Abstract
The study aims to present the incidence of COVID-19 in pediatric patients undergoing renal replacement therapy (RRT) and to compare the severity and outcomes of the disease between the dialysis and kidney transplant (KTx) groups. This multicenter observational study was conducted between 1 April and 31 December 2020 in Istanbul. Members of the Istanbul branch of the Turkish Pediatric Nephrology Association were asked to report all confirmed cases of COVID-19 who were on RRT, as well as the number of prevalent RRT patients under the age of 20. A total of 46 confirmed cases of COVID-19 were reported from 12 centers, of which 17 were dialysis patients, and 29 were KTx recipients. Thus, the incidence rate of COVID-19 was 9.3% among dialysis patients and 9.2% among KTx recipients over a 9-month period in Istanbul. Twelve KTx recipients and three dialysis patients were asymptomatic (p = 0.12). Most of the symptomatic patients in both the dialysis and KTx groups had a mild respiratory illness. Only two patients, one in each group, experienced a severe disease course, and only one hemodialysis patient had a critical illness that required mechanical ventilation. In the entire cohort, one hemodialysis patient with multiple comorbidities died.
Conclusion: While most cases are asymptomatic or have a mild disease course, pediatric patients undergoing dialysis and a kidney transplant are at increased risk for COVID-19.
|
What is Known: • In adult population, both dialysis patients and kidney transplant recipients are at increased risk for severe illness of COVID-19 and have higher mortality rate. • Children with kidney transplantation are not at increased risk for COVID-19 and most have mild disease course. • Data on children on dialysis are scarce. | |
|
What is New: • Pediatric patients undergoing dialysis and kidney transplantation have an increased risk for COVID-19. • Most patients undergoing renal replacement therapy either on dialysis or transplanted develop asymptomatic or mild COVID-19 disease with a favorable outcome. |
Keywords: Children, COVID-19, Dialysis, Kidney transplantation, Pediatric, Renal replacement therapy, RRT
Introduction
Currently, the overall mortality rate of COVID-19 is about 2.1% worldwide [1], and this rate varies from country to country, age, and specific patient groups. Uremia is a known risk factor for impaired immune function as evidenced by an increased susceptibility to infections [2]. Evidence from adult studies indicates that chronic kidney disease (CKD), particularly end-stage kidney disease (ESKD), is associated with severe illness and a high mortality rate of COVID-19 [3]. Mortality rates rise to 32% among dialysis patients [4–6]. Similarly, it has been reported that kidney transplant (KTx) recipients have an increased risk of critical COVID-19, with a remarkably high mortality rate of up to 28% [7, 8].
In contrast to adults, most evidence to date suggests that children with advanced stages of CKD, dialysis, or a KTx are not at high risk for COVID-19 and generally have a mild disease course [9–12]. Despite the growing data on COVID-19, it is still of great importance to collect national and international data on this challenging disease from both children and adults [13]. In Turkey, a nationwide study has shown that the mortality rate is higher in hospitalized COVID-19 patients with stages 3–5 CKD, hemodialysis, and KTx than in patients without kidney disease [14]. Therefore, in this multicenter study we conducted in Istanbul, the most populated city in Turkey, we aimed to reveal the incidence of COVID-19 in pediatric patients undergoing renal replacement therapy (RRT) and to compare the disease course and outcomes between the dialysis and KTx groups.
Materials and methods
This multicenter observational study was based on the data collected from children and adolescents with COVID-19 who were undergoing RRT. The study was announced in April 2020 following ethical approval (number 57697, April 29, 2020), and the patient registration form was sent to all members of the Istanbul branch of the Turkish Pediatric Nephrology Association. The members were requested to submit all confirmed cases of COVID-19 (inpatients and outpatients) under the age of 20 years who were receiving dialysis and a KTx. The data collection was completed on December 31, 2020. A total of 12 pediatric nephrology centers reported their COVID-19 cases during the study period. In addition, these centers were asked to report all prevalent ESKD patients under the age of 20 who were either on dialysis or having a functioning KTx that they have actively followed up.
Patient registration forms included information on anthropometric measurements, primary kidney disease, dialysis duration and modality, transplant properties, comorbid conditions, vaccination status for pneumococcus and influenza, and medications. Regarding comorbid conditions, obesity was defined as a height-specific body mass index above the 95th percentile according to the national pediatric growth percentiles [15]. Hypertension was defined as an office blood pressure greater than the 95th percentile according to age-, sex-, and height-specific normative values in the Fourth Report [16]. Patients receiving antihypertensive medication were also described as hypertensive.
COVID-19 was diagnosed by laboratory confirmation using the reverse transcriptase-polymerase chain reaction or serology tests. The possible source of COVID-19, presenting symptoms, clinical, radiological, and available laboratory findings at the time of diagnosis, drug therapy, and outcomes was documented. The severity of the disease was classified as mild, moderate, severe, or critical illness [17]. The number of patients with the multisystem inflammatory syndrome (MIS-C) was also reported. Acute kidney injury (AKI) was defined for KTx recipients as an increase of serum creatinine by 0.3 mg/dL or 50% from baseline within 7 days, according to the Kidney Disease: Improving Global Outcomes [18].
Statistical analyses were performed using SPSS version 21.0 (IBM, Armonk, NY, USA). Data were analyzed by descriptive statistics and presented as a median [(quartile 1 (Q1)–quartile 3 (Q3)] or number (percentage). The two groups were compared using the Mann–Whitney U test or Fisher’s exact test. Statistical significance was defined as a two-tailed p value < 0.05.
Results
A total of 46 confirmed cases of COVID-19 were reported from 12 centers between April 1 and December 31, of which 29 were KTx recipients and 17 were dialysis patients (10 hemodialysis and seven peritoneal dialysis). Table 1 shows the demographics and clinical characteristics of the study population. There was no difference in age, gender, primary kidney disease, or comorbid conditions between the dialysis and KTx groups. Transplant-specific features are also summarized in Table 2.
Table 1.
The demographics and clinical characteristics of the patients
| KTx group n = 29 |
Dx group n = 17 |
P value | |
|---|---|---|---|
| Age*, years | 13.0 (10.5–18.2) | 13.7 (7.2–17) | 0.67 |
| Sex, female/male, n | 14/15 | 6 / 11 | 0.54 |
| RRT durationa, months | 24.4 (5.2–63.8) | 15.9 (10.0–23.1) | 0.18 |
| Primary kidney disease, n | 0.46 | ||
| CAKUT | 9 | 7 | |
| Glomerular disease | 8 | 6 | |
| Ciliopathies | 8 | 2 | |
| Others | 4 | 2 | |
| Comorbid conditions, n | |||
| Hypertension | 18 | 12 | 0.75 |
| Obesity | 9 | 1 | 0.07 |
| Other comorbid conditions | 6 | 8 | 0.10 |
| Pulmonary disease | 3 | 1 | |
| Cardiac problems | 1 | 3 | |
| Malignancies | 2 | 2 | |
| Others | 0 | 2 | |
| Vaccination status, n | |||
| Pneumococcus | 19 | 11 | 1.0 |
| Influenza | 11 | 3 | 0.25 |
KTx kidney transplantation, Dx dialysis, RRT renal replacement therapy, CAKUT congenital anomalies of the kidney and urinary tract
aData presented as median (quartile 1–quartile 3)
Table 2.
Transplant-specific properties of 29 kidney transplant recipients
| Donor type, living/deceased, n | 25/4 |
|---|---|
| Number of HLA mismatchesa | 3.0 (3.0–4.0) |
| Time from transplantationa, months | 24.4 (5.2–64.8) |
| Induction therapy, n | |
| ATG | 10 |
| Basiliximab | 14 |
| None | 5 |
| Maintenance immunosuppression, n | |
| PRED + TAC + Mycophenolate (MMF/MPA) | 25 |
| PRED + TAC + mTORi | 2 |
| PRED + CsA + MMF | 1 |
| TAC + MMF | 1 |
HLA human leukocyte antigen, ATG anti-thymocyte globulin, PRED prednisolone, TAC tacrolimus, MMF mycophenolate mofetil, MPA mycophenolate sodium, mTORi the mammalian target of rapamycin inhibitor, CsA cyclosporine
aData presented as median (quartile 1–quartile 3)
Incidence of COVID-19
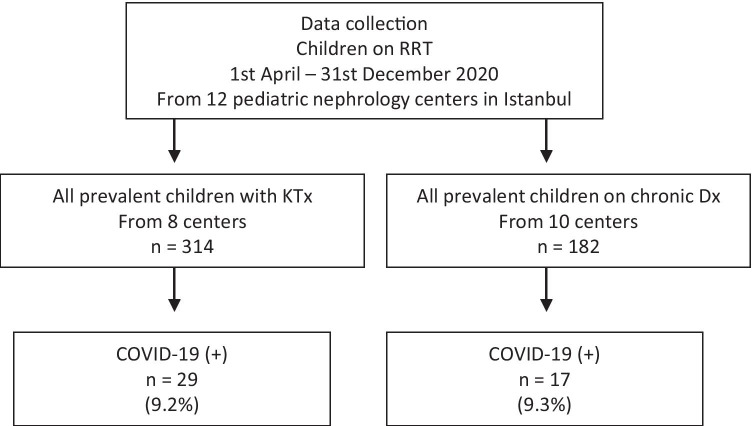
The total number of prevalent patients reported by the 12 centers was 182 for dialysis and 314 for KTx. As shown in Fig. 1, confirmed cases of COVID-19 throughout a 9-month period represented 9.3% of prevalent dialysis patients and 9.2% of prevalent KTx recipients. Two-thirds of the cases (n = 33) were diagnosed between September and December 2020.
Fig. 1.
Flowchart of the study population. RRT renal replacement therapy, KTx kidney transplantation, Dx dialysis
Clinical course and treatment
Table 3 shows COVID-19-specific characteristics of the patients in comparison between the two groups. Fever was the most common presenting symptom in the dialysis group (9/17); however, it was less common in the KTx group (7/29) (p = 0.06). Fifteen patients were asymptomatic in the cohort; all had been tested due to close contact with a person with confirmed COVID-19. The asymptomatic disease was more common in the KTx group than in the dialysis group, but the difference was not statistically significant (12/29 vs. 3/17, p = 0.12). In both groups, most of the symptomatic patients had a mild pulmonary illness. Severe respiratory symptoms requiring oxygen support were uncommon. Only two patients, one in each group, experienced a severe disease course, and only one hemodialysis patient had a critical illness that required mechanical ventilation.
Table 3.
COVID-19 specific features and treatment details in the study population
| KTx group n = 29 |
Dx group n = 17 |
|
|---|---|---|
| Diagnosis of COVID-19, n | ||
| Positive PCR test | 27 | 17 |
| Positive IgM test | 2 | 0 |
| Possible source of COVID-19, n | ||
| Household | 22 | 10 |
| Healthcare | 0 | 2 |
| Unknown | 7 | 5 |
| Presenting symptoms, n | ||
| Fever | 7 | 9 |
| Cough | 7 | 2 |
| Weakness or myalgia | 9 | 6 |
| Headache | 5 | 4 |
| Gastrointestinal symptoms | 5 | 2 |
| Upper respiratory tract symptoms | 3 | 4 |
| Shortness of breath or hypoxia | 4 | 2 |
| Severity of the disease, n | ||
| Asymptomatic disease | 12 | 3 |
| Mild disease | 14 | 12 |
| Moderate disease | 2 | 0 |
| Severe disease | 1 | 1 |
| Critical illness | 0 | 1 |
| Abnormal radiologic findings, n/N | 8/19 | 6/15 |
| Lymphopenia (< 1500 cells/µL), n/N | 9/22 | 10/16 |
| Respiratory support, n | ||
| No respiratory support | 26 | 15 |
| Oxygen treatment | 3 | 1 |
| Mechanical ventilation | 0 | 1 |
| Drug treatment, n | ||
| Favipiravir | 12 | 3 |
| Hydroxychloroquine | 2 | 5 |
| Antibiotics* | 4 | 8 |
| Oseltamivir | 1 | 0 |
| IVIG | 3 | 1 |
| Immunosuppressive modification, n | 18 | |
| Mycophenolate withdrawal | 9 | |
| Mycophenolate dose reduction | 9 | |
| TAC withdrawal or dose reduction | 2 | |
| Increase in steroid dose | 4 | |
| Hospitalization**, n | 8 | 14 |
| ICU admission, n | 3 | 1 |
| Outcome, n | ||
| Recovery | 29 | 16 |
| Death | 0 | 1 |
KTx kidney transplantation, Dx dialysis, PCR polymerase chain reaction, IVIG intravenous immunoglobulin, TAC tacrolimus, ICU intensive care unit
*P = 0.019; **P = 0.001 using Fisher’s exact test, other parameters did not differ between the two groups
Twenty-two patients received antiviral treatment according to the national treatment strategy, and 12 patients received antibiotics. In addition, the immunosuppressive therapy was modified in 18 KTx recipients. The most common modification was withdrawal or dose reduction of mycophenolate. The treatment details are shown in Table 3.
Outcomes
The hospitalization rate was significantly higher among dialysis patients than among KTx recipients (14/17 vs. 8/29, p = 0.001); however, the median length of hospital stays did not differ between the dialysis and KTx groups [11.5 days (4.8–16.3) vs. 6.5 days (5.25–21.0), p = 0.64]. A total of three patients were admitted to the intensive care unit; one of them was due to critical illness of COVID-19 in the dialysis group, and the other two were due to MIS-C in the KTx group. One patient who was receiving hemodialysis died during the study period. This patient had an immune deficiency and constrictive pericarditis and a history of bone marrow transplantation for aplastic anemia.
Renal outcome in KTx recipients
Two patients had proteinuria, but none had hematuria. There was a significant increase in median serum creatinine from baseline [0.84 mg/dL (0.67–1.10)] to peak level [0.95 mg/dL (0.70–1.30)] in 22 KTx recipients who had available data (p = 0.003). Acute kidney injury developed in 8 out of 22 KTx recipients; all had AKI stage 1. There was no difference between the patients with or without AKI in terms of sex, age, comorbidity, medications, or disease severity; however, the hospitalization rate was significantly higher in patients with AKI (6/8 vs. 2/14) (p = 0.008).
Discussion
This multicenter study from Istanbul provided an important opportunity to determine the incidence of COVID-19 in a cohort of pediatric patients receiving RRT over 9 months and to compare disease severity and outcomes between the dialysis and KTx groups. Our results reveal that the incidence of COVID-19 is higher in pediatric patients with RRT, either dialysis or a KTx, than in the general pediatric population. Our study also shows that most patients in both groups develop asymptomatic or mild forms of the disease with a favorable outcome, and death is exceptional.
It is challenging to determine and effectively compare the incidence of COVID-19 due to differences in testing strategies between countries and centers and the presence of many asymptomatic cases. The ERA-EDTA registry showed that the incidence of diagnosed COVID-19 was 1.4% in KTx recipients and 2.9% in the dialysis population at the beginning of the pandemic [5]. For children with ESKD (on dialysis or transplanted), the Spanish Pediatric Association estimated an incidence of COVID-19 of 0.61% [10]. Similarly, the Improving Renal Outcome Collaboratives (IROC) registry reported an overall incidence of COVID-19 of 0.6% among pediatric KTx recipients and 4.4% among tested KTx recipients during the study period of April to September 2020 [19]. Our results demonstrated that the incidence of COVID-19 was 9.3% among dialysis patients and 9.2% among KTx recipients during the first 9-month period of the pandemic; of note, two-thirds of patients were diagnosed between September and December. Considering the overall rate of the disease was 1.2 (1–5)% around the world on December 31 [1], and lower infection rates for children than adults, our result revealed that the incidence of COVID-19 was higher in pediatric RRT patients with either dialysis or a KTx than in the general pediatric population. Consistent with adult studies, but unlike previous pediatric studies, our study shows that children and adolescents with ESKD have an increased risk of COVID-19. We think that this high incidence rate of COVID-19 in our cohort cannot be explained by the disease-specific factors alone, such as the uremic environment, chronic immunosuppression, and higher risk of infection exposure caused by ongoing clinical care. Screening of asymptomatic patients with a contact history due to serious illness concerns may also be a contributing factor to this high rate. On the other hand, it is considered that regional factors may also affect our results. Istanbul, which is the most populous city in Turkey, has been one of the riskiest cities during the pandemic. Therefore, living conditions in Istanbul, crowded households, and low socioeconomic status may also have contributed to the higher incidence rate of COVID-19 in this cohort.
Children are most likely to develop asymptomatic or mild disease due to COVID-19 [15, 20]; however, selected pediatric populations with high rates of comorbidities may have a considerable clinical course [21]. Previous reports demonstrated that most children with CKD or a functioning KTx had a mild or moderate illness of COVID-19 [9–12, 19, 22]. The IROC registry reported that 37% of KTx children were asymptomatic [19]. In our cohort, a significant number of KTx recipients developed an asymptomatic disease, and most symptomatic patients in both groups had mild forms of the disease. Severe respiratory symptoms were uncommon. Although patients in both groups had similar severity of COVID-19, the hospitalization rate was much higher among dialysis patients than among KTx recipients. This difference may be explained by a high proportion of asymptomatic disease in KTx recipients.
Early evidence of the pandemic revealed a high mortality rate of up to 32% in adult patients on RRT, which was strongly associated with older age and comorbidities [5, 7, 23–25]. Comparing to adults, the pediatric population had lower rates of COVID-19-associated mortality. According to a multicenter European Study, the reported mortality rate of COVID-19 is 0.7% among the general pediatric population [26]. This rate ranges from 0 to 3.5% in children with coexisting kidney disease [9, 10, 12, 22]. In our cohort, one hemodialysis patient who had severe comorbidities died from COVID-19. This finding contributes additional evidence that death is quite exceptional in the pediatric population, but patients with comorbidities may have an increased risk for mortality from COVID-19.
Previous studies have demonstrated that COVID-19-associated AKI develops in 27 to 52% of KTx recipients [23, 24, 27–29]. In our cohort, eight out of 22 KTx recipients demonstrated AKI; none required dialysis. However, it is important to note that the hospitalization rate was higher in transplanted patients with AKI than in patients without AKI. Another challenge posed by the COVID-19 pandemic for KTx recipients has been to maintain optimal immunosuppressive therapy. It is recommended to continue their calcineurin inhibitors and glucocorticoids and stop anti-proliferative drugs [30]. In our cohort, two-thirds of KTx recipients had a change in their immunosuppressive regimen. All of these had a modification in mycophenolate as an anti-proliferative drug. Mycophenolate withdrawal was preferred in half, while mycophenolate dose reduction was performed in the other half. None of our KTx recipients experienced allograft rejection, serious bacterial infections, or critical illness during COVID-19.
Our study has several limitations. First, we did not have any control group of the general pediatric population with COVID-19 from Istanbul to compare the infection rate and disease course to minimize regional differences. Another limitation is that we could not obtain any information regarding how many children with RRT were tested for COVID-19. Lastly, some variation may have been introduced due to the multicenter design since individual centers had their own indications for hospitalization and different approaches for the immunosuppressive modifications in KTx recipients.
Conclusion
The incidence of COVID-19 is higher in pediatric patients who receive dialysis or a KTx than in the general pediatric population. At the same time, most cases are asymptomatic or have a mild disease course. Our study also contributes additional evidence that patients with severe comorbidities may have a high risk of mortality. More comprehensive and collaborative reports are needed to elucidate the course and impact of COVID-19 in this patient population.
Acknowledgements
We thank all members of the Istanbul branch of the Turkish Pediatric Nephrology Association for their contributions.
Abbreviations
- AKI
Acute kidney injury
- CKD
Chronic kidney disease
- COVID-19
Coronavirus disease 2019
- Dx
Dialysis
- ESKD
End-stage kidney disease
- KTx
Kidney transplant
- RRT
Renal replacement therapy
Authors’ contributions
Conceptualization and methodology: N. Canpolat, Z.Y. Yıldırım, N. Yıldız; data collection and analysis: N. Canpolat, Z.Y. Yıldırım, N. Yıldız, Z. Eynep Yürük M. Taşdemir, N. Göknar, H. Evrengül, R. Gülmez, B. Aksu, H. Dursun, G. Özçelik, Ö. Yavaşcan, R.Y. Çiçek, S. Tülpar, D.Ö. Hacıhamdioğlu, A. Nayır, H. Alpay. Writing of the first draft: N. Canpolat; review and editing: N. Canpolat, H. Alpay. All the authors read and approved the final manuscript.
Data availability
Data may be shared upon reasonable requests.
Declarations
Ethics approval
This study was conducted retrospectively from data obtained for clinical purposes and approved by the Clinical Research Ethical Committee of Cerrahpaşa Faculty of Medicine (Date: April 29, 2020/No: 57697).
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nur Canpolat, Email: nur.canpolat@iuc.edu.tr.
Zeynep Yürük Yıldırım, Email: znyuruk@gmail.com.
Nurdan Yıldız, Email: nbilgenyildiz@gmail.com.
Mehmet Taşdemir, Email: drmtasdemir@gmail.com.
Nilüfer Göknar, Email: nilufergoknar@gmail.com.
Havva Evrengül, Email: havva.evrengul@isu.edu.tr.
Bağdagül Aksu, Email: bagdagul@yahoo.com.
Hasan Dursun, Email: dursunhs@yahoo.com.
Gül Özçelik, Email: ozcelik62@gmail.com.
Önder Yavaşcan, Email: oyavascan@hotmail.com.
Rümeysa Yasemin Çiçek, Email: dr-rumeysayasemincicek@hotmail.com.
Sebahat Tülpar, Email: stulpar76@yahoo.com.
Duygu Övünç Hacıhamdioğlu, Email: duyguovunc@gmail.com.
Ahmet Nayır, Email: nayir@ttmail.com.
Harika Alpay, Email: harika.alpay@gmail.com.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int/covid-19-data.page. Accessed June 11, 2021
- 2.Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 3.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valeri AM, Robbins-Juarez SY, Stevens JS, Ahn W, Rao MK, Radhakrishnan J, Gharavi AG, Mohan S, Husain SA. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31:1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA-EDTA registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberici F, Delbarba E, Manenti C, et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98:20–26. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARSCoV2 pneumonia. Kidney Int. 2020;97:1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plumb L, Benoy-Deeney F, Casula A, et al. COVID-19 in children with chronic kidney disease: findings from the UK renal registry. Arch Dis Child. 2021;106(3):e16. doi: 10.1136/archdischild-2020-319903. [DOI] [PubMed] [Google Scholar]
- 10.Melgosa M, Madrid A, Alvárez O, Lumbreras J, Nieto F, Parada E, Perez-Beltrán V, Association SPN. SARS-CoV-2 infection in Spanish children with chronic kidney pathologies. Pediatr Nephrol. 2020;35:1521–1524. doi: 10.1007/s00467-020-04597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angeletti A, Trivelli A, Magnasco A et al (2020) Risk of COVID-19 in young kidney transplant recipients. Results from a single-center observational study. Clin Transplant 34:e13889. 10.1111/ctr.13889 [DOI] [PubMed]
- 12.Marlais M, Wlodkowski T, Vivarelli M, et al. The severity of COVID-19 in children on immunosuppressive medication. Lancet Child Adolesc Health. 2020;4:e17–e18. doi: 10.1016/S2352-4642(20)30145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritz N, de Winter JP. COVID-19 in children: patiently and critically evaluate the scientific evidence. Eur J Pediatr. 2020;179:1179–1180. doi: 10.1007/s00431-020-03708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozturk S, Turgutalp K, Arici M, et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis, and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant. 2020;35:2083–2095. doi: 10.1093/ndt/gfaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neyzi O, Bundak R, Gokcay G, et al. Reference values for weight, height, head circumference, and body mass index in Turkish children. J Clin Res Pediatr Endocrinol. 2015;7:280–293. doi: 10.4274/jcrpe.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 17.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellum JA, Lameire N, Group KAGW Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varnell C Jr, Harshman LA, Smith L et al (2021) COVID-19 in pediatric kidney transplantation: the improving renal outcomes collaborative. Am J Transplant January 16. 10.1111/ajt.16501 [DOI] [PMC free article] [PubMed]
- 20.Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A, Vidal E, Cogo P. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029–1046. doi: 10.1007/s00431-020-03684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parri N, Magistà AM, Marchetti F, Cantoni B, Arrighini A, Romanengo M, Felici E, Urbino A, Da Dalt L, Verdoni L, Armocida B, Covi B, Mariani I, Giacchero R, Musolino AM, Binotti M, Biban P, Fasoli S, Pilotto C, Nicoloso F, Raggi M, Miorin E, Buonsenso D, Chiossi M, Agostiniani R, Plebani A, Barbieri MA, Lanari M, Arrigo S, Zoia E, Lenge M, Masi S, Barbi E, Lazzerini M; CONFIDENCE and COVID-19 Italian Pediatric Study Networks Characteristic of COVID-19 infection in pediatric patients: early findings from two Italian Pediatric Research Networks. Eur J Pediatr. 2020;179:1315–1323. doi: 10.1007/s00431-020-03683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastrangelo A, Morello W, Vidal E, et al. Impact of COVID-19 pandemic in children with CKD or immunosuppression. Clin J Am Soc Nephrol. 2021;16:449–451. doi: 10.2215/CJN.13120820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberici F, Delbarba E, Manenti C, et al. management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep. 2020;5:580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cravedi P, Mothi SS, Azzi Y. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140–3148. doi: 10.1111/ajt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goicoechea M, Sánchez Cámara LA, Macías N, et al. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98:27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Heal. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bossini N, Alberici F, Delbarba E, et al. Kidney transplant patients with SARS-CoV-2 infection: the Brescia Renal COVID task force experience. Am J Transplant. 2020;20:3019–3029. doi: 10.1111/ajt.16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair V, Jandovitz N, Hirsch JS, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lum E, Bunnapradist S, Multani A, et al. Spectrum of Coronavirus disease 2019 outcomes in kidney transplant recipients: a single-center experience. Transplant Proc. 2020;52:2654–2658. doi: 10.1016/j.transproceed.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kronbichler A, Gauckler P, Windpessl M, Il Shin J, Jha V, Rovin BH, Oberbauer R. COVID-19: implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol. 2020;16:365–367. doi: 10.1038/s41581-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be shared upon reasonable requests.