis
All results were expressed as mean ± S.D. and analyzed statistically with Graphpad Prism 6.02(Graphpad Software, Inc., San Diego, USA). The student t-test was used to analyze the differences between two groups. One-way ANOVA followed post-hoc comparison (Tukey’s HSD) test were used to compare the differences in more than two groups. Statistical significance was defined as P < 0.05.
3. Results
3.1. IMQ-induced psoriasiform dermatitis is alleviated in TRPV1 KO mice
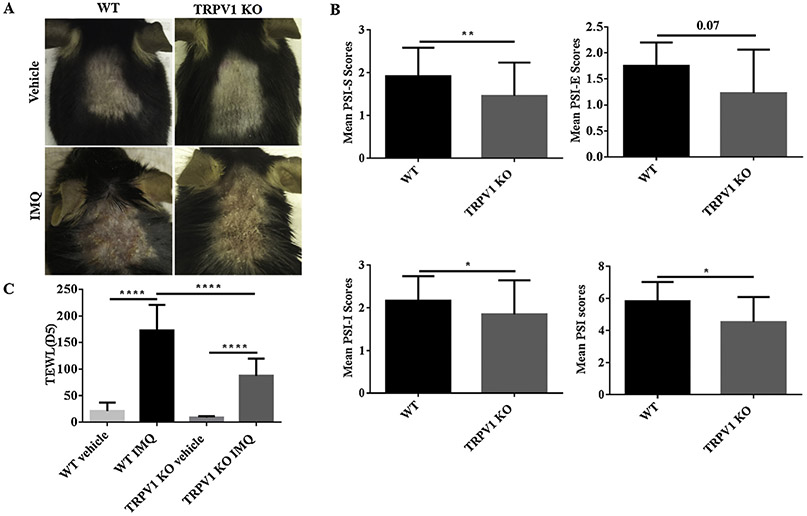
We compared the clinical and immunologic differences in the skin of TRPV1 KO mice compared to WT control littermates using a well-established model of PsD. After topically applying IMQ for consecutive 5 days, we noticed that the clinical manifestations of PsD were less severe in the TRPV1 KO group, compared with WT group (Fig. 1A). To confirm this observation, PSI scores were assigned to mice by a blinded observer. Compared with WT mice, the total PSI scores of TRPV1 KO mice (P < 0.05) were decreased, which was shown for both the scores of scaling (PSI-S) (P < 0.01) and induration (PSI-I) (P < 0.05) (Fig. 1B).
Fig. 1.
PSI and TEWL scores of IMQ-induced PsD. (A) Representative images are shown of the back skin of vehicle- and IMQ-treated in WT mice vs. TRPV1 KO mice on Day 5. (B) The PSI scores, including scales (S), erythema (E), induration (I) are shown in mice treated with IMQ for 5 days in WT and TRPV1 KO group, respectively. (C) TEWL Scores in WT or TRPV1 KO mice with IMQ treatment are shown after 5 days (n = 12 in each vehicle group, n = 13 in each IMQ group). P-value scores for PSI scores were calculated by student t-test; TEWL scores by one-way ANOVA followed by post-hoc comparison (Tukey’s HSD) test. Mean ± S.D. values are indicated (*P < 0.05, **P < 0.01, ****P < 0.0001).
Disruption of the skin barrier function can be measured objectively through a device that quantifies TEWL. An increase in TEWL, for example, would reflect a loss of barrier function and is accompanied by perceived skin dryness and visible surface scales. TEWL has been reported to increase in psoriasis patients and to go down following successful therapy [17]. As might be expected, TEWL increased significantly in WT mice treated with IMQ upon development of psorisiform changes. TEWL, however, in TRPV1 KO mice was decreased by 49.55% (P < 0.0001) after topical application of IMQ for 5 days, as compared with WT mice (Fig. 1C), suggesting better preservation of skin barrier function in the KO mice. Thus, TRPV1 contributes to the disrupted skin barrier damage following IMQ application, thus potentially explaining the reduction in scaling in psoriatic lesions of IMQ-treated TRPV1 KO mice.
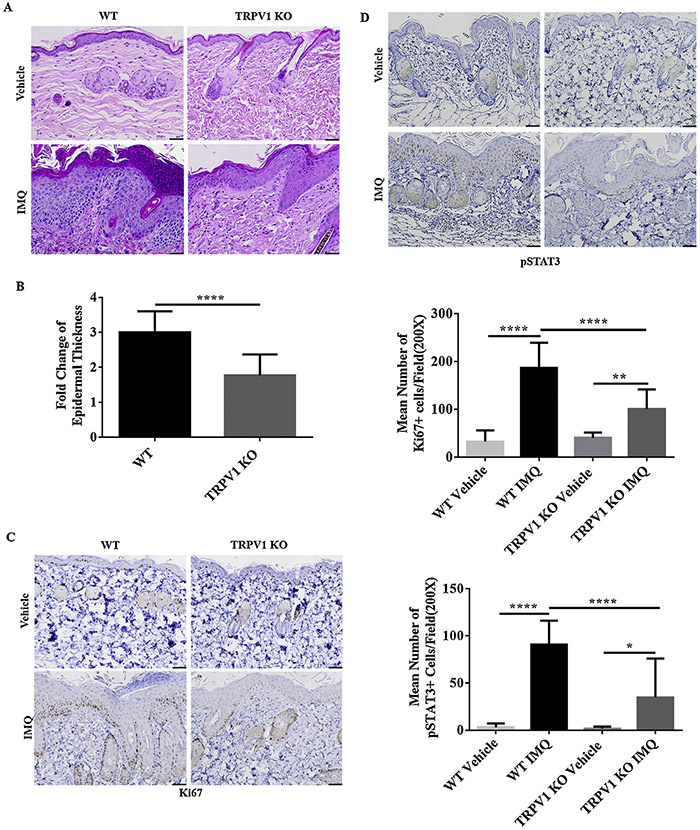
3.2. Reduced epidermal thickness and proliferation in TRPV1 KO mice (compared to WT mice) following application of IMQ
Epidermal hyperproliferation and thickening is a hallmark of psoriatic inflammation. Histopathological examination showed prominent hyperkeratosis and acanthosis after treatment by IMQ on the back of mice for 5 days (Fig.2A). We measured epidermal thickness with Image J analysis from H&E skin sections. Epidermal hyperplasia was reduced in TRPV1 KO mice by 41.03% (P < 0.0001) compared with WT mice (Fig.2B). Weakly positive staining of Ki67 (Fig.2C) and pSTAT3 (Fig.2D) were observed, respectively, in basal keratinocytes and dermis in both TRPV1 KO and WT mice treated with vehicle. In IMQ-treated groups, however, the numbers of Ki67+ and pSTAT3+ cells per microscopic field in WT mice were significantly greater than that in TRPV1 KO mice (Fig.2C,2D) (P < 0.0001).
Fig. 2.
Epidermal hyperplasia, pSTAT3, and Ki67 expression following IMQ treatment. (A) After IMQ treatment for 5 days, histopathology showed significant changes in hyperkeratosis and acanthosis in WT vs. TRPV1 KO mice. 200X magnification (scale bar = 50 μm). (B) Quantitative epidermal thickness changes are shown for TRPV1 KO mice and WT mice (n = 12 in each vehicle group, n = 13 in each IMQ group). (C) Ki67 staining and quantification per microscopic field (200X) are shown from treated skin of TRPV1 KO vs. WT mice treated with IMQ/vehicle (5 days of treatment), n = 6 in each group. (D) pSTAT3 staining and quantification per microscopic field (200X) are shown from treated skin of TRPV1 KO vs. WT mice treated with IMQ/vehicle (5 days of treatment), n = 6 in each group (scale bar = 50 μm). Statistical testing included student t-test for epidermal thickness, one-way ANOVA followed by post-hoc comparison (Tukey’s HSD) test for Ki67 and pSTAT3 analysis. Mean ± S.D. values are indicated (*P < 0.05, **P < 0.01, ****P < 0.0001).
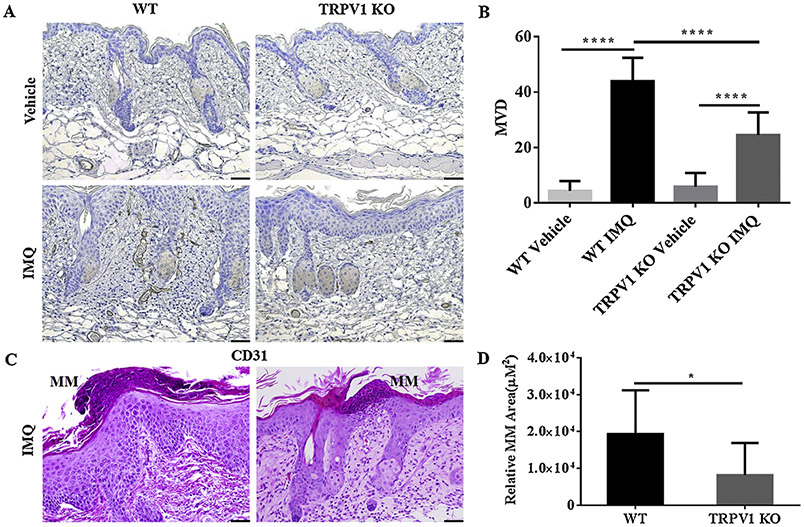
3.3. Angiogenesis and MM are decreased in TRPV1 KO mice compared to WT mice
Next, we determined if angiogenesis and the presence of MM, two prominent features of human psoriasis, were altered in TRPV1 KO mice. Evaluation of microvascular density (MVD) was done by immunohistochemical staining for CD31 (Fig. 3A). The average MVD in psoriatic lesions in WT group increased markedly (P < 0.0001) with IMQ treatment compared with vehicle. Although TRPV1 KO mice seemed to have more angiogenesis in IMQ-treated mice than those treated with vehicle (P < 0.0001), the extent of augment of MVD in TRPV1 KO mice were reduced by 44.28% (P < 0.0001) compared to WT mice (Fig.3B). Thus, the profound angiogenesis in the skin of IMQ-treated mice appears to be partially dependent on TRPV1.
Fig. 3.
Changes in angiogenesis and MM formation in WT and TRPV1 KO mice. (A) Skin sections from indicated mice were stained with anti-CD31 antibodies as described in Materials and Methods and (B) quantified with respect to mean vessel density (MVD) (n = 6 in each group). (C) MM in IMQ-treated skin from WT and TRPV1 KO mice were (D) quantified in terms of relative MM area after 5 days of IMQ or vehicle treatment (n = 12 in each vehicle group, n = 13 in each IMQ group) in TRPV1 KO mice/WT mice treated with IMQ/vehicle for 5 days. (200X magnification; scale bar = 50 μm). Statistical analysis included a student t-test for MM and one-way ANOVA followed by post-hoc comparison (Tukey’s HSD) test for MVD. Mean ± S.D. values are indicated (*P < 0.05, ****P < 0.0001).
Finally, we measured MM formation in lesional psoriatic lesion of WT and TRPV1 KO mice and found that MM were diminished in the latter group (Fig. 3C). The average area of total MM per mouse was reduced in TRPV1 KO mice by 57.61% (P < 0.05) compared with WT mice (Fig. 3D), demonstrating that TRPV1 plays a role in neutrophils accumulation in the IMQ-treated skin.
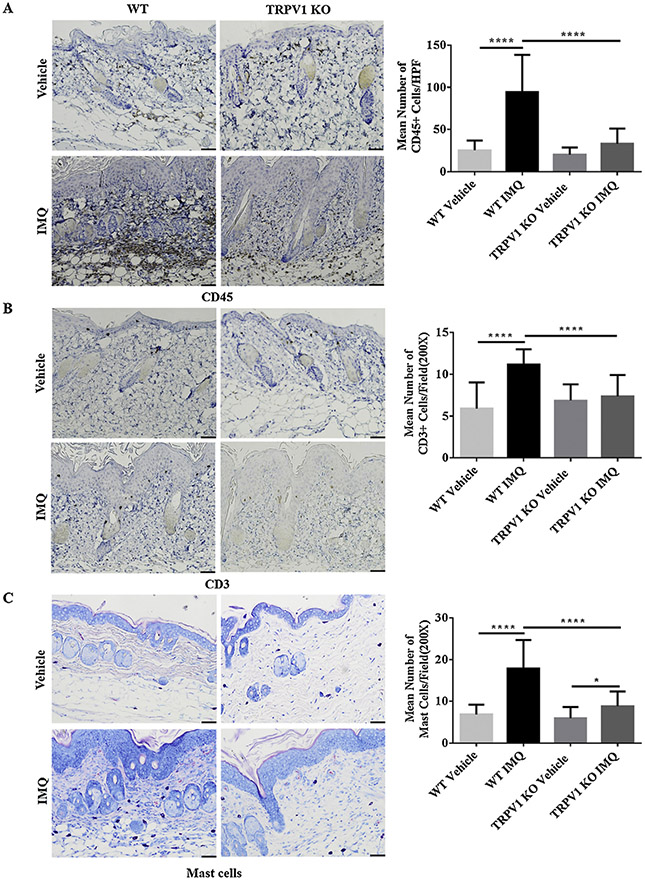
3.4. Reduction in infiltration of inflammatory cells in IMQ-treated skin of TRPV1 KO mice
In order to assess the clinical and pathological influence of TRPV1 on the composition of dermal inflammatory cells following IMQ treatment, we used IHC or Giemsa staining (for mast cells) in WT and TRPV1 KO mice following induction of PsD. We observed large numbers of CD45+ leukocytes (Fig. 4A), scattered staining of CD3 + T cells (Fig. 4B) and mast cells (Fig. 4C) in the IMQ-treated lesional skin. The average numbers of CD45+, mast cells and CD3+ cells per microscopic field showed significant increases in WT mice following IMQ treatment compared to vehicle treatment. Interestingly, the numbers in IMQ-treated WT mice were significantly higher (P < 0.0001) than that in IMQ-treated TRPV1 KO mice (Fig. 4A, 4B, 4C). These data suggest that dermal inflammatory cell infiltration, which may include proliferation, was inhibited with the absence of TRPV1 in the psoriasis model.
Fig. 4.
Immunohistochemical and Giemsa staining of IMQ- and vehicle-treated skin in WT and TRPV1 KO mice. (A) CD45 staining and quantification per HPF (400X). (B) CD3+ T cell staining and quantification and (C) mast cell staining with Giemsa and quantification after 5 days of treatment. Representative photos are shown with 200X magnification (scale bar = 50 μm). Statistical analysis was performed using one-way ANOVA followed by post-hoc comparison (Tukey’s HSD) test. N = 6 per group. Mean ± S.D. values are indicated (*P < 0.05, ****P < 0.0001).
3.5. mRNA expression of related genes in psoriasis lesions
To understand the mechanism whereby TRPV1 mediates psoriasiform inflammation, we measured mRNA expression for key inflammation genes that are known to regulate psoriatic skin inflammation. RT-qPCR showed relatively decreased mRNA levels of IL-1β (P < 0.001), IL-6(P < 0.05), IL-23(P < 0.05), S100A8 (P < 0.05), CXCL1(P = 0.06) and increased level of IL-10 (P < 0.01) in the psoriasiform skin lesions of TRPV1 KO mice as compared with WT mice. The mRNA levels of IL-17 A, IL-17 F, IL-22 and DEFB4 were somewhat lower in TRPV1 KO (vs. WT) mice although this did not reach statistical significance (P > 0.05) (Fig. 5). Thus, TRPV1 impacts skin inflammatory changes by regulating important pro-inflammatory cytokine genes such as IL-1β and IL-6, but not necessarily IL-17 A/F, in this experimental model of psoriasis.
Fig. 5.
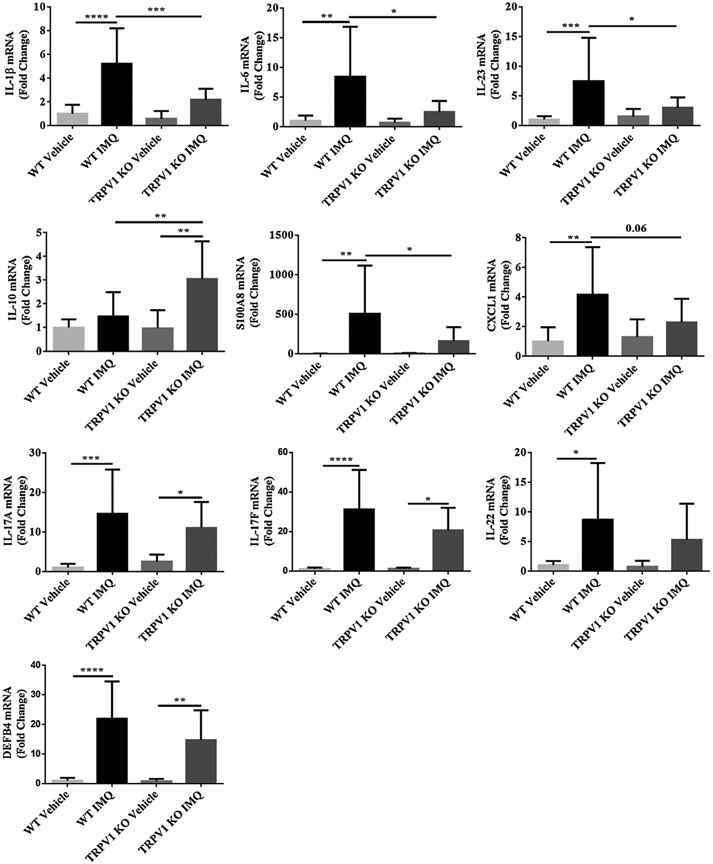
Transcriptional mRNA expression of psoriasis-related genes in lesions. Relative skin mRNA expression of IL-1β, IL-6, IL-23, S100A8, CXCL1, IL-10, IL-17 A/F, IL-22 and DEFB4 in TRPV1 KO mice/WT mice treated with IMQ/vehicle were analyzed and quantified as indicated in Material and Methods. Results are normalized to GAPDH expression. N = 12 in each vehicle group, n = 13 in each IMQ group. Statistical analysis was performed using one-way ANOVA followed by post-hoc comparison (Tukey’s HSD) test. Mean ± S.D. values are indicated (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
4. Discussion
There has been interest in the role of TRP channel receptors in inflammatory skin diseases such as psoriasis. Von Andrian et al. reported that resiniferatoxin (RTX) reduced IL-23/IL-17 responses in IMQ-induced psoriasiform inflammation. They concluded that NaV1.8+TRPV1+ nociceptors are driving to induce IL-23 production. Subsequently, IL-17 A, IL-17 F and IL-22 were reduced significantly in RTX-treated mice with ELISA [7]. Because TRPA1 and TRPV1 are co-expressed in sensory neurons, however, treatment of TRPV1+ neurons with RTX reduces the expression of both TRPV1 and TRPA1 [14], and RTX treatment decreases mRNA expression of nociceptor markers, including not only TRPV1 and TRPA1 but also Substance P and TRPM8 in dorsal root ganglia (DRGs) in IMQ induced PsD [7]. More recently, Ágnes Kemény et al. reported that TRPV1 KO mice also showed fewer changes in skin thickness and blood perfusion in comparison to WT mice following IMQ treatment [18], but their treatment period was somewhat shorter than that reported by other investigators. Herein, we used a variety of methodologies, including PSI scores, TEWL, dermal inflammatory infiltrates, as well as gene expression levels to investigate the role of TRPV1 in IMQ-induced psoriasiform dermatitis.
Psoriatic plaques appear characteristically dry and have fine silvery scales. To objectively measure skin barrier function, we took advantage of technology that can measure TEWL. Elevated TEWL scores in lesional skin of psoriasis patients has been reported to normalize after use of emollients [19] and after effective light therapy [20]. We found that TEWL scores increased markedly in mice treated with IMQ on day 5. This elevation was reduced by almost a half in TRPV1 KO mice. Our data indicate that TRPV1 may contribute in the epidermal barrier dysfunction that has been reported in psoriasis. Yun et al. reported that pharmacologic blockade of TRPV1 activation can inhibit the symptoms of an AD-like condition as manifested by a lower TEWL score [21]. The inhibition is based on restoration of a neutral lipid layer and reversal of changes in loricrin and filaggrin expression [21]. Both of these two important epidermal barrier proteins are decreased in psoriasis [22]. We speculate that TRPV1′s role in skin barrier damages may be related with above mechanisms or as consequence of inflammation.
We also found TRPV1 may participate in the recruitment of neutrophils, T cells and mast cells in skin as well as angiogenesis. A recent study has suggested that TRPV1 is expressed in CD4 + T cells where it contributes to T cell receptor-induced Ca2+ influx, signaling and T cell activation [23]. In addition, mast cells may be major producers of IL-22 in patients with psoriasis [24]. Our observations may be related to reduced mRNA levels of inflammatory cytokines, such as IL-1β, IL-6, IL-23, S100A8, and CXCL1 in the psoriatic lesion of TRPV1 KO mice induced by IMQ. The decrease of CXCL1 and other neutrophil chemoattractants in the TRPV1 KO mice may explain the marked diminished numbers of MM in the KO animals. It is notable that IL-10 mRNA is actually increased in the TRPV1 KO mice compared to WT littermates after IMQ treatment. IL-10 has been reported to have immunosuppressive functions. IMQ-treated IL-10 KO mice have been reported to show more severe psoriasiform skin features compared to WT controls [25], implying that IL-10 may have protective effect in psoriasis. Our data suggest that TRPV1 is a negative regulator of IL-10 expression.
Taken together, our study provides evidence that TRPV1 contributes to the development of psoriasiform dermatitis via its role in maximizing expression of several critical inflammatory cytokines such as IL-6, IL-23 and IL-1β. Interestingly, we did not detect a significant reduction IL-17 A/F in TRPV1 KO mice, but this may be due to larger variation in the mRNA levels of these cytokines under the conditions tested. Our TEWL studies strongly suggest that epidermal barrier function defects which are found in psoriasiform dermatitis may be improved if TRPV1 is genetically ablated. We suggest that TRPV1 should be further investigated as a candidate target for psoriatic skin disease in humans.
Acknowledgements
This work was supported by the Grant from National Psoriasis Foundation of USA; Natural Science Foundation of China (Grant No. 81703130); the Science and Technology Research and Development Program of Shaanxi Province of China (Grant No. 2016SF-238).
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- [1].Chiricozzi A, Romanelli P, Volpe E, Borsellino G, Romanelli M, Scanning the immunopathogenesis of psoriasis, Int. J. Mol. Sci 19 (1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Saggini A, Chimenti S, Chiricozzi A, IL-6 as a druggable target in psoriasis: focus on pustular variants, J. Immunol. Res 2014 (2014)964069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Giang J, Seelen MAJ, van Doorn MBA, Rissmann R, Prens EP, Damman J, Complement activation in inflammatory skin diseases, Front. Immunol 9 (2018) 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hunter HJ, Griffiths CE, Kleyn CE, Does psychosocial stress play a role in the exacerbation of psoriasis? Br. J. Dermatol 169 (5) (2013) 965–974. [DOI] [PubMed] [Google Scholar]
- [5].Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R, Neuroimmunology of stress: skin takes center stage, J. Invest. Dermatol 126 (8) (2006) 1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Choi JE, Di Nardo A, Skin neurogenic inflammation, Semin. Immunopathol 40 (3) (2018) 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH, Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation, Nature 510 (7503) (2014) 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, Soumelis V, Feld M, Alenius H, Dillon SR, Carstens E, Homey B, Basbaum A, Steinhoff M, A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1, J. Allergy Clin. Immunol 133 (2) (2014) 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bonchak JG, Swerlick RA, Emerging therapies for atopic dermatitis: TRPV1 antagonists, J. Am. Acad. Dermatol 78 (3S1) (2018) S63–S66. [DOI] [PubMed] [Google Scholar]
- [10].Huang WX, Yu F, Sanchez RM, Liu YQ, Min JW, Hu JJ, Bsoul NB, Han S, Yin J, Liu WH, He XH, Peng BW, TRPV1 promotes repetitive febrile seizures by pro-inflammatory cytokines in immature brain, Brain Behav. Immun 48 (2015) 68–77. [DOI] [PubMed] [Google Scholar]
- [11].Gouin O, L’Herondelle K, Lebonvallet N, Le Gall-Ianotto C, Sakka M, Buhe V, Plee-Gautier E, Carre JL, Lefeuvre L, Misery L, Le Garrec R, TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization, Protein Cell 8 (9) (2017) 644–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roblin D, Yosipovitch G, Boyce B, Robinson J, Sandy J, Mainero V, Wickramasinghe R, Anand U, Anand P, Topical TrkA kinase inhibitor CT327 is an effective, novel therapy for the treatment of pruritus due to psoriasis: results from experimental studies, and efficacy and safety of CT327 in a phase 2b clinical trial in patients with psoriasis, Acta Derm. Venereol 95 (5) (2015) 542–548. [DOI] [PubMed] [Google Scholar]
- [13].Nattkemper LA, Tey HL, Valdes-Rodriguez R, Lee H, Mollanazar NK, Albornoz C, Sanders KM, Yosipovitch G, The genetics of chronic itch: gene expression in the skin of patients with atopic dermatitis and psoriasis with severe itch, J. Invest. Dermatol 138 (6) (2018) 1311–1317. [DOI] [PubMed] [Google Scholar]
- [14].Pecze L, Pelsoczi P, Kecskes M, Winter Z, Papp A, Kaszas K, Letoha T, Vizler C, Olah Z, Resiniferatoxin mediated ablation of TRPV1+ neurons removes TRPA1 as well, Can. J. Neurol. Sci 36 (2) (2009) 234–241. [DOI] [PubMed] [Google Scholar]
- [15].Mohammed D, Matts PJ, Hadgraft J, Lane ME, Depth profiling of stratum corneum biophysical and molecular properties, Br. J. Dermatol 164 (5) (2011) 957–965. [DOI] [PubMed] [Google Scholar]
- [16].Feldman SR, Bushnell DM, Klekotka PA, Scanlon M, Martin ML, Wade SW, Yang W, Pinto L, Kircik L, Viswanathan HN, Differences in psoriasis signs and symptom severity between patients with clear and almost clear skin in clinical practice, J. Dermatolog. Treat 27 (3) (2016) 224–227. [DOI] [PubMed] [Google Scholar]
- [17].Lee Y, Je YJ, Lee SS, Li ZJ, Choi DK, Kwon YB, Sohn KC, Im M, Seo YJ, Lee JH, Changes in transepidermal water loss and skin hydration according to expression of aquaporin-3 in psoriasis, Ann. Dermatol 24 (2) (2012) 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kemeny A, Kodji X, Horvath S, Komlodi R, Szoke E, Sandor Z, Perkecz A, Gyomorei C, Setalo G, Kelemen B, Biro T, Toth BI, Brain SD, Pinter E, Gyulai R, TRPA1 acts in a protective manner in imiquimod-induced psoriasiform dermatitis in mice, J. Invest. Dermatol 138 (8) (2018) 1774–1784. [DOI] [PubMed] [Google Scholar]
- [19].Liu M, Li X, Chen XY, Xue F, Zheng J, Topical application of a linoleic acid-ceramide containing moisturizer exhibit therapeutic and preventive benefits for psoriasis vulgaris: a randomized controlled trial, Dermatol. Ther 28 (6) (2015) 373–382. [DOI] [PubMed] [Google Scholar]
- [20].Darlenski R, Hristakieva E, Aydin U, Gancheva D, Gancheva T, Zheleva A, Gadjeva V, Fluhr JW, Epidermal barrier and oxidative stress parameters improve during in 311nm narrow band UVB phototherapy of plaque type psoriasis, J. Dermatol. Sci 91 (1) (2018) 28–34. [DOI] [PubMed] [Google Scholar]
- [21].Yun JW, Seo JA, Jeong YS, Bae IH, Jang WH, Lee J, Kim SY, Shin SS, Woo BY, Lee KW, Lim KM, Park YH, TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery, J. Dermatol. Sci 62 (1) (2011) 8–15. [DOI] [PubMed] [Google Scholar]
- [22].Kim BE, Howell MD, Guttman-Yassky E, Gilleaudeau PM, Cardinale IR, Boguniewicz M, Krueger JG, Leung DY, TNF-alpha downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-alpha antagonists to improve skin barrier, J. Invest. Dermatol 131 (6) (2011) 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bertin S, Aoki-Nonaka Y, de Jong PR, Nohara LL, Xu H, Stanwood SR, Srikanth S, Lee J, To K, Abramson L, Yu T, Han T, Touma R, Li X, Gonzalez-Navajas JM, Herdman S, Corr M, Fu G, Dong H, Gwack Y, Franco A, Jefferies WA, Raz E, The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4(+) T cells, Nat. Immunol 15 (11) (2014) 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mashiko S, Bouguermouh S, Rubio M, Baba N, Bissonnette R, Sarfati M, Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis, J. Allergy Clin. Immunol 136 (2) (2015) 351–9 e1. [DOI] [PubMed] [Google Scholar]
- [25].Jin SP, Koh SJ, Yu DA, Kim MW, Yun HT, Lee DH, Yoon HS, Cho S, Park HS, Imiquimod-applied Interleukin-10 deficient mice better reflects severe and persistent psoriasis with systemic inflammatory state, Exp. Dermatol 27 (1) (2018) 43–49. [DOI] [PubMed] [Google Scholar]