Abstract
Background
We investigated potential predictive factors for mortality and disease severity from demographic and clinical data, comorbidities, and laboratory findings in patients with confirmed COVID-19 who were consecutively admitted to our tertiary hospital.
Methods
In this retrospective, single-center, observational study, we enrolled consecutive 540 adult patients who had COVID-19 confirmed by a molecular method. Patients were categorized into three groups based on disease severity. Patients’ demographic and clinical characteristics, mortality rates, and mortality-associated factors were analyzed.
Results
The overall mortality rate was 4.3% (23/540). Disease severity was mild in 40.9% (n = 221), severe in 53.7% (n = 290), and critical in 5.4% (n = 29) of the patients. There were significant differences among groups in terms of median white blood cell (WBC), hemoglobin, neutrophil, lymphocyte, and thrombocyte counts, as well as C-reactive protein (CRP), procalcitonin, lactate dehydrogenase (LDH), creatinine, albumin, D-dimer, ferritin, troponin, and fibrinogen levels. Furthermore, there were significant differences between surviving and non-surviving patient groups in terms of median WBC, hemoglobin, neutrophil, and lymphocyte counts, as well as CRP, procalcitonin, LDH, creatinine, albumin, D-dimer, and ferritin levels. CRP level (odds ratio [OR]: 1.020, 95% confidence interval [CI]: 1.009–1.032; p < 0.001), and CURB-65 score (OR: 4.004, 95% CI: 1,288–12,447; p = 0.017) were independently associated with disease severity and mortality.
Conclusion
On admission, WBC, neutrophil, lymphocyte, and platelet counts can be used to predict disease severity in patients with COVID-19. CRP, ferritin, LDH, creatinine, troponin, D-dimer, fibrinogen, and albumin levels can also be used to predict disease severity in these patients. Finally, elevated CRP level and high CURB-65 score were predictors of disease severity and mortality.
Keywords: COVID-19, clinical characteristics, demographic characteristics, disease severity, mortality
Introduction
The coronavirus disease 2019 (COVID-19) outbreak began in Hubei Province, China, in December 2019 and spread rapidly to every continent except Antarctica. In Turkey, the first affected patient was reported on March 11, 2020.1 The most common reported comorbidities in patients with COVID-19 are hypertension (HT) and diabetes mellitus (DM). Furthermore, old age and comorbid conditions are risk factors for severe disease.2 Lippi et al reported that an increased procalcitonin level and a low platelet count were associated with an increased risk of severe disease in patients with COVID-19.3,4 Serum inflammatory markers (eg, C-reactive protein [CRP] and interleukin-6) are elevated in patients with severe disease. However, research is ongoing regarding routine blood test markers that can predict disease severity.5,6
In this study, we investigated potential predictive factors for mortality and disease severity from among demographic and clinical data, comorbidities, and laboratory findings in patients with confirmed COVID-19 who were admitted to the Emergency Department (ED).
Materials and Methods
Study Design and Patients
This retrospective, single-center, observational study was conducted in accordance with the 1989 Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Haseki Research and Training Hospital in Istanbul, Turkey (no. 120). The Advisory Board on Coronavirus Research of the Turkish Ministry of Health approved the study. Patient consent to review their medical records was not required by the IRB, because, there were no potentially identifying marks and no patient identifiers in the images or accompanying text.
This study included consecutive 540 Turkish adult patients aged ≥18 years who were admitted to the pandemic ward or pandemic intensive care unit (ICU) with signs of COVID-19 confirmed by real-time polymerase chain reaction (RT-PCR) of nasopharyngeal or oropharyngeal swab specimens. Treatment was performed and specimens were obtained in the ED of our tertiary pandemic hospital from March 11, 2020 (when the first patient was reported in Turkey) to May 31, 2020. Patients were not included in this study if they were younger than 18 years of age, were not diagnosed via RT-PCR test, or had insufficient available information in the medical records.
Variable Selection
Data were collected by searching for U06.0 and U07.3 International Classification of Disease codes in the hospital’s automation systems and archives. We assessed patients’ demographics (age and sex), vital signs on admission (systolic blood pressure [SBP, N: 90–120 mmHg], SpO2 [N: 93–100], heart rate [HR, N: 60–100 beats/min], and body temperature [N: 36.1–37.2 °C]), complaints at admission (fever, cough, nausea/vomiting, sore throat, confusion, shortness of breath, fatigue, weakness, diarrhea, and chest pain), comorbidities (HT, DM, chronic renal failure, coronary artery disease [CAD], cerebrovascular disease, smoking, chronic obstructive pulmonary disease, and asthma), COVID-19 RT-PCR test result, laboratory parameters, hematological findings (white blood cell [WBC, N: 4.5–10.0 10^3/uL], neutrophil [N: 1.5–8.0 10^3/uL], lymphocyte [N: 0.8–5.0 10^3/uL], and platelet counts [N: 150–45,010^3/uL]), and biochemical findings (lactate dehydrogenase [LDH, N: 0–248 U/L], CRP [N < 5 mg/L], procalcitonin [N < 0.05 µg/L], creatinine [N: 0.84–1.21 mg/dL], albumin [N: 35–52 g/L], D-dimer [N < 550 µg/L], ferritin [N: 20–400 µg/L], troponin [N < 14.5 pg/mL], and fibrinogen [N: 200–400 mg/dL] levels).
Patients were separated into three groups according to their clinical severity, based on the definitions in the “COVID-19 Clinical Management Guidelines” printed by World Health Organization (WHO).7 According to the WHO guidelines, mild disease was characterized by features such as fever, muscle/joint pain, cough, sore throat, and nasal congestion, but no signs of severe pneumonia, including SpO2 ≥ 90% on room air. Severe disease was characterized by pneumonia findings, with a respiratory rate > 30 breaths/min and O2 saturation < 90% while breathing room air. Critical disease was characterized by severe pneumonia (confusion, respiratory distress, respiratory rate ≥ 30 breaths/min, SpO2 ≤ 90% while breathing room air, bilateral lung involvement > 50% on chest radiography or computed tomography (CT), SBP < 65 mmHg, and HR > 100 beats/min) or requirement for ICU admission.
We compared demographic data, vital signs, hematological and biochemical parameters, and CURB-65 (new-onset confusion, blood urea nitrogen > 42.8 mg/dL, respiratory rate > 30 breaths/min, blood pressure < 90/60 mmHg, and age ≥ 65 years) score among patient groups to identify factors associated with mortality and disease severity.
Outcome Definition
We evaluated predictors of mortality in adult patients with COVID-19 who were admitted to a pandemic ward or pandemic ICU. We also examined relationships between hematological and biochemical findings and COVID-19 severity.
Statistical Analysis
The required sample size was calculated by power analysis before data collection based on information from previous studies.8–10 It was estimated that at least 353 patients would be required to detect significant differences among the groups based on disease severity with a power of 95% and an alpha error of 5%.
All data analyses were conducted using SPSS statistical software (version 15.0 for Windows; SPSS Inc., Chicago, IL, USA). Numerical data (eg, WBC, hemoglobin neutrophil, lymphocyte, and thrombocyte counts; CRP, procalcitonin, D-dimer, ferritin, troponin, and fibrinogen levels; and CURB-65 score) were expressed as median and interquartile range (IQR) values. Categorical variables (sex and age) were expressed as numbers of patients (n) and percentages (%). The Kruskal–Wallis test was used to assess numerical data among patients grouped according to disease severity (eg, mild, severe, critical) when the data were not normally distributed, according to the Kolmogorov–Smirnov test. Subgroup analyses (mild vs severe, mild vs critical, and severe vs critical) were conducted using the chi-squared test for normally distributed data and the Mann–Whitney U-test with Bonferroni correction for non-normally distributed data. Intragroup comparisons (survivors vs non-survivors) were made using the chi-squared test and Mann–Whitney U-test, as appropriate. Independent variables were analyzed using multivariate logistic regression analysis. The threshold for statistical significance was defined as p < 0.05.
Results
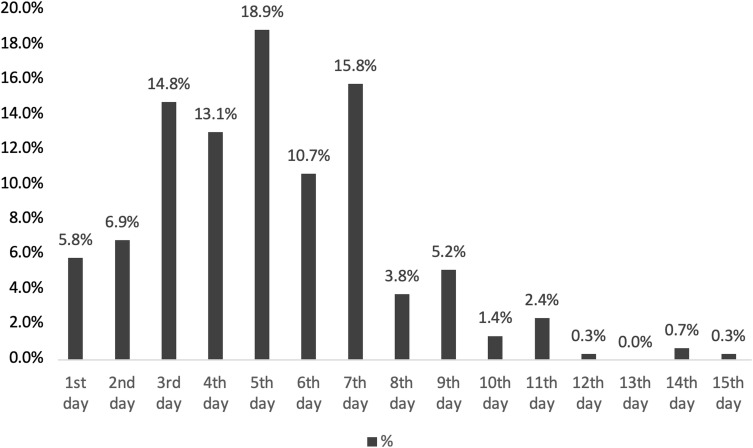
Table 1 presents the demographic and clinical characteristics of the patients in this study. In total, 540 patients were included: 302 men (55.9%) and 238 women (44.1%). The mean age was 48.00 ± 14.60 years (median, 47 years). The number of patients > 30 years of age was 487, which represented 90.2% of the total patient population. The overall mortality rate was 4.3% (n = 23): mortality rates were 5.0% in men (n = 15) and 3.4% in women (n = 8). The lowest mortality rate was among patients aged 30–39 years, and the highest mortality rate was among patients aged 70–79 years. Notably, 65.2% of non-surviving patients (n = 15) were aged > 60 years. Furthermore, 202 patients (37.4%) were ambulatory at the follow-up visit, while 308 patients (57.0%) had inpatient treatment in isolated wards and 30 patients (5.6%) were treated in the ICU. The mean lengths of stay were 10.10 ± 5.40 days in the ward and 11.53 ± 7.30 days in the ICU. Overall, 28.9% of patients (n = 156) had at least one chronic medical condition. The most common comorbid diseases were DM (15.4%), HT (15.2%), and CAD (6.1%). At least one comorbid disease was present in 14 (60.8%) of the 23 non-surviving patients. The most common complaints on admission to the ED were cough (n = 390, 72.2%), fatigue (n = 379, 70.2%), fever (n = 317, 58.7%), and shortness of breath (n = 230, 42.6%). Regarding the interval between symptom onset and positive PCR test results, patients who had a positive test result in the first 7 days were 85.9% (n = 464) of all patients (Figure 1). The mean interval between symptom onset and the positive test result was 5.20 ± 2.55 days.
Table 1.
Patient Demographic and Clinical Characteristics
| Patients | Mortality | |||
|---|---|---|---|---|
| n | % | n | % | |
| Total | 540 | 100.0 | 23 | 4.3 |
| Sex | ||||
| Male | 302 | 55.9 | 15 | 5.0 |
| Female | 238 | 44.1 | 8 | 3.4 |
| Age Distribution (years) | ||||
| < 30 | 53 | 9.8 | 2 | 3.8 |
| 30–39 | 98 | 18.1 | 1 | 1.0 |
| 40–49 | 141 | 26.1 | 2 | 1.4 |
| 50–59 | 124 | 23.0 | 3 | 2.4 |
| 60–69 | 86 | 15.9 | 5 | 5.8 |
| 70–79 | 25 | 4.6 | 7 | 28.0 |
| > 80 | 13 | 2.4 | 3 | 23.1 |
| Monitoring | ||||
| Ambulatory monitoring | 202 | 37.4 | ||
| Hospital treatment | 308 | 57.0 | ||
| ICU support | 30 | 5.6 | ||
| Comorbidities | ||||
| No additional disease | 384 | 71.1 | 9 | 2.3 |
| Additional disease | 156 | 28.9 | 14 | 9.0 |
| Diabetes mellitus | 83 | 15.4 | ||
| Hypertension | 82 | 15.2 | ||
| Coronary artery disease | 33 | 6.1 | ||
| Chronic respiratory disease | 18 | 3.3 | ||
| Chronic renal disease | 11 | 2.0 | ||
| Stroke | 6 | 1.1 | ||
| Malignancy | 4 | 0.7 | ||
| Symptoms at Admission | ||||
| Cough | 390 | 72.2 | ||
| Fatigue | 379 | 70.2 | ||
| Fever (> 37.8°C) | 317 | 58.7 | ||
| Shortness of breath | 230 | 42.6 | ||
| Phlegm | 117 | 21.7 | ||
| Headache | 91 | 16.9 | ||
| Myalgia | 81 | 15.0 | ||
| Diarrhea | 46 | 8.5 | ||
| Sore throat | 45 | 8.3 | ||
| Stomachache | 20 | 3.7 | ||
| Nausea/vomiting | 18 | 3.3 | ||
| Appetite loss | 17 | 3.1 | ||
| Chest pain | 4 | 0.7 | ||
Note: Data are expressed as numbers (n) and percentages (%).
Abbreviation: ICU, intensive care unit.
Figure 1.
Distribution of the time between symptom onset and positive PCR test result.
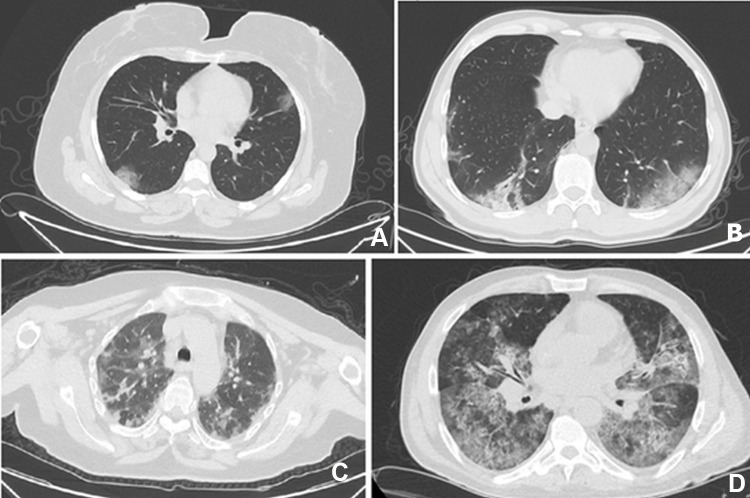
Examination of radiological findings showed that 86.9% (467/537) of the patients did not have any abnormalities on chest radiographs. However, at least one abnormality was detected in 96.8% (n = 519) of the patients with thoracic CT. The most common CT findings were bilateral ground-glass opacities (85.8%), consolidation (37.9%), and diffuse infiltration (25.7%). The lesions tended to be located generally bilateral (n = 389, 74.9%), peripheral (n = 393, 75.7%), and in lower lobes (n = 372, 71.6%) (Table 2). Typical chest CT findings of patients with COVID-19 at presentation were shown in Figure 2.
Table 2.
Distribution of Thoracic CT Findings
| n | % | ||
|---|---|---|---|
| Thoracic CT Findings | GGO | 461 | 85.8 |
| GGO with mixed consolidation | 165 | 30.8 | |
| Consolidation | 205 | 38.2 | |
| Diffuse infiltration | 138 | 25.7 | |
| Others | 34 | 6.3 | |
| No abnormality | 17 | 3.2 | |
| Radiological Distribution | Peripheral | 393 | 75.7 |
| Central | 20 | 3.9 | |
| Involvement | Bilateral | 389 | 74.9 |
| Unilateral | 114 | 21.9 | |
| Lobar Distribution | Right lower lobe | 220 | 42.4 |
| Left lower lobe | 152 | 29.2 | |
| Right middle lobe | 120 | 23.1 | |
| Left lingula | 58 | 11.2 | |
| Right upper lobe | 62 | 11.9 | |
| Left upper lobe | 50 | 9.6 | |
Note: Data are expressed as numbers (n) and percentages (%).
Abbreviations: CT, high-resolution computed tomography; GGO, ground-glass opacity; Others, atelectasis, fibrosis, lymph node enlargement, pleural plaques, effusion, mediastinal lymphadenopathy.
Figure 2.
Typical thoracic CT findings: (A) Peripheral ground-glass opacifications; (B) Bilateral ground-glass opacifications; (C) Multifocal involvement; (D) Diffuse involvement.
The distribution of disease severity was mild in 40.9% (n = 221), severe in 53.7% (n = 290), and critical in 5.4% (n = 29) of patients (Table 3). The median patient age was significantly lower among patients with mild disease than among patients with severe or critical disease (p < 0.001 for both comparisons). In this study, there were more male patients (55.9%) than female patients (44.1%). However, no significant sex-related difference was observed in terms of disease severity (p = 0.178). Smoking frequency (ie, median packs/year) was significantly lower in patients with mild disease than in patients with severe or critical disease (p < 0.001 and p = 0.016, respectively).
Table 3.
Comparisons of Age, Sex, Smoking, Vital Signs and Risk Score in Patient Groups Classified According to the Disease Severity (Mild, Moderate, and Severe)
| Mild (n = 221) | Severe (n = 290) | Critical (n = 30) | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p* | |
| Sex | |||||||
| Female | 101 | 42.4 | 129 | 54.2 | 8 | 3.4 | 0.178 |
| Male | 120 | 39.7 | 161 | 53.3 | 21 | 6.9 | |
| Median | IQR (25-75) | Median | IQR (25-75) | Median | IQR (25-75) | p* | |
| Age (years) | 40.00 | 33.00–50.00 | 53.50 | 44.00–61.00 | 63.00 | 54.50–72.50 | < 0.001 |
| Cigarettes (packs/year) | 20.00 | 10.00–25.00 | 30.00 | 20.00–30.00 | 30.00 | 15.00–30.00 | < 0.001 |
| Vital Signs | |||||||
| Systolic blood pressure (mmHg) | 120.00 | 110.00–130.00 | 120.00 | 110.00–130.00 | 100.00 | 80.00–140.00 | < 0.001 |
| Respiratory rate (breaths/min) | 14.00 | 12.00–16.00 | 14.00 | 14.00–16.00 | 22.00 | 18.00–24.00 | < 0.001 |
| Heart rate (beats/min) | 88.00 | 88.00–98.00 | 88.00 | 84.25–98.00 | 102.00 | 93.00–118.00 | < 0.001 |
| Body temperature (°C) | 37.80 | 37.20–38.40 | 37.85 | 37.20–38.80 | 38.80 | 38.40–38.95 | < 0.001 |
| Risk Score | |||||||
| CURB-65 | 0.00 | 0.00–0.00 | 0.00 | 0.00–1.00 | 2.00 | 1.00–3.00 | < 0.001 |
Note: Data are expressed as numbers (n), percentages (%), or median and interquartile range (IQR). *Kruskal–Wallis test was used to assess numerical data of patients grouped according to disease severity (eg, mild, severe, or critical).
Abbreviations: CURB-65 (new-onset confusion, blood urea nitrogen > 42.8 mg/dL, respiratory rate > 30 breaths/min, blood pressure < 90/60 mmHg, age ≥ 65 years)
Laboratory findings on admission revealed significant differences among disease severity groups in terms of median WBC, hemoglobin, neutrophil, lymphocyte, and thrombocyte counts (p = 0.035, p = 0.002, p = 0.004, p < 0.001, and p = 0.013, respectively; Table 4), as well as CRP, procalcitonin, LDH, creatinine, albumin, D-dimer, ferritin, troponin, and fibrinogen levels (p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001, and p = 0.001, respectively; Table 4). Additionally, significant differences were observed among groups in terms of median neutrophil–lymphocyte ratio (NLR) and CRP/albumin ratio (p < 0.001 for both comparisons; Table 4).
Table 4.
Comparison of Laboratory Findings in Patient Groups Classified According to the Disease Severity (Mild, Moderate, and Severe)
| Mild | Severe | Critical | |||||
|---|---|---|---|---|---|---|---|
| Median | IQR (25-75) | Median | IQR (25-75) | Median | IQR (25-75) | p* | |
| Hematological Parameters | |||||||
| WBC (10^3/uL) | 6.70 | 5.25–8.43 | 6.27 | 5.02–7.90 | 6.88 | 5.82–10.06 | 0.035 |
| Hemoglobin (g/dL) | 13.8 | 12.50–15.10 | 13.60 | 12.50–14.60 | 12.80 | 11.50–13.80 | 0.002 |
| Neutrophil (10^3/uL) | 3.76 | 2.63–5.09 | 4.06 | 2.87–5.58 | 4.72 | 3.72–7.36 | 0.004 |
| Lymphocyte (10^3/uL) | 2.00 | 1.48–2.74 | 1.51 | 1.08–1.97 | 1.08 | 0.72–1.23 | < 0.001 |
| Thrombocyte (10^3/uL) | 216.00 | 184.50–264.00 | 205.00 | 173.00–258.25 | 179.00 | 141.50–246.50 | 0.013 |
| Biochemical Parameters | |||||||
| CRP (mg/L) | 8.50 | 3.95–19.80 | 42.00 | 17.00–83.00 | 136.00 | 107.70–199.00 | < 0.001 |
| Procalcitonin (µg/L) | 0.03 | 0.02–0.05 | 0.04 | 0.03–0.08 | 0.305 | 0.15–1.55 | < 0.001 |
| Lactate dehydrogenase (U/L) | 218.50 | 177.00–259.00 | 274.00 | 217.00–341.00 | 357.00 | 267.00–480.00 | < 0.001 |
| Creatinine (mg/dL) | 0.76 | 0.62–0.88 | 0.84 | 0.68–0.99 | 1.02 | 0.86–1.78 | < 0.001 |
| Albumin (g/L) | 43.00 | 40.00–45.00 | 36.00 | 33.00–38.00 | 31.00 | 26.00–35.00 | < 0.001 |
| D-dimer (µg/L) | 500.00 | 270.00–880.00 | 740.00 | 470.00–1065.00 | 1120.00 | 750.00–3220.00 | < 0.001 |
| Ferritin (µg/L) | 86.85 | 39.07–183.55 | 184.00 | 99.00–333.50 | 247.00 | 112.00–541.00 | < 0.001 |
| Troponin (pg/mL) | 2.70 | 1.45–4.00 | 3.70 | 2.15–5.52 | 6.40 | 3.60–22.85 | < 0.001 |
| Fibrinogen (mg/dL) | 382.00 | 280.00–559.00 | 500.00 | 421.00–583.00 | 562.00 | 405.00–643.00 | 0.001 |
| NLR | 1.80 | 1.30–2.79 | 2.74 | 1.66–4.26 | 5.73 | 3.59–9.37 | < 0.001 |
| CRP/Abumin Ratio | 0.20 | 0.08–0.71 | 1.29 | 0.48–2.68 | 4.86 | 3.22–6.04 | < 0.001 |
Note: Data are expressed as median and interquartile range (IQR). *Kruskal–Wallis test was used to assess numerical data of patients grouped according to disease severity (eg, mild, severe, or critical).
Abbreviations: WBC, white blood cell; NLR, neutrophil–lymphocyte ratio; CRP, C-reactive protein.
Subgroup analysis revealed that the median neutrophil count was significantly higher among patients with critical disease than among patients with mild or severe disease (p < 0.001 and p = 0.013, respectively; Table 5). However, the median lymphocyte count was significantly lower among patients with critical disease than among patients with mild or severe disease (p < 0.001 for both comparisons; Table 5). The median CRP, procalcitonin, LDH, creatinine, D-dimer, and troponin levels were significantly higher among patients with critical disease, whereas the median albumin levels were significantly higher among patients with mild or severe disease (Table 5). Moreover, the median ferritin and fibrinogen levels were significantly lower among patients with mild disease than among patients with severe or critical disease. There were no significant differences between patients with severe disease and patients with critical disease in terms of median ferritin and fibrinogen levels (Table 5). The median CURB-65 score was significantly higher among patients with critical disease than among patients with mild disease (p < 0.001). Additionally, median body temperature, respiratory rate, and HR were significantly higher among patients with critical disease than among patients with mild or severe disease, while SBP was significantly lower among patients with critical disease (p < 0.001 for all comparisons; Table 5).
Table 5.
Subgroups Analyses
| Mild vs Severe | Mild vs Critical | Severe vs Critical | |
|---|---|---|---|
| p* | p* | p* | |
| Age (years) | <0.001 | <0.001 | 0.002 |
| Cigarettes (packs/year) | <0.001 | 0.016 | 0.790 |
| Hematological Parameters | |||
| WBC (10^3/uL) | 0.052 | 0.248 | 0.040 |
| Hemoglobin (g/dL) | 0.063 | 0.001 | 0.007 |
| Neutrophil (10^3/uL) | 0.085 | 0.001 | 0.013 |
| Lymphocyte (10^3/uL) | <0.001 | <0.001 | <0.001 |
| Thrombocyte (10^3/uL) | 0.046 | 0.009 | 0.084 |
| Biochemical Parameters | |||
| CRP (mg/L) | <0.001 | <0.001 | <0.001 |
| Procalcitonin (µg/L) | 0.008 | <0.001 | <0.001 |
| Lactate dehydrogenase (U/L) | <0.001 | <0.001 | 0.001 |
| Creatinine (mg/dl) | <0.001 | <0.001 | <0.001 |
| Albumin (g/L) | <0.001 | <0.001 | <0.001 |
| D-dimer (µg/L) | <0.001 | <0.001 | 0.001 |
| Ferritin (µg/L) | 0.001 | <0.001 | 0.132 |
| Troponin (pg/mL) | <0.001 | <0.001 | 0.001 |
| Fibrinogen (mg/dl) | 0.001 | 0.002 | 0.194 |
| NLR | <0.001 | <0.001 | <0.001 |
| CRP/Albumin Ratio | <0.001 | <0.001 | <0.001 |
| Vital Signs | |||
| Respiratory rate (breaths/min) | <0.001 | <0.001 | <0.001 |
| Heart Rate (beats/min) | 0.644 | <0.001 | <0.001 |
| Body temperature (°C) | 0.007 | <0.001 | <0.001 |
| Risk Score | |||
| CURB-65 | <0.001 | <0.001 | <0.001 |
Note: *Subgroup analyses (mild vs severe, mild vs critical, and severe vs critical) were conducted using chi-squared and the Mann–Whitney U-tests, as appropriate.
Abbreviations: WBC, white blood cell; NLR, neutrophil–lymphocyte ratio; CRP, C-reactive protein; CURB-65 (new-onset confusion, blood urea nitrogen > 42.8 mg/dL, respiratory rate > 30 breaths/min, blood pressure < 90/60 mmHg, age ≥ 65 years)
The multivariate logistic regression analysis demonstrated that elevated CRP level, and CURB-65 score (odds ratio [OR]: 4.004, 95% confidence interval [CI]: 1.288–12.447; p = 0.017) remained independent predictors of disease severity in patients with COVID-19. According to multivariate logistic regression analysis, CRP is the most important predictor for disease severity, with an OR of 1.020 (95% CI: 1.009–1.032, p < 0.001; Table 6).
Table 6.
Multivariate Logistic Regression Analysis to Determine Disease Severity
| p | OR | 95% CI | ||||
|---|---|---|---|---|---|---|
| Backward Method | WBC (10^3/uL) | 0.282 | 0.297 | 0.032 | 2.712 | |
| CRP (mg/L) | <0.001 | 1.020 | 1.009 | 1.032 | ||
| Lactate dehydrogenase (U/L) | 0.282 | 0.998 | 0.996 | 1.001 | ||
| D-dimer (µg/L) | 0.171 | 1.000 | 1.000 | 1.000 | ||
| Troponin (pg/mL) | 0.474 | 2.379 | 0.222 | 25.467 | ||
| NLR | 0.505 | 1.123 | 0.798 | 1.582 | ||
| CRP/Albumin | 0.523 | 0.543 | 0.083 | 3.535 | ||
| CURB-65 | 0.017 | 4.004 | 1.288 | 12.447 | ||
Abbreviations: OR, odds ratio; CI, confidence interval; WBC, white blood cell; CRP, C-reactive protein; NLR, neutrophil–lymphocyte ratio; CURB-65 (new-onset confusion, blood urea nitrogen > 42.8 mg/dL, respiratory rate > 30 breaths/min, blood pressure < 90/60 mmHg, age ≥ 65 years)
In total, 23 patients died: 15 men (65.2%) and eight women (34.8%) (Table 7). The median ages of the non-surviving and surviving patients were 70.00 years (IQR, 51.00–76.00) and 56.00 years (IQR, 38.00–58.00; p < 0.001), respectively. The median lengths of hospital stay were 6.00 days (IQR, 0.00–9.00) in surviving patients and 13.00 days (IQR, 8.00–17.00) in non-surviving patients. This difference was statistically significant (p < 0.001; Table 7). However, there was no significant difference between surviving and non-surviving patients regarding the median number of cigarette packs/year (p = 0.583; Table 7). The median CURB-65 score was significantly higher among non-surviving patients than among surviving patients (p < 0.001). Median body temperature, respiratory rate, and HR were significantly higher among non-surviving patients, but SBP was significantly lower among non-surviving patients (p < 0.001 for all comparisons; Table 7).
Table 7.
Comparisons of Age, Sex, Smoking, Length of Hospital Stay, Vital Signs and Risk Score in Survivors versus Non-survivors
| Survivors | Non-survivors | ||||
|---|---|---|---|---|---|
| n | % | n | % | p* | |
| Gender | |||||
| Female | 230 | 96.6 | 8 | 3.4 | 0.359 |
| Male | 287 | 95.1 | 15 | 4.9 | |
| Median | IQR (25-75) | Median | IQR (25-75) | p* | |
| Age (years) | 47.00 | 38.00–58.00 | 64.00 | 51.00–76.00 | <0.001 |
| Length of hospital stay (days) | 8.00 | 0.00–9.00 | 4.00 | 8.00–17.00 | <0.001 |
| Cigarettes (packs/year) | 20.00 | 15.00–30.00 | 20.00 | 15.00–40.00 | 0.583 |
| Vital Signs | |||||
| Systolic blood pressure (mmHg) | 120.00 | 110.00–130.00 | 90.00 | 80.00–130.00 | <0.001 |
| Respiratory rate (breaths/min) | 14.00 | 14.00–16.00 | 22.00 | 18.00–24.00 | <0.001 |
| Heart Rate (beats/min) | 88.00 | 88.00–98.00 | 102.00 | 98.00–118.00 | <0.001 |
| Body temperature (°C) | 37.80 | 37.20–38.60 | 38.80 | 38.50–39.00 | <0.001 |
| Risk Score | |||||
| CURB-65 | 0.00 | 0.00–0.00 | 2.00 | 1.00–3.00 | <0.001 |
Note: Data are expressed as numbers (n), percentages (%), or median and interquartile range (IQR). *Intragroup comparisons (survivors vs non-survivors) were made using chi-squared and Mann–Whitney U-tests, as appropriate.
Abbreviations: CURB-65 (new-onset confusion, blood urea nitrogen > 42.8 mg/dL, respiratory rate > 30 breaths/min, blood pressure < 90/60 mmHg, age ≥ 65 years)
There were significant differences between surviving and non-surviving patients in terms of median WBC, hemoglobin, neutrophil, and lymphocyte counts (p = 0.034, p = 0.021, p = 0.001, and p < 0.001, respectively; Table 8). Furthermore, there were significant differences between surviving and non-surviving patients in terms of median CRP, procalcitonin, LDH, creatinine, albumin, D-dimer, and ferritin levels (p < 0.001, p = 0.001, p < 0.001, p < 0.001, p < 0.001, p = 0.027, and p < 0.001, respectively; Table 8). Additionally, significant differences were observed between surviving and non-surviving patients in terms of median NLR and CRP/albumin ratio (p < 0.001 for both comparisons).
Table 8.
Comparison of Laboratory Findings in Survivors versus Non-survivors
| Survivors | Non-survivors | ||||
|---|---|---|---|---|---|
| Median | IQR (25-75) | Median | IQR (25-75) | p* | |
| Hematological Parameters | |||||
| WBC (10^3/uL) | 6.40 | 5.10–8.13 | 6.88 | 5.83–11.74 | 0.034 |
| Hemoglobin (g/dL) | 13.70 | 12.50–14.80 | 12.70 | 11.20–14.10 | 0.021 |
| Neutrophil (10^3/uL) | 3.99 | 2.77–5.23 | 5.04 | 4.20–10.37 | 0.001 |
| Lymphocyte (10^3/uL) | 1.68 | 1.21–2.29 | 1.08 | 0.88–1.55 | <0.001 |
| Thrombocyte (10^3/uL) | 207.00 | 174.00–259.00 | 190.00 | 156.00–307.00 | 0.488 |
| Biochemical Parameters | |||||
| CRP (mg/L) | 20.00 | 7.20–62.00 | 127.00 | 88.00–163.00 | <0.001 |
| Procalcitonin (µg/L) | 0.04 | 0.03–0.08 | 0.26 | 0.11–0.44 | 0.001 |
| Lactate dehydrogenase (U/L) | 249.00 | 200.00–312.00 | 349.00 | 263.50–463.50 | <0.001 |
| Creatinine (mg/dl) | 0.80 | 0.66–0.95 | 1.23 | 0.86–1.61 | <0.001 |
| Albumin (g/L) | 36.50 | 34.00–40.00 | 31.00 | 26.00–35.00 | <0.001 |
| D-dimer (µg/L) | 710.00 | 420.00–1050.00 | 970.00 | 500.00–2940.00 | 0.027 |
| Ferritin (µg/L) | 172.00 | 80.00–313.00 | 195.50 | 102.25–528.50 | 0.307 |
| Troponin (pg/mL) | 3.50 | 1.80–4.60 | 6.40 | 3.70–24.70 | <0.001 |
| Fibrinogen (mg/dl) | 494.00 | 388.00–580.75 | 557.00 | 394.00–663.50 | 0.111 |
| NLR | 2.19 | 1.49–3.58 | 5.74 | 3.81–8.50 | <0.001 |
| CRP/Albumin Ratio | 1.00 | 0.34–2.27 | 4.80 | 2.83–5.78 | <0.001 |
Note: Data are expressed as median and interquartile range (IQR). *Intragroup comparisons (survivors vs non-survivors) were made using chi-squared and Mann–Whitney U-tests, as appropriate.
Abbreviations: WBC, white blood cell; NLR, neutrophil–lymphocyte ratio; CRP, C-reactive protein (mg/L).
The multivariate logistic regression analysis demonstrated that elevated CRP level (OR: 1.012, 95% CI: 1.002–1.022; p = 0.016), and CURB-65 score (OR: 4.848, 95% CI: 1.378–17.062; p = 0.014) remained independent predictors of mortality in patients with COVID-19 (Table 9).
Table 9.
Multivariate Logistic Regression Analysis to Determine Mortality
| p | OR | 95% CI | |||
|---|---|---|---|---|---|
| Backward Method | WBC (10^3/uL) | 0.480 | 2.183 | 0.250 | 19.033 |
| CRP (mg/L) | 0.016 | 1.012 | 1.002 | 1.022 | |
| Lactate dehydrogenase (U/L) | 0.550 | 0.999 | 0.995 | 1.003 | |
| D-dimer (µg/L) | 0.855 | 1.000 | 1.000 | 1.000 | |
| Troponin (pg/mL) | 0.961 | 1.061 | 0.099 | 11.362 | |
| Neutrophil/lymphocyte | 0.107 | 1.454 | 0.922 | 2.292 | |
| CRP/Albumin | 0.228 | 0.237 | 0.023 | 2.457 | |
| CURB-65 | 0.014 | 4.848 | 1.378 | 17.062 | |
Abbreviations: OR, odds ratio; CI, confidence interval; WBC, white blood cell; CRP, C-reactive protein; NLR, neutrophil–lymphocyte ratio; CURB-65 (new-onset confusion, blood urea nitrogen > 42.8 mg/dL, respiratory rate > 30 breaths/min, blood pressure < 90/60 mmHg, age ≥ 65 years).
Discussion
This study investigated potential predictive factors for mortality and disease severity in patients with confirmed COVI9-19 who were admitted to the ED. The key findings were as follows. First, the overall mortality rate was 4.3%, and more than half of non-surviving patients were men > 60 years of age. Second, the most common complaints on admission to the ED were cough, fatigue, and fever. Third, nearly one-third of the patients had at least one comorbid disease, and DM and HT were the most common comorbidities. Fourth, the distribution of disease severity was approximately 41% mild, 54% severe, and 5% critical. Fifth, the median body temperature, respiratory rate, and HR were significantly higher among patients with critical disease than among patients with mild or severe disease, while SBP was significantly lower among patients with critical disease. Sixth, the median neutrophil count was significantly higher among patients with critical disease than among patients with mild or severe disease, while the median lymphocyte count was significantly lower among patients with critical disease. Seventh, the median CRP, procalcitonin, LDH, creatinine, D-dimer, and troponin levels were significantly higher among patients with critical disease than among patients with mild or severe disease, while median albumin levels were significantly lower among patients with critical disease. Eighth, the median WBC and neutrophil counts on admission to the ED were significantly higher among non-surviving patients than among surviving patients, while the median lymphocyte and hemoglobin counts were significantly lower among non-surviving patients. However, there was no significant difference between surviving and non-surviving patients in terms of median platelet count. Ninth, the median CRP, procalcitonin, LDH, creatinine, albumin, D-dimer, ferritin, and procalcitonin levels, as well as the CRP/albumin ratio, were significantly higher in non-surviving patients. However, the median albumin level was significantly lower in non-surviving patients. Finally, elevated CRP level and high CURB-65 score were independently associated with both mortality and disease severity.
The prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is reportedly higher in men.11,12 Zhao et al suggested that this higher prevalence in men may be related to greater lung ACE2 expression in men, compared with women.13 In our study, the proportion of men was considerably greater than the proportion of women, although there were no significant differences between men and women in terms of disease severity and mortality.
Leung et al reported that increased ACE2 expression levels in smokers and patients with chronic obstructive pulmonary disease might contribute to greater severity of COVID-19.14 Similarly, we observed that the median number of packs/year was significantly lower among patients with mild disease than among patients with severe or critical disease (p < 0.001 and p = 0.016, respectively). Furthermore, the median number of packs/year was similar among non-surviving patients than among surviving patients (20.00 with IQR, 15.00–40.00 packs/year vs 20.00 with IQR, 15.00–30.00 packs/year). However, there was no significant difference between non-surviving and surviving patients in terms of smoking. Therefore, smoking presumably increases disease severity in patients with COVID-19, but there is not a clear relationship between smoking and mortality.
In a study of 94 patients with confirmed COVID-19, He et al reported that the highest viral loads were observed in nasopharyngeal swabs immediately after symptom onset.15 Our analysis of time intervals between symptom onset and positive PCR test results showed that 85.9% of patients had a positive test result in the first 7 days (5.9% on the 1st day, 7% on the 2nd day, 14.7% on the 3rd day, 13% on the 4th day, 18.6% on the 5th day 10.6% on the 7th day, and 18.8% on the 7th day). Our study also showed the highest viral loads immediately after symptom onset.
In a meta-analysis conducted by Fu et al, the most common complaints in patients with COVID-19 on admission to the ED were fever (83.3%), cough (60.3%), and fatigue (38.0%), respectively. These were followed by phlegm, shortness of breath, and myalgia.12 Similarly, in our study, the most common complaints were cough, fatigue, and fever. These were also followed by shortness of breath, phlegm, and myalgia.
Previous studies showed that HT and DM were the most common comorbid diseases among patients with COVID-19. Additionally, older age and comorbidities were reported as risk factors for mortality in these patients.12,16 In our study, the most common comorbid diseases were DM, HT, and CAD. The distributions of comorbidities in non-surviving patients were 22.2% chronic obstructive pulmonary disease, 15.2% CAD, 13.4% HT, and 10.8% DM.
Chest radiographs reportedly have low sensitivity for COVID-19 diagnosis.17 In our study, > 85% of the patients had no chest radiograph abnormalities. Common abnormal CT findings were ground-glass opacities, consolidation, and diffuse infiltration with bilateral, peripheral, and lower lung zone distributions. Although these findings are common in patients with COVID-19, they are not pathognomonic and are frequently observed in patients with other types of viral pneumonia. Accordingly, the American College of Radiology does not recommend the use of chest CT for screening or diagnosis of COVID-19.18
According to the “COVID-19 Diagnosis and Treatment Guide” printed by the Turkish Ministry of Health, poor prognostic factors in laboratory tests on admission including ferritin (> 500 ng/mL), D-dimer (> 1000 ng/mL), and CRP (> 10× above the upper limit of normal) levels, as well as lymphocyte count (< 800/µL), can be used for hospitalization and treatment decisions.1 Sharifpour et al identified elevated CRP and procalcitonin levels as potential determinants of disease severity in patients with SARS-CoV-2 infection.19 In another study, increased D-dimer (> 1 μg/mL) levels and hypoalbuminemia were identified as risk factors for disease severity in patients with COVID-19.20 In our study, the median neutrophil counts on admission were significantly higher among patients with critical disease than among patients with mild or severe disease, and the median lymphocyte counts on admission were significantly lower in patients with critical disease. Additionally, the median CRP, procalcitonin, LDH, creatinine, D-dimer, and troponin levels were significantly higher among patients with critical disease. However, median albumin levels were significantly lower among patients with critical disease, compared with patients with mild or severe disease. Furthermore, the median CURB-65 score was significantly higher among patients with critical disease than among patients with mild disease. We found that elevated CRP level and high CURB-65 score was independent predictors of disease severity. These parameters can be used to determine the severity of COVID-19 on admission.
In a Chinese Centers for Disease Control and Prevention report involving approximately 44,500 patients, the overall mortality was 2.3%.21 In that report, 81% of the patients had mild disease, while 19% had severe or critical disease. In our study, 40.9% of the patients had mild disease, while 59.1% had severe or critical disease. Moreover, the overall mortality in our study was 4.3%. Because our hospital is a tertiary hospital, the patient distribution considerably differed with respect to the Chinese report. In particular, more patients had severe or critical disease in our hospital. However, among patients with severe or critical disease, the mortality rate was 12.4% in the Chinese report, while our mortality rate among these patients was 7.2%.
In a similar study involving 2,449 patients from the USA, stratification according to age group showed that 80% of deaths occurred in patients aged ≥ 65 years.22 In our study, 65.2% of deaths occurred in patients aged ≥ 60 years. Furthermore, we found a significant age difference between non-surviving and surviving patients. In an Italian study, the overall mortality rate was 7.2%, and individuals aged ≥ 70 years represented 37.6% of patients.23 Conversely, individuals aged ≥ 70 years represented only 8.0% of patients in our study. A review of the distribution of mortality by age in our study population compared to the other studies22,23 reveals that it does not accumulate in the elderly population. At the onset of the COVID-19 outbreak in Turkey, the lockdown was specifically applied to individuals aged > 65 years, followed by the expansion of the curfew for those with the age of 20 and younger. Therefore, the spread of disease in people aged > 65 and ≤ 20 years, and the overall mortality rate, were lower in our study than in the Italian study.
Previous studies have indicated that old age; smoking; increased D-dimer, CRP, ferritin, and troponin levels; decreased lymphocyte count were associated with mortality in patients with COVID-19.24–26 In the present study, the median WBC and neutrophil counts were significantly higher among non-surviving patients, while the median lymphocyte count and hemoglobin level were significantly lower among these patients. In addition, the median CRP, LDH, troponin, creatinine, D-dimer, and procalcitonin levels, as well as the NLR and CRP/albumin ratio, were significantly higher among non-surviving patients than among surviving patients. Finally, the median albumin level was significantly lower among non-surviving patients.
Pre-COVID-19 analyses demonstrated that a CURB-65 score of < 2 was consistently associated with low 30-day mortality rates of 0.4–2.8% days. However, studies of patients with COVID-19 have shown that 17% mortality occurs among patients with a CURB-65 score of < 2.27 Bradley et al stated that low CURB-65 scores may not support early COVID-19 discharge, but higher scores are useful for predicting poor outcomes, consistent with our findings.27 In our study, CURB-65 score on admission was also significantly higher among non-surviving patients than among surviving patients.
Liu et al stated that NLR is an independent predictor for disease severity and mortality in hospitalized patients.28 In another study, Liu et al reported that NLR levels > 3.13 effectively predict critical disease.29 In our study, 186 patients had an NLR of > 3.13, and 19 of these patients died. The mortality rate among this group was 10.2%. Furthermore, 82.6% of non-surviving patients had an NLR of > 3.13. Similarly, there were 31 patients with a CRP/albumin ratio of > 4.50, and 11 of these patients died. The mortality rate among this group was 35.4%. Notably, 47.8% of non-surviving patients had a CRP/albumin ratio of > 4.50.
Our study found that elevated CRP level and high CURB-65 score predict both mortality and disease severity in patients with COVID-19. Physicians may use these parameters to identify patients at risk of fatal or severe COVID-19.
There are some limitations to this study, the most important being the small cohort of non-surviving patients with COVID-19 and single-center design.
Conclusion
Our findings indicate that median body temperature, respiratory rate, HR, and SBP, as well as WBC, neutrophil, lymphocyte, and platelet counts, may be useful to predict disease severity in patients with COVID-19. In addition, CRP, ferritin, LDH, creatinine, troponin, D-dimer, fibrinogen, and albumin levels can be used to predict disease severity. Finally, high CURB-65 score was useful in determining disease severity on admission. We concluded that elevated CRP level and high CURB-65 score predict both mortality and disease severity.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.T. C. Ministry Of Health General Directorate Of Public Health. Covid-19 (Sars-Cov2 Infection) Directory. Turkey: Coronavirus Scientific Advisory Board,; 2020. [Google Scholar]
- 2.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0 [DOI] [PubMed] [Google Scholar]
- 3.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu GQ, Zhang Q, Wang RC, Jiang SQ. Predictive value of neutrophil-to-lymphocyte ratio and other inflammatory indicators in estimating clinical severity of coronavirus disease. World J Emerg Med. 2021;12(1):79–80. doi: 10.5847/wjem.j.1920-8642.2021.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92(7):791–796. doi: 10.1002/jmv.25770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. COVID-19 Clinical Management: Living Guidance. Geneva; 2021. [Google Scholar]
- 8.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323((15):):1488–1494. doi: 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80(6):656–665. doi: 10.1016/j.jinf.2020.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei X, Xiao YT, Wang J, et al. Sex differences in severity and mortality among patients with COVID-19: evidence from pooled literature analysis and insights from integrated bioinformatic analysis. arXiv Preprint arXiv. 2020;2003:13547. [Google Scholar]
- 13.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-Cell RNA expression profiling of ACE2, the Receptor of SARS-CoV-2]. Am J Respir Crit Care Med. 2020;202(5):756–759. doi: 10.1164/rccm.202001-0179LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(5):2000688. doi: 10.1183/13993003.00688-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19 [published correction appears in Nat Med. 2020 Sep;26(9):1491-1493]. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 16.Imam Z, Odish F, Gill I, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288(4):469–476. doi: 10.1111/joim.13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong HYF, Lam HYS, Fong AH-T, et al. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology. 2020;296(2):E72–E78. doi: 10.1148/radiol.2020201160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radiology, ACR. [Internet]. ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19. Infection. ACR website. Available from: https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed June16, 2021.
- 19.Sharifpour M, Rangaraju S, Liu M, et al. C-Reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLoS One. 2020;15(11):e0242400. doi: 10.1371/journal.pone.0242400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Sha J, Meng M, et al. Risk factors for severe COVID-19 in middle-aged patients without comorbidities: a multicentre retrospective study. J Transl Med. 2020;18(1):461. doi: 10.1186/s12967-020-02655-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 22.CDC COVID-19 Response Team. Severe outcomes among patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. doi: 10.1183/13993003.00524-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley P, Frost F, Tharmaratnam K, Wootton DG, NW Collaborative Organisation for Respiratory Research. Utility of established prognostic scores in COVID-19 hospital admissions: multicentre prospective evaluation of CURB-65, NEWS2 and qSOFA. BMJ Open Respir Res. 2020;7(1):e000729. doi: 10.1136/bmjresp-2020-000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6–e12. doi: 10.1016/j.jinf.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1):206. doi: 10.1186/s12967-020-02374-0 [DOI] [PMC free article] [PubMed] [Google Scholar]