Most crop plants have undergone a long process of domestication and each crop plant has its own unique domestication story. In the case of watermelon, its ancestral relatives produced small and sour fruits, a far cry from the large sweet melons we enjoy today. So how did watermelons get their sweetness? In their recent article in The Plant Cell, Yi Ren et al. (2021) use genetic and metabolomic tools to uncover major domestication events that altered watermelon fruit carbohydrate metabolism and transport.
Throughout development, plants transport sugars via the phloem from photosynthetic source organs (like leaves) to sink organs (like fruits and tubers). These source–sink relationships have major implications for agricultural yields and have been greatly impacted by crop domestication. Watermelons and other Cucurbitaceae are special crop plants because the major carbohydrate transported from source to sink organs is not sucrose, but raffinose family oligosaccharides (RFO), which consist of sucrose linked to one or more additional galactose units. Nevertheless, current watermelon cultivars accumulate high amounts of sucrose, fructose, and glucose, but not RFO, in their large fruits. The metabolic steps and genes that mobilize the RFO transported by the phloem into the simple sugars that accumulate in the vacuoles of fruit parenchymal cells have not been determined.
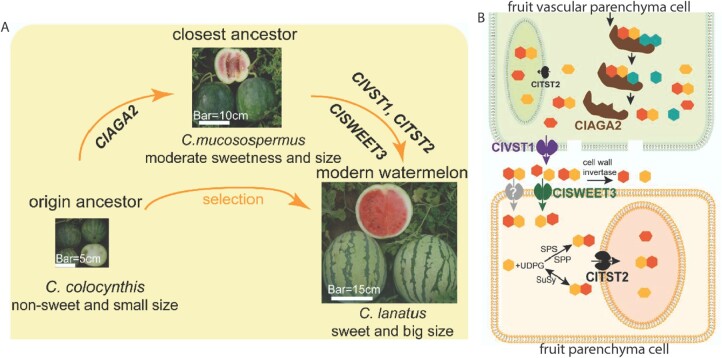
To directly assess changes to RFO metabolism during domestication, the authors first used reciprocal grafting of watermelon fruits onto stems from domesticated or ancestral species. The hydrolysis of RFO to sucrose and galactose was greatly reduced in nonsweet wild watermelons compared to cultivated sweet watermelons, regardless of stem origin. This indicates that sugar metabolism is regulated within fruits independently of source and stem tissues. A genome-wide association study across 135 watermelon accessions then identified a correlation between fruit RFO content and functional loci in the promoter region of ClAGA2, which encodes an alpha-galactosidase that hydrolyzes RFO. The expression of ClAGA2 localized mainly to fruit vascular bundles during the period of fruit sugar accumulation and, due to an enhanced transcription factor interaction with the ClAGA2 promoter, domesticated fruits displayed greater alpha-galactosidase activity. Furthermore, altered expression of ClAGA2 in transgenic sweet and nonsweet varieties directly correlated with fruit sucrose, fructose, and glucose content, indicating an important role for ClAGA2 promoter selection during watermelon domestication.
Following the hydrolysis of RFOs, sucrose and galactose must be transported out of the phloem and into the fruit parenchymal cells (see Figure 1). The authors previously found that the transporter ClVST1 functions to unload sucrose from the vascular tissues (Ren et al., 2020). Here, they found that the expression of the transporter ClSWEET3 correlated with fruit sugar content across watermelon accessions. ClSWEET3 was then determined to transport hexoses and to localize to the plasma membrane fruit parenchymal cells. Overexpression and knockdown of ClSWEET3 in nonsweet and sweet varieties, respectively, caused a reciprocal increases and decreases in fruit sugar levels, strongly implicating this hexose transporter in the sugar accumulation of watermelon fruit.
Figure.
Schematic of the domestication watermelon carbohydrate storage. A, Visual phenotype of sweet vs. unsweet watermelon varieties. B, The sequential role of ClAGA2, ClVST1, ClSWEET3, and ClTST2 in mobilizing sugar from phloem to the fruit parenchymal cell vacuole (Adapted from Ren et al. [2021] Supplemental Figure 5 [A] and Figure 7 [B]).
Once inside the fruit parenchyma cells, hexose sugars must be stored in the vacuole. The authors previously identified a tonoplast sugar transporter, ClTST2, whose expression levels correlated with watermelon sugar content (Ren et al., 2018). As expected, disruption of the ClTST2 gene substantially decreased sugar accumulation in cultivated watermelon fruit. The authors then created double and triple lines of claga2, clsweet3, and cltst2 that demonstrated the additive effects of ClAGA2, ClSWEET3, and ClSTS2 on sugar accumulation in domesticated watermelon. The accumulated insights suggest that increased fruit-localized expression of four sequential RFO metabolizing and transport genes played a major role in creating the increased sink strength and sugar accumulation that typifies sweet watermelon fruits (see Figure).
REFERENCES
- Ren Y, Guo S, Zhang J, He H, Sun H, Tian S, Gong G, Zhang H, Levi A, Tadmor Y, et al. (2018) A tonoplast sugar transporter underlies a sugar accumulation QTL in watermelon. Plant Physiol 176: 836–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Li M, Guo S, Sun H, Zhao J, Zhang J, Lui G, He H, Tian S, Gong G, et al. (2021) Evolutionary gain of oligosaccharide hydrolysis and sugar transport enhanced carbohydrate partitioning in sweet watermelon fruits. Plant Cell 33: 1554–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Sun H, Zong M, Guo S, Ren Z, Zhao J, Li M, Zhang J, Tian S, Wang J, et al. (2020) Localization shift of a sugar transporter contributes to phloem unloading in sweet watermelons. New Phytol 227: 1858–1871 [DOI] [PubMed] [Google Scholar]