The annual nitrogen-fixing legumes soybean (Glycine max) and common bean (Phaseolus vulgaris) are crops of major societal importance and represent considerable sources of protein and essential nutrients for mankind. Since diverging from common bean, wild soybean (Glycine soja) underwent specific polyploidy and diploidization (Lavin et al., 2005) before being domesticated ∼6,000–9,000 years ago. (Soy)beans thus represent an excellent model to study genome evolution.
Polyploidy or whole-genome duplication is a widespread key driver of genome evolution. However, doubling the genome also brings challenges such as dosage compensation or chromosome paring errors during meiosis. The 3D structure of the genome is intricately linked to such processes but it remains unclear how chromosome architecture has evolved. To address this question, Wang et al. (2021) used genome-wide chromosome conformation capture (Hi-C ) to generate high-resolution maps for cultivated soybean, wild soybean, and common bean (Lieberman-Aiden et al., 2009). They found that changes in chromatin architecture influence transcriptional and epigenetic patterns during post-polyploid diploidization and domestication.
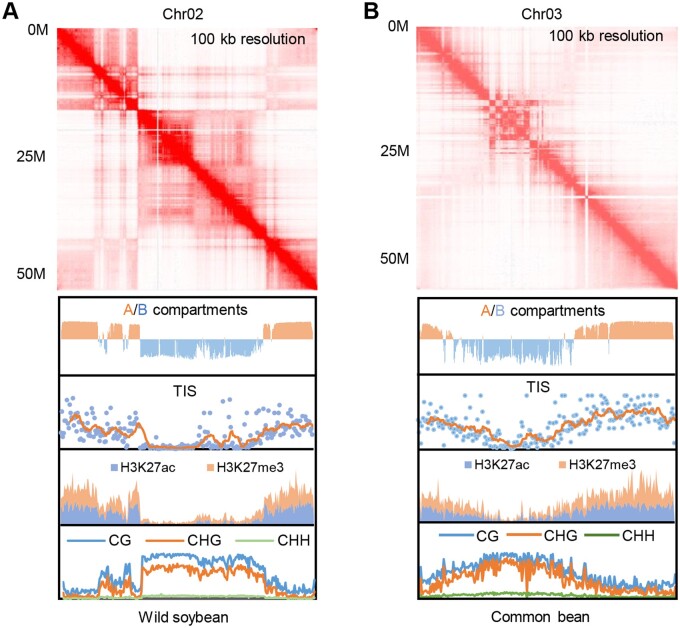
Hi-C analysis identified many interesting features of the G. max genome. Thirty five percent of the genome was compartmented into 2,621 topologically associated domains (TADs) with a median length of 105 kb. TAD boundaries were marked by high chromatin accessibility and active epigenetic marks, suggesting that these features may be involved in TAD establishment. Like in animals, the G. max genome was partitioned into A/B compartments (chromosome arms versus centromeres), supporting a conserved 3D spatial chromatin structure between Kingdoms (see Figure). Hi-C also determined intra- and interchromosomal loops. While intrachromosomal gene–gene loops connected 10,133 genes (one-sixth of all protein-coding genes), gene–distal loops were rarer. Interestingly, the latter had significantly higher expression levels compared to genes without loops, indicating that these types of chromatin loops contribute to coexpression of anchored genes. By contrast, most interchromosomal loops were located in the heterochromatic B compartment and these also displayed significantly higher expression levels than genes without loops. Together, this indicates that both inter- and intrachromosomal loops were positively associated with changes in gene expression.
Figure.
Genome architecture evolution between wild soybean (G. soja) and common bean (P. vulgaris). Genome-wide chromosome capture maps (Hi-C) and profiling of chromatin accessibility (displayed as Tn5 transposon insertion sites), histone post-translational marks (H3K27ac, and H3K27me3) and DNA methylation revealed the compartmentalization and features of wild soybean (A) and common bean (B). Data for Chromosomes 2 and 3 are shown. Adapted from Wang et al. (2021); Supplemental Figure S2.
Because of polyploidization, 75% of the G. max genes are present in multiple copies (Kim et al., 2009). Duplicated genes were highly enriched in the A compartments, indicating that genes located in chromosomal arms were more retained over time compared to genes in pericentromeric regions. Duplicated genes had significantly increased expression compared to single-copy genes, likely due to increased loop contacts, higher chromatintty ccessibilities, and the presence of active epigenetic marks. Duplicated gene pairs with one copy located in each type of compartment showed changes in expression between both compartments and were accompanied by increased chromosomal interactions, higher chromatin accessibility, and active epigenetic marks. Altogether, these data suggest that chromatin interactions may integrate epigenetic marks to maintain expression divergence between duplicated genes.
Wang et al. also compared Hi-C maps between G. max, G. soja, and P. vulgaris. They found that no significant differences in the proportions of genes located in TADs between the three species. However, half of the homologous genes with one copy in common bean and two copies in soybean were not associated with TADs in common bean. This result strongly suggests that polyploidization may induce changes in TAD architecture. During post-polyploid genome diploidization, chromosomes are fractured and rearranged. Identified chromosomal breakpoints were mostly located in genes and showed significant gene–gene contact differences between common bean and soybean. Break locations were void of TADs, had higher gene–gene contacts and chromatin accessibility, and were enriched in active epigenetic marks. Taken together, these results support a dramatic rearrangement of 3D chromatin architecture during polyploidization and diploidization.
Compared to polyploidization, domestication (G. max versus G. soja) seemed to have an overall weaker effect on genome architecture as most homologous genes remained within the same compartment. However, many divergent genes (wherein chromatin loops were exclusively detected in wild or cultivated soybean) showed a significant change in expression. This suggests a potential role of chromatin architecture in differential gene expression during domestication. In conclusion, this study nicely illustrates the potential of large-scale genomics to understand how genome architecture evolved during polyploidization and domestication and how this 3D evolution, together with epigenetic marks altered gene expression.
References
- Kim KD, Shin JH, Van K, Kim DH, Lee SH (2009) Dynamic rearrangements determine genome organization and useful traits in soybean. Plant Physiol 151: 1066–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin M, Herendeen PS, Wojciechowski MF (2005) Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the tertiary. Syst Biol 54: 575–594 [DOI] [PubMed] [Google Scholar]
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO. et al. (2009) Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326: 289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang l, Jia G, Jiang X, Cao S, Chen ZJ, Song Q (2021) Altered chromatin architecture and gene expression during polyploidization and domestication of soybean. Plant Cell 33: 1430–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]