Abstract
BACKGROUND AND OBJECTIVES:
CT measurements of sarcopenia have been proposed as biomarkers associated with outcomes in various cancers and have typically been evaluated at the L3 vertebral level. However, staging imaging for patients with extremity and truncal soft tissue sarcoma (STS) often only includes chest CT imaging which precludes evaluation at L3. Therefore, we sought to evaluate muscle metrics at T12 on standard staging chest CT scans and evaluate for correlation with overall and event-free survival in patients with STS.
METHODS:
CT Chest imaging for 89 patients with intermediate and high-grade STS (53 M, 36 F; 58.5±19.0 years old, follow up 37.4±27.1 months) was reviewed on PACS at T12 for skeletal muscle density (SMD) and skeletal muscle index (SMI).
RESULTS:
Overall survival increased with increased SMD on univariate (HR = 0.61 [0.43, 0.86]) and age-adjusted analysis (HR = 0.65 [0.42, 0.89]. Event-free survival also increased with increased SMD in univariate analyses (HR = 0.68 [0.49, 0.95]) but did not maintain significance after adjusting for age (HR = 0.68 [0.43, 1.07]). SMI was not a predictor of overall or event-free survival.
CONCLUSIONS:
Higher SMD measured on routinely obtained staging chest CTs in STS patients are associated with improved survival.
Keywords: sarcoma, sarcopenia, myosteatosis, muscle metrics
INTRODUCTION
Sarcopenia, as defined by proposed CT biomarkers, has been associated with poor prognosis in many forms of cancers including hepatocellular carcinoma, non-small cell lung carcinoma, renal cell carcinoma, esophageal carcinoma, breast carcinoma, bladder carcinoma, melanomas, and lymphomas [1–9]. In these patients, sarcopenia has been associated with multiple endpoints, including treatment failure, chemotherapy toxicity, and earlier tumor progression [10]. Clinically, sarcopenia is defined by the degenerative loss of skeletal muscle mass and function associated with aging and/or disease [10]. The most common imaging method for diagnosing sarcopenia in patients with cancer is computed tomography (CT), which allows objective measurements for assessment of muscle mass and adiposity [10]. Muscle adiposity, as an indicator of muscle quality, is measured using of skeletal muscle density (SMD) whereas muscle mass, as an indicator of muscle quantity, is measured using skeletal muscle index (SMI) [6].
Due to its impact on various cancer types, Wilson et. al hypothesized that muscle mass would also affect overall survival in soft tissue sarcoma (STS) [11]. Wilson et. al assessed skeletal muscle mass using SMI at the L3 vertebral level [11]. Recently, Hendrickson et. al similarly evaluated the association of SMI on mortality in both soft tissue and primary bone tumors [12]. However, the CT protocols for staging of extremity STS often do not image at the L3 level used in those studies [7, 10–12]. Moreover, there is limited data on SMI and SMD on pelvis and abdomen CTs in patients with soft tissue sarcoma [13–14]. Due to the proclivity for lung metastases, the staging and surveillance of STS primarily includes chest CT imaging, which includes the T12 vertebral level, while routine surveillance CT of the abdomen and pelvis is less common [15–16]. Although less often investigated than at the L3 level, muscle metrics at the T12 level have also been validated in assessment of outcomes in patients with other forms of cancer besides STS [17–19]. Direct comparisons of muscle metrics measured at T12 versus L3 yield similar results in patients undergoing thoracic endovascular aortic repair, with agreement assessed yielding nearly perfect Cohen κ values (>0.85) [20]. Our study aims to analyze both muscle quantity (i.e., SMI) and quality (i.e., SMD) at the T12 vertebral level on staging chest CTs and determine their association with overall survival and event-free survival in patients with grade 2 or 3 extremity and trunk STS undergoing surgical resection.
MATERIALS AND METHODS
Prior to initiation of the study, all procedures were reviewed and approved by the IRB of the University of California, Davis. From an initial 388 patients collected based on CPT codes for surgical resection for sarcoma of the extremities, pelvis, and trunk, demographic information, physical exam findings, laboratory results, imaging, operative reports, and pathology were retrospectively extracted from patients’ electronic medical records. In particular, we collected age, sex (M or F), ethnicity (white or other), Charlson-Deyo score (0 or >0), date of diagnosis, date of surgery, date of chest CT, date of locoregional or distant recurrence, date of last follow up, disease status at last follow-up (Alive with Disease (AWD), Died of Disease (DOD), or No Evidence of Disease (NED)), neo-adjuvant or adjuvant treatment status, tumor location (extremity or trunk), tumor size (≤10 cm or >10 cm), tumor grade (1, 2, or 3 according to the French Federation of Cancer Centers (FNCLCC)), tumor stage (II, IIIA, IIIB, or IV according to AJCC 8th ed.), surgical margins (negative or positive), as well as muscle metrics from CT images.
Patients with non-STS as well as grade 1 STS were excluded given the decreased proclivity for systemic disease. Patients who did not have a pre-operative baseline staging CT scan within 120 days prior to resection available for review were also excluded. For patients with multiple pre-operative CT scans, the CT scans closest to date of diagnosis was chosen except for two that did not capture the T12 interval.
89 patients (average age at diagnosis 58.5 ± 19.0 years with 59.6% male) were included in this single institution, retrospective review of patients diagnosed with grade 2 or 3 extremity and truncal STS between 2004–2017 for overall survival as shown in Figure 1. For event-free survival, where an event is defined as local or distant disease recurrence after surgical resection, ten patients with stage IV disease by the time of surgical resection were not included. This exclusion left 79 patients of whom 41 had NED at last follow up and 38 had DOD or had a disease recurrence either locally or distally.
Figure 1.
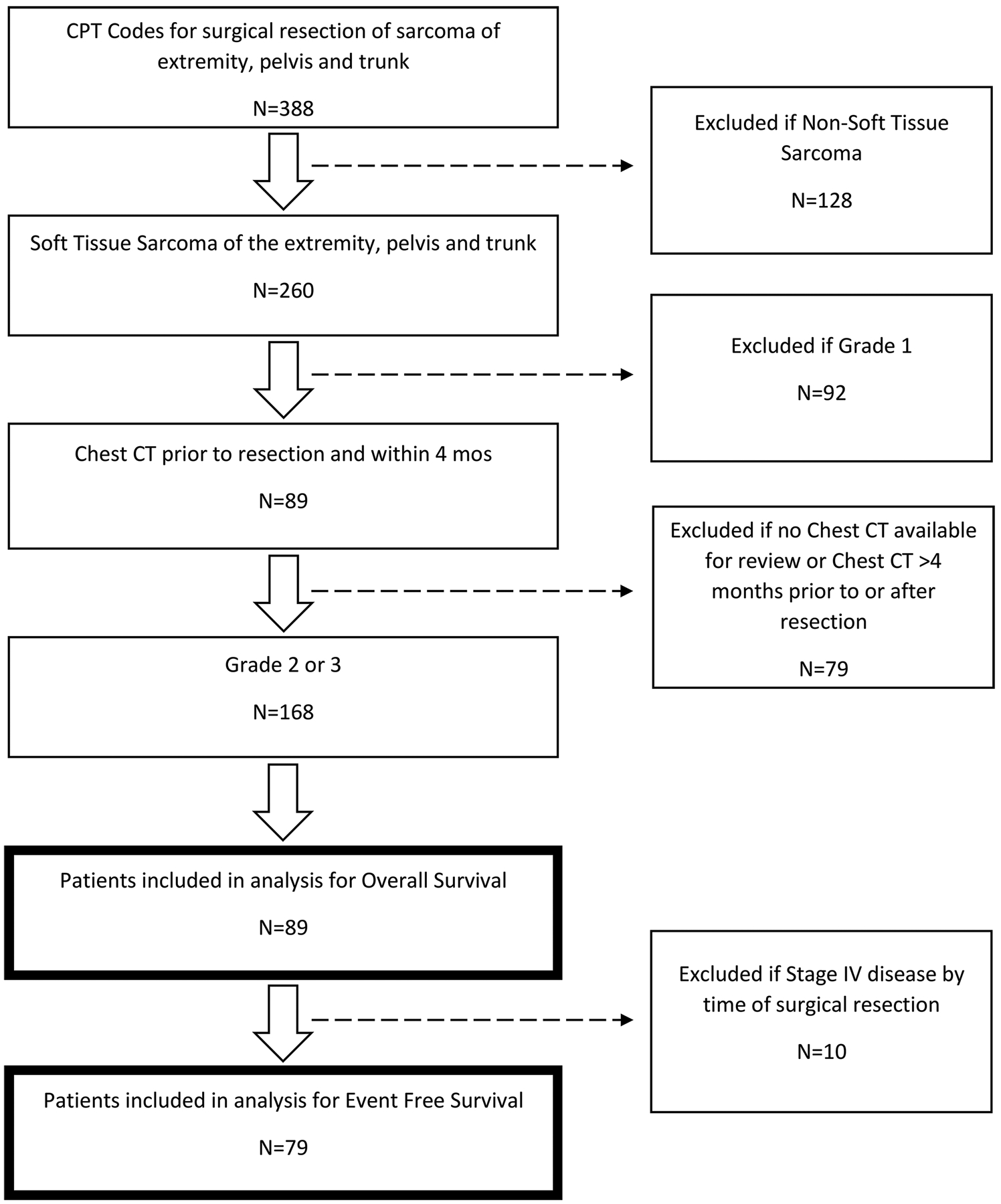
CONSORT diagram depicting patient exclusion criteria frequencies and final cohort size for overall survival and event free survival.
Muscle metrics were calculated by manually outlining the paravertebral muscles at T12 for SMD (measured in Hounsfield units, HU) and cross-sectional area (measured in cm2) as shown in Figure 2. Left and right paravertebral muscles were averaged in each patient. Muscle adiposity was evaluated based on CT density of the skeletal muscle, where lower SMD (HU) represents greater fat content within the muscle. Values were adjusted by −4.6 HU for CTs performed with intravenous contrast [21]. SMI (cm2/m2) was determined by adjusting the muscle area for patient height in meters squared, similar to BMI. Two common types of viewing software were used to make CT measurements, both the enterprise-wide PACS (which allows any viewer to make measurements) and OsiriX (which allows for semi-automated analysis of muscle). Images on PACS were analyzed by a single trained reader (R1) and images on OsiriX were analyzed by a different trained reader (R2). Both readers were blinded to patient clinical status and outcomes.
Figure 2.
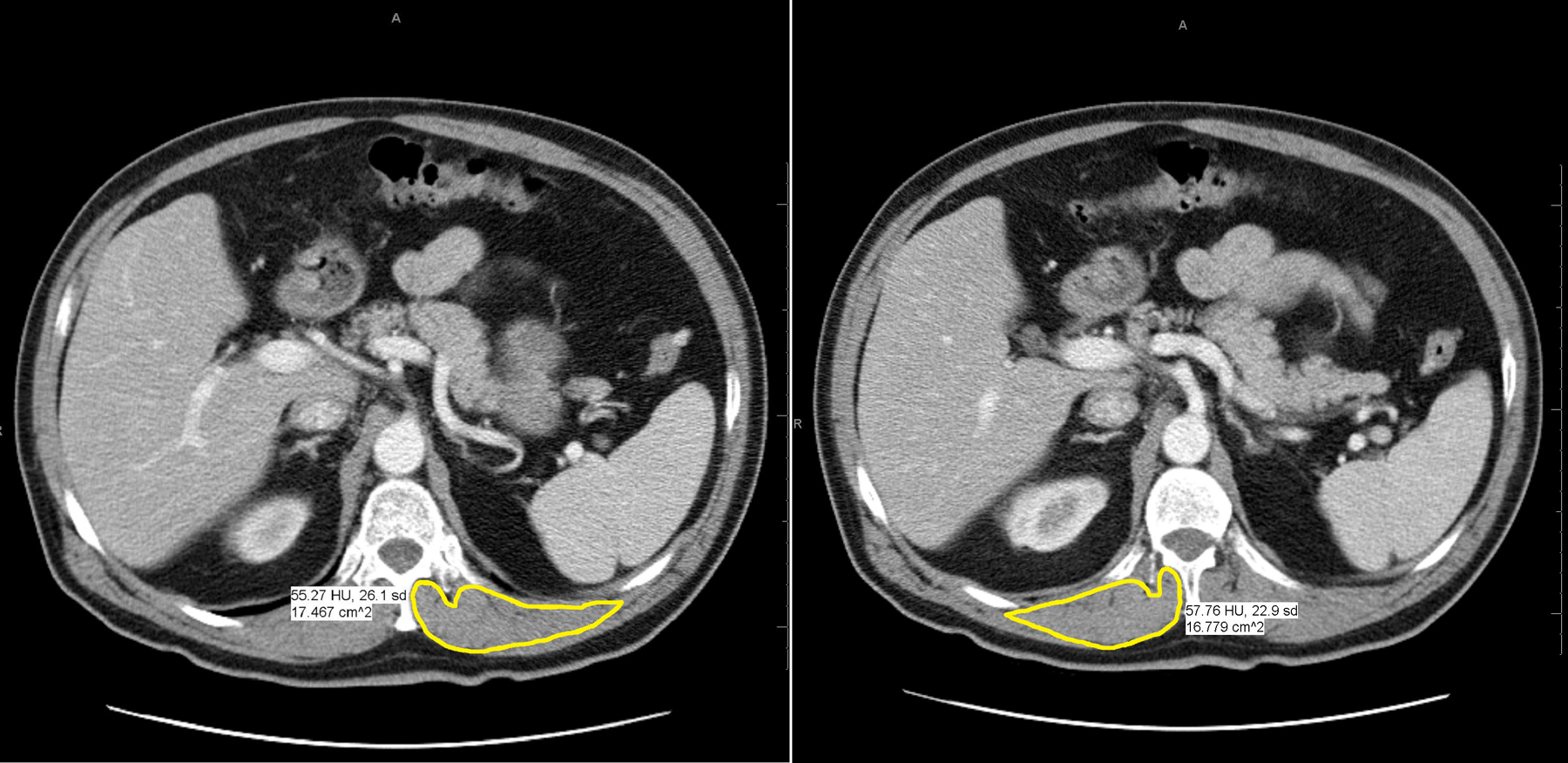
CT images of left and right paravertebral muscle density (HU) and cross-sectional area (cm2) using PACS viewing software.
All 89 patients’ vertebral levels were assessed for consensus between the two readers. 20% of images were measured twice by each reader to obtain intra-reader reliability. Average muscle metrics was calculated across both sides of the patient using each reader’s first observation for use as predictors in survival analysis. Observations from the two readers were not combined because they used different measuring software.
The effect of age on overall and event-free survival was evaluated with proportional hazard models. Log-rank tests were used to evaluate the association between overall and event-free survival with the following patient characteristics: sex (M vs. F), ethnicity (white vs. other), Charlson-Deyo score (0 vs. >0), tumor location (trunk vs. extremity), tumor size (≤10 cm vs. >10 cm), tumor grade (2 vs. 3), AJCC 8th ed. stage (II, IIIA, IIIB, or IV), and surgical margins (0 vs. 1). Proportional hazard models were used to evaluate the effect of each muscle metric on overall and event-free survival univariately and then with age as a covariate. Due to the relatively small number events which decreased even further for some categories of patient characteristics, no additional covariates were included in the modeling. Age was centered at 0 and scaled to a standard deviation of 1. Survival modeling was conducted separately using each reader’s muscle measurements. For the statistical analysis, SMD was centered at 0 and scaled to a standard deviation of 1. SMI was similarly centered and scaled within sex to remove sex differences. Kaplan-Meier plots for overall survival and event-free survival were plotted for each muscle metric stratified into three groups based on standard deviations by standardized (Z) scores: Z ≤ −1, −1 > Z < 1, Z ≥ 1. Intra-reader reliability was estimated with the intraclass class correlation assuming fixed assessors and random subjects. All statistical analyses were two-tailed at a significance level of 0.05 and performed using R Statistical Computing Software Version 3.6.1.
RESULTS
Table I summarizes patients’ pre-operative and disease characteristics as well as CT muscle metrics by the following classifications: AWD, DOD, NED.
Table I.
Patient Demographics
| AWD | DOD | NED | |
|---|---|---|---|
| Age | |||
| Average | 52.9 ± 20.3 | 61.9 ± 14.1 | 58.2 ± 21.9 |
| Sex | |||
| Female | 3 (23.1%) | 14 (41.2%) | 19 (45.2%) |
| Male | 10 (76.9%) | 20 (58.8%) | 23 (54.8%) |
| Ethnicity | |||
| African American | 3 (23.1%) | 14 (41.2%) | 19 (45.2%) |
| Asian | 10 (76.9%) | 20 (58.8%) | 23 (54.8%) |
| Caucasian | 6 (46.2%) | 21 (61.8%) | 31 (73.8%) |
| Hispanic or Latino | 3 (23.1%) | 4 (8.8%) | 5 (11.9%) |
| Other | 2 (15.4%) | 5 (14.7%) | 3 (7.1%) |
| Charlson-Deyo Score | |||
| 0 | 7 (53.8%) | 23 (67.6%) | 27 (64.3%) |
| 1 | 5 (38.5%) | 7 (20.6%) | 8 (19%) |
| 2 | 1 (7.7%) | 4 (11.8%) | 7 (16.7%) |
| Tumor Location | |||
| Extremity | 12 (92.3%) | 26 (76.5%) | 36 (85.7%) |
| Trunk | 1 (7.7%) | 8 (23.5%) | 6 (14.3%) |
| Tumor Size | |||
| ≤ 10 cm in greatest dimension | 4 (30.8%) | 19 (55.9%) | 32 (76.2%) |
| >10 cm in greatest dimension | 9 (69.2%) | 15 (44.1%) | 10 (23.8%) |
| Tumor Grade | |||
| 2 | 2 (15.4%) | 2 (5.9%) | 5 (11.9%) |
| 3 | 11 (84.6%) | 32 (94.1%) | 37 (88.1%) |
| Tumor Stage | |||
| II | 1 (7.7%) | 3 (8.8%) | 16 (38.1%) |
| IIIA | 3 (23.1%) | 15 (44.1%) | 16 (38.1%) |
| IIIB | 6 (46.2%) | 10 (29.4%) | 10 (23.8%) |
| IV | 3 (23.1%) | 6 (17.6%) | 0 (0%) |
| Surgical Margins | |||
| Negative | 10 (76.9%) | 29 (85.3%) | 39 (92.9%) |
| Positive | 3 (23.1%) | 5 (14.7%) | 3 (7.1%) |
| Tumor Pre-Op Treatment | |||
| Both | 2 (15.4%) | 8 (23.5%) | 8 (19%) |
| Chemo | 2 (15.4%) | 1 (2.9%) | 2 (4.8%) |
| None | 5 (38.5%) | 11 (32.4%) | 15 (35.7%) |
| RT | 4 (30.8%) | 14 (41.2%) | 17 (40.5%) |
| Tumor Post-Op Treatment | |||
| Both | 3 (23.1%) | 4 (11.8%) | 2 (4.8%) |
| Chemo | 5 (38.5%) | 13 (38.2%) | 4 (9.5%) |
| None | 3 (23.1%) | 11 (32.4%) | 31 (73.8%) |
| RT | 2 (15.4%) | 6 (17.6%) | 5 (11.9%) |
| Length of Follow-Up | |||
| Average | 31.96 ± 19.23 | 23.53 ± 18.63 | 50.21 ± 29.13 |
| PACS SMD (HU) | |||
| Average | 37.3±16.6 | 34.2±13.2 | 41.2±13.2 |
| PACS SMI (cm2/m2) | |||
| Average | 4.9±1.5 | 4.5±1.3 | 4.4±1.3 |
| OsiriX SMD (HU) | |||
| Average | 39.4 ± 15.9 | 36.9 ± 12.9 | 43.7 ± 12.8 |
| OsiriX SMI (cm2/m2) | |||
| Average | 3.9 ± 1.2 | 3.9 ± 2.0 | 3.5± 1.2 |
Overall Survival
Two-year and five-year overall survival was 69% [95% CI, 59% – 80%] and 54% [95% CI, 42–68] respectively for the entire cohort. Overall survival was associated with stage (p = 0.008). (Table II). Sex, race, Charlson-Deyo, location, grade, tumor size and margins were not significantly associated with survival (Table II).
Table II.
Association between patient and tumor characteristics with Overall Survival and Event Free Survival.
| Overall Survival | Event-free Survival | |
|---|---|---|
| Variable | P-value | P-value |
| Age | 0.060b | 0.03b |
| Sex (M vs. F) | 0.80a | 0.30a |
| Ethnicity (White vs. other) | 0.40a | 0.80a |
| Charlson-Deyo (0 vs. > 0) | 0.70a | 0.30a |
| Location (Extremity vs. Trunk/Other) | 0.70a | 0.50a |
| Size (≤ 10 cm vs.>10 cm) | 0.080a | 0.40a |
| Grade (2 vs. 3) | 0.40a | 0.30a |
| Stage (II, IIIa,IIIb IV) | 0.008a | 0.20a |
| Margins (− vs. +) | 0.50a | 0.20a |
Log rank test
Proportional hazard model
In univariate analyses of the effects of muscle metrics, overall survival was significantly related to SMD for both readers (HR 0.61 [0.43, 0.86]) with increased survival associated with increased SMD (Table III). This relationship persisted after adjusting for age (HR 0.65 [0.42, 0.98] on PACS and HR 0.64 [0.42, 0.98] on OsiriX) (Table III). For SMI, however, there was insufficient evidence to reject the null hypothesis of no association with overall survival with or without adjusting for age for either reader (Table III). The risk of death generally increased with increasing age (HR = 1.02 [1.00, 1.04]) although this did not reach statistical significance.
Table III.
Hazard ratios [95% confidence limits] and p-values for one standard deviation change in sarcopenia metric from proportional hazard model analysis of the effect of muscle metrics on overall survival with (Age-adjusted) and without (Univariate) adjusting for age
| SMD | SMI | |||
|---|---|---|---|---|
| HR [95% CI] | p-value | HR [95% CI] | p-value | |
| Univariate | ||||
| PACS | 0.61 [0.43, 0.86] | 0.005 | 1.09 [0.74, 1.6] | 0.673 |
| OsiriX | 0.61 [0.43, 0.86] | 0.005 | 1.09 [0.79, 1.5] | 0.603 |
| Age-adjusted | ||||
| PACS | 0.65 [0.42, 0.98] | 0.041 | 1.26 [0.85, 1.88] | 0.249 |
| OsiriX | 0.64 [0.42, 0.98] | 0.038 | 1.21 [0.89, 1.63] | 0.225 |
Figure 3 shows Kaplan-Meier curves for each reader and muscle metric. Consistent with the proportional hazard modeling, KM curves demonstrated overall survival was greatest for patients with higher values (Z ≥ 1) one standard deviation or greater above the mean for SMD but with no apparent relationship for SMI (Figure 3). At 2 years, overall survival was 27%, 69.6%, and 100%, for SMD one standard deviation or less below the mean, SMD within a standard deviation of the mean, and SMD one standard deviation or higher above the mean respectively. Similarly, overall survival 5 years showed a progressive relationship of increasing survival: 27%, 52.5%, and 83.3%, with higher standardized deviations of SMD.
Figure 3.
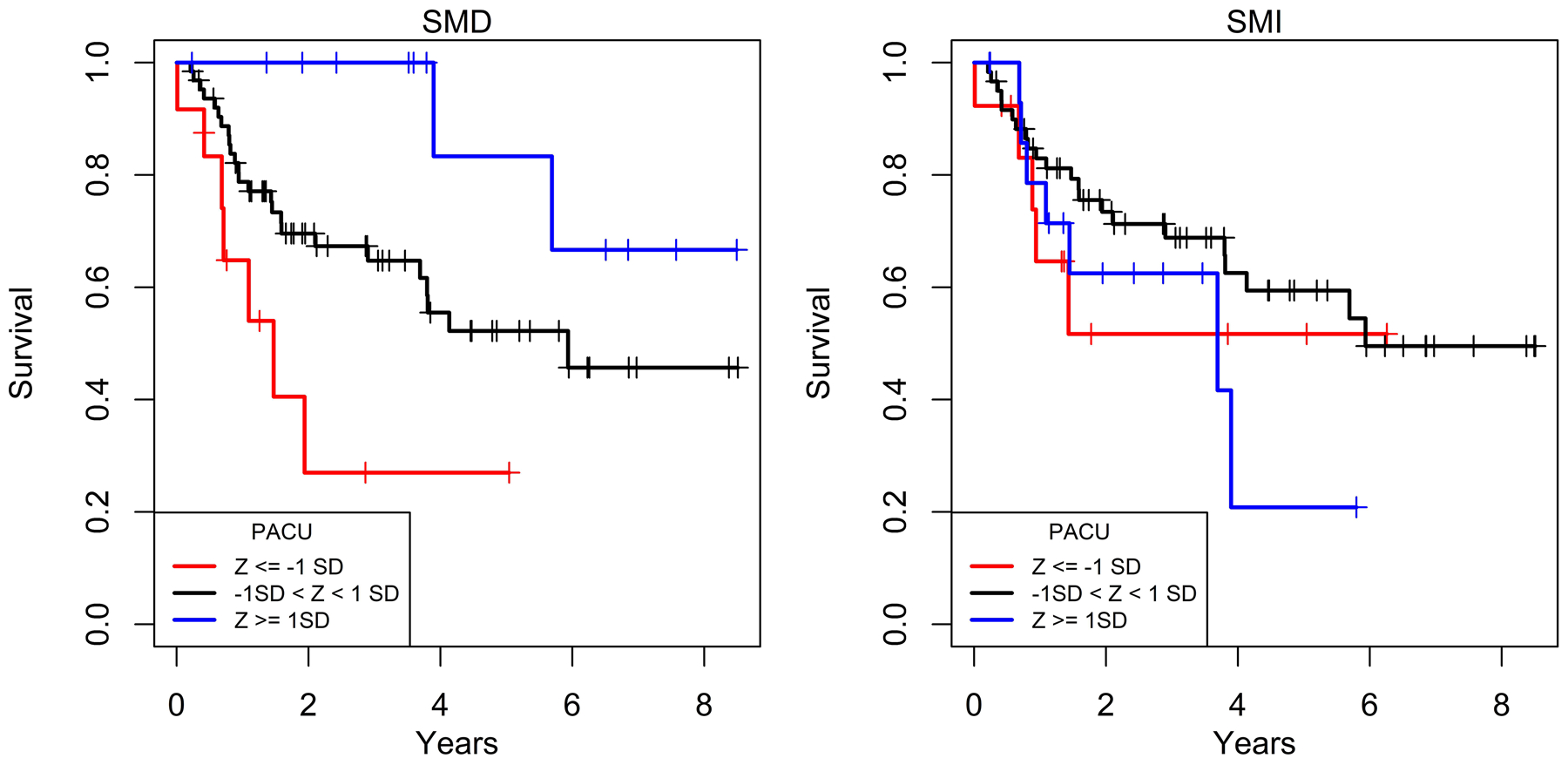
Kaplan-Meier curves of overall survival relative to PACS by standardized deviations of SMD and SMI. Overall survival progressively increased with higher standardized deviation categories above the mean SMD but did not change in association with standardized deviation categories of SMI.
Event-Free Survival
Like overall survival, event-free survival was also significantly associated with tumor stage (Table II). Although the estimated hazard of death or recurrence generally increased with age (HR = 1.01 [0.99, 1.03]), this did not reach statistical significance.
In univariate analyses, event-free survival increased with SMD for both observers (Table IV). When age was included the estimated effect of SMD remained similar, but the confidence intervals widened encompassing a hazard ratio of 1.0. Thus, a potential independent effect of SMD on event-free survival after adjusting for age could not be determined. Event-free survival was not significantly related to SMI without or with accounting for age, for either reader.
Table IV.
Hazard ratios [95% confidence limits] and p-values for one standard deviation change in sarcopenia metric from proportional hazard model analysis of the effect of muscle metrics on event-free survival with (Age-adjusted) and without (Univariate) adjusting for age.
| SMD | SMI | |||
|---|---|---|---|---|
| HR [95% CI] | p-value | HR [95% CI] | p-value | |
| Univariate | ||||
| PACS | 0.68 [0.49, 0.95] | 0.024 | 1.28 [0.89, 1.85] | 0.182 |
| OsiriX | 0.66 [0.48, 0.92] | 0.015 | 1.13 [0.85, 1.49] | 0.404 |
| Age-adjusted | ||||
| PACS | 0.68 [0.43, 1.07] | 0.098 | 1.35 [0.88, 2.08] | 0.165 |
| OsiriX | 0.69 [0.45, 1.08] | 0.102 | 1.25 [0.9, 1.74] | 0.187 |
Similarly, to overall survival, event-free survival was greatest for patients with higher values (Z ≥ 1) one standard deviation or greater above the mean for SMD but with no apparent relationship for SMI (Figure 4). At 2 years, overall survival was 12.5%, 55.4%, and 90%, respectively, for SMD one standard deviation or less below the mean, SMD within a standard deviation of the mean, and SMD one standard deviation or higher above the mean. At 5 years, overall survival also showed a progressive relationship of increasing survival: 12.5%, 46.4%, and 80%, with higher standardized deviations of SMD.
Figure 4.
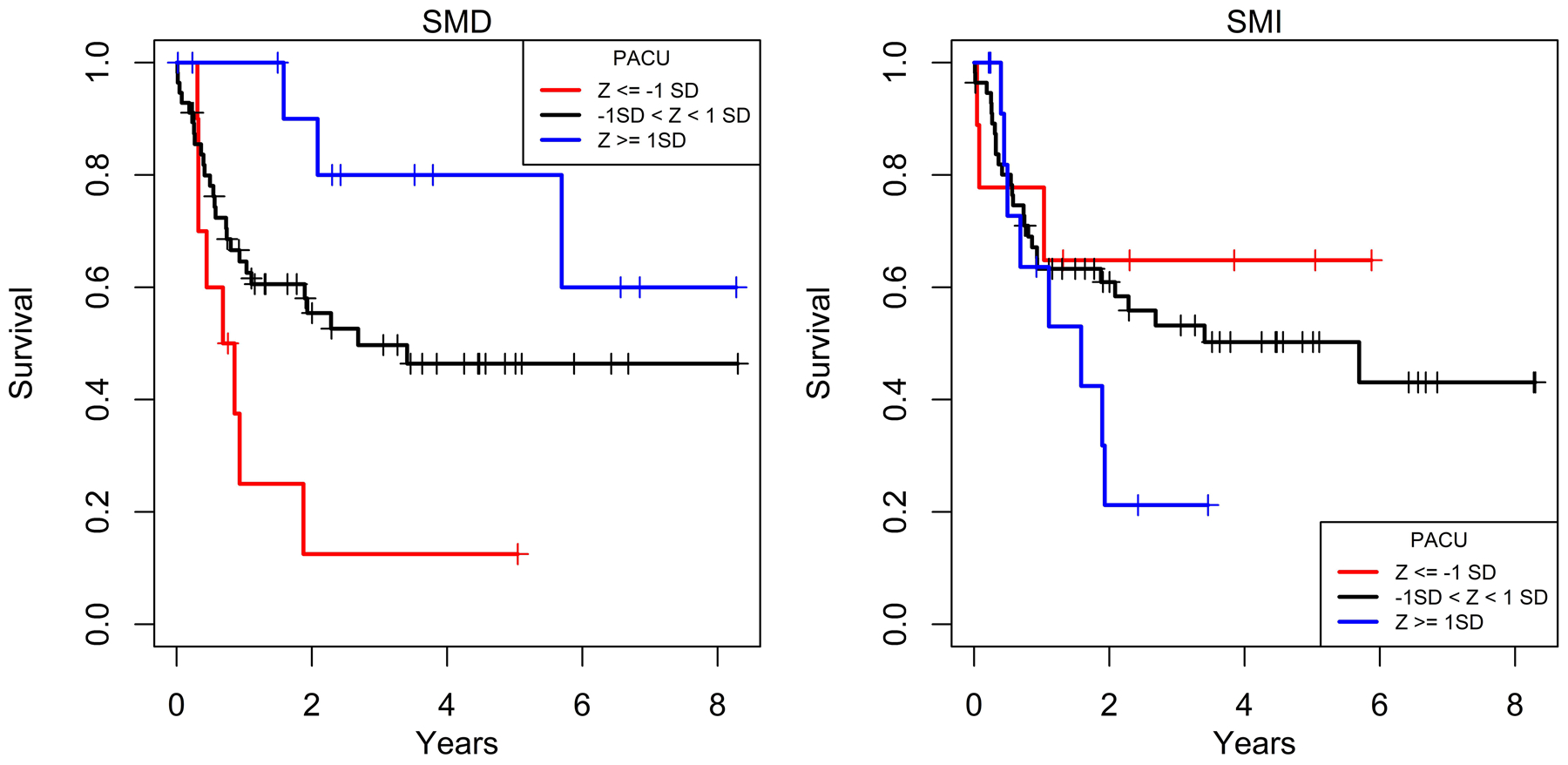
Kaplan-Meier curves of event-free survival relative to PACS by standardized deviations of SMD and SMI. Event-free survival progressively increased with higher standardized deviation categories with SMD but did not change in association with standardized deviation categories of SMI.
Intra-rater Reliability
Both readers had high intra-class correlation, indicating excellent reliability. For Reader 1 using PACS, intra-class correlation was ≥ 0.9 for measures of SMD and SMI. For Reader 2 using OsiriX, intra-class correlation was above 0.9 for all measures of SMD. SMI was less reliable on OsiriX, with intra-class correlation ranging from 0.47–0.94.
DISCUSSION
SMD, a measure of muscle quality, can be reliably determined at the T12 level on staging chest CT scans. Patients with higher SMD, representing decreased muscle adiposity and increased muscle quality, had increased overall and event-free survival compared to patients with lower SMD. This relationship persisted with inclusion of age for overall survival. For event-free survival, the estimated mean effect of SMD also was similar after controlling for age although the relationship did not remain statistically significant. This change in significance may be due to the relatively small sample size with limited statistical power. These results suggest an association between survival and muscle quality at the T12 level on chest CT and support the work of Veld et al [13] who reported psoas SMD on abdominal CT (L4 level) was associated with mortality in fully adjusted models. A larger series would be necessary to determine the relative contributions of age and muscle adiposity as measured by SMD.
SMI, as a measure of paravertebral muscle quantity at the T12 level, was not significantly associated with overall or event-free survival. Despite recent findings by Hendrickson et al that SMI is an independent predictor of 1-year mortality in patients with sarcomas in the extremities, our results are consistent with previous results reported by Wilson et al where SMI was not a predictor of overall survival (p = 0.746), after controlling for sex (p = 0.712) in a Cox proportional hazard model [11]. Unlike Wilson et al and our current study, Hendrickson et al included both bone and soft tissue sarcomas in their analysis, which may result in greater heterogeneity in the cohort due to disease and treatment differences [12]. Unlike both prior studies that evaluated the psoas muscle on abdominal CTs at the L3 level, our study assessed the paravertebral muscle on chest CTs at the T12 level. Although skeletal muscles are most commonly evaluated by CT at the L3 level, measurements at T10-L5 have been validated [19, 22]. Importantly, paravertebral muscle density and size can now be measured automatically with machine learning algorithms in large cohorts [23], such as in the National Lung Screening Trial [24].
Inherent limitations of our study include the small sample size which is reflective of the rarity of STS as well as our restriction to Grade 2 and 3 disease. The non-significant findings of SMI’s influence on overall survival in patients with STS in our study and Wilson’s may be due to the lack of power. Moreover, our study is a single-centered, retrospective evaluation of STS with significant histological heterogeneity. Insufficient follow-up data and the relatively small sample size further limited our statistical analysis. In line with previous literature where STS stage was important in survival outcomes of STS, univariate analysis demonstrated STS stage was significantly associated with overall and event-free survival, confirming the validity of our patient data [25–26]. Multi-variate modeling was not performed due to limitations of the cohort size. However, age was adjusted for due to the expected confounding between age and muscle metrics [10].
Strengths of our study include the use of two image analysis programs by two trained independent readers demonstrating high intra-reader reliability. Furthermore, compared to most prognostic tumor biomarkers such as tumor grading, staging, and histology that account for tumor aggressiveness, proxy measures of muscle by SMD and SMI may reflect both tumor and patient characteristics [6]. Sarcopenia can develop secondarily from tumor-induced systemic inflammation or metabolic disturbances representing an intrinsically weakened, functionally impaired patient condition. These metabolic disturbances have been demonstrated in multiple studies with various forms of cancer using a prognostic value of the CRP/Alb ratio [6, 27–28]. Due to its ability to assess both tumor and patient status, CT-derived muscle metrics can serve as opportunistic prognostic biomarkers of survival outcomes in STS. At the same time, skeletal muscle represents a potentially modifiable risk factor, regardless of the cause of muscle depletion (e.g., age-related sarcopenia or cancer-related cachexia). Diagnosis using opportunistic CT could allow interventions earlier in the disease course, when the window of anabolic potential is open [29], which may help improve sarcopenia and thus outcomes in patients with STS. Currently, there are 116 trials in progress evaluating nutrition, supplements, exercise (e.g., pre-habilitation), diet, and pharmaceuticals for sarcopenia (listed at clinicaltrials.gov) [30].
Discrete categorical values for muscle metrics in STS staging chest CTs could be useful in screening patients, thereby identifying those patients at risk of poor prognosis for possible intervention and adjustments to treatment including nutritional support and adjuvant therapies. While this study found survival to generally increase with increasing SMD values, given the small sample size and inherent patient variability, a considerably larger sample size would be needed to identify specific thresholds for clinical application.
CONCLUSIONS
Our study is the first to show that muscle quality (i.e., SMD) on chest CTs obtained for routine staging is a significant predictor of survival in patients with STS. Furthermore, these measurements were reproducible between different readers and different image analysis platforms. We did not find any significant association between paravertebral muscle quantity (i.e., SMI) at T12 and survival in patients with STS, mirroring the results of previous studies evaluating psoas SMI at L3 on CTs of the abdomen [11]. Future studies are needed to confirm our findings that SMD on routine chest CT scans is an independent predictor of mortality in STS and investigate clinically relevant cut-off values that may aid in risk stratification (e.g., predict treatment failure during adjuvant therapy), contribute to personalized medical management, and ultimately optimize patient outcomes.
Synopsis:
Our study evaluates muscle metrics, measured at the T12 vertebral level on staging chest CT scans, as prognostic indicators of overall and event-free survival in patients with soft tissue sarcomas.
Acknowledgements:
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health through grant # UL1 TR001860
Disclosures and Funding Sources:
Statistical analysis was supported by the National Center for Advancing Translational Sciences, National Institutes of Health through grant # UL1 TR001860
ABBREVIATIONS
- STS
Soft Tissue Sarcoma
- SMD
Skeletal Muscle Density in Hounsfield Units (HU) as a measure of muscle quality
- SMI
Skeletal Muscle Index in (cm2/m2) as a measure of muscle quantity adjusted for patient height
- FNCLCC
French Federation of Cancer Centers
- AJCC
American Joint Committee on Cancer
- CI
Confidence Interval
- CT
Computed Tomography
- HR
Hazard Ratio
- AWD
Alive with Disease
- DOD
Died of Disease
- NED
No Evidence of Disease
Footnotes
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Harimoto N, Shirabe K, Yamashita YI, et al. : Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg 2013; 1523–1530. [DOI] [PubMed] [Google Scholar]
- 2.Prado CM, Lieffers JR, McCargar LJ, et al. : Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008; 629–635. [DOI] [PubMed] [Google Scholar]
- 3.Psutka SP, Boorjian SA, Moynagh MR, et al. : Decreased Skeletal Muscle Mass is Associated with an Increased Risk of Mortality after Radical Nephrectomy for Localized Renal Cell Cancer. J Urol 2016; 270–276. [DOI] [PubMed] [Google Scholar]
- 4.Harada K, Ida S, Baba Y, et al. : Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus 2016; 627–633. [DOI] [PubMed] [Google Scholar]
- 5.Villaseñor A, Ballard-Barbash R, Baumgartner K, et al. : Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv 2012; 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukushima H, Takemura K, Suzuki H, and Koga F: Impact of Sarcopenia as a Prognostic Biomarker of Bladder Cancer. Int J Mol Sci 2018; 2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabel MS, Lee J, Cai S, et al. : Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 2011; 3579–3585. [DOI] [PubMed] [Google Scholar]
- 8.Go S, Park MJ, Song HN, et al. : Prognostic impact of sarcopenia in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Cachexia Sarcopenia Muscle 2016; 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukagoshi M, Yokobori T, Yajima T, et al. : Skeletal muscle mass predicts the outcome of nivolumab treatment for non-small cell lung cancer. Medicine (Baltimore) 2020; 99(7):e19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prado CM, Baracos VE, McCargar LJ, et al. : Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009; 2920–2926. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RJ, Alamanda VK, Harley KG, et al. : Sarcopenia Does Not Affect Survival or Outcomes in Soft-Tissue Sarcoma. Sarcoma 2015; 146481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendrickson NR, Mayo Z, Shamrock A, et al. : Sarcopenia is associated with increased mortality but not complications following resection and reconstruction of sarcoma of the extremities. J Surg Oncol 2020. [DOI] [PubMed] [Google Scholar]
- 13.Veld J, Vossen JA, De Amorim Bernstein K, et al. : Adipose tissue and muscle attenuation as novel biomarkers predicting mortality in patients with extremity sarcomas. Eur Radiol 2016;26(12):4649–4655. [DOI] [PubMed] [Google Scholar]
- 14.DeAmorim BK, Bos SA, Veld J, et al. : Body composition predictors of therapy response in patients with primary extremity soft tissue sarcomas. Acta Radiol 2018;59(4):478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crago AM, Brennan MF: Principles in Management of Soft Tissue Sarcoma. Adv Surg 2015; 107–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casali PG, Jost L, Sleijfer S, et al. : Soft tissue sarcomas: ESMO clinical recommendations for diagnosis, treatment and follow up. Ann Oncol 2008; 89–93. [DOI] [PubMed] [Google Scholar]
- 17.Lee CS, Cron DC, Terjimanian MN, et al. : Dorsal muscle group area and surgical outcomes in liver transplantation. Clin Transplant 2014; 1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutin RD, Bamrungchart S, Bateni CP, et al. : CT of patients with hip fracture: muscle size and attenuation help predict mortality. Am J Roentgenol 2017; W208–W215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derstine BA, Holcombe SA, Ross BE, et al. : Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep 2018; 11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panthofer AM, Olson SL, Harris DG, Matsumura JS:. Derivation and validation of thoracic sarcopenia assessment in patients undergoing thoracic endovascular aortic repair. J Vasc Surg. 2019;69(5):1379–1386. [DOI] [PubMed] [Google Scholar]
- 21.Boutin RD, Kaptuch JM, Bateni CP, et al. : Influence of IV Contrast Administration on CT Measures of Muscle and Bone Attenuation: Implications for Sarcopenia and Osteoporosis Evaluation. Am J Roentgenol 2016; 1046–1054. [DOI] [PubMed] [Google Scholar]
- 22.Amini B, Boyle SP, Boutin RD, Lenchik L: Approaches to Assessment of Muscle Mass and Myosteatosis on Computed Tomography: A Systematic Review. J Gerontol A Biol Sci Med Sci 2019; 74:1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenchik L, Heacock L, Weaver AA, et al. : Automated Segmentation of Tissues Using CT and MRI: A Systematic Review. Acad Radiol 2019; 26:1695–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnard R, Tan J, Roller B, et al. : Machine Learning for Automatic Paraspinous Muscle Area and Attenuation Measures on Low-Dose Chest CT Scans. Acad Radiol 2019; 26:1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brennan MF, Antonescu CR, Moraco N, Singer S: Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg 2014; 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maretty-Nielsen K: Prognostic factors in soft tissue sarcoma. Dan Med J 2014; B4957. [PubMed] [Google Scholar]
- 27.Liu Z, Jin K, Guo M, et al. : Prognostic Value of the CRP/Alb Ratio, a Novel Inflammation-Based Score in Pancreatic Cancer. Ann Surg Oncol 2017; 561–568. [DOI] [PubMed] [Google Scholar]
- 28.Shen Y, Chen J, Chen X, et al. : Prevalence and Associated Factors of Sarcopenia in Nursing Home Residents: A Systematic Review and Meta-analysis. J Am Med Dir Assoc 2019; 5–13. [DOI] [PubMed] [Google Scholar]
- 29.Prado CM, Purcell SA, Laviano A: Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle 2020. January 8. doi: 10.1002/jcsm.12525. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/home Accessed 2/17/2020.
- 31.[dataset] Phan EN, Thorpe SW, Wong FS, et al. ; 2020; Opportunistic Muscle Measurements on Staging Chest CT for Extremity and Truncal Soft Tissue Sarcoma Are Associated with Survival; Data stored at University of California Davis School of Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]


