Abstract
Group B Streptococcus (GBS) is an encapsulated gram-positive human pathogen which causes invasive infections in pregnant hosts and neonates, as well as immunocompromised individuals. Colonization of the human host requires the ability to adhere to mucosal surfaces and circumnavigate the nutritional challenges and antimicrobial onslaught associated with the innate immune response. Biofilm formation is a critical process to facilitate GBS survival and establishment of a replicative niche in the vertebrate host. Previous work has shown that the host responds to GBS infection by producing the innate antimicrobial glycoprotein lactoferrin, which has been implicated in repressing bacterial growth and biofilm formation. Additionally, lactoferrin is highly abundant in human breast milk and could serve a protective role against invasive microbial pathogens. Our work demonstrates that human breast milk lactoferrin has antimicrobial and anti-biofilm activity against GBS and inhibits GBS adherence to human gestational membranes. Together these results indicate that human milk lactoferrin could be utilized as a prebiotic chemotherapeutic strategy to limit the impact of bacterial adherence and biofilm formation on GBS-associated disease outcomes.
Keywords: lactoferrin, streptococcus, infection, innate immunity, antimicrobial, biofilm
Introduction
Group B Streptococcus (GBS), or Streptococcus agalactiae, is an encapsulated gram-positive bacterium and one of the leading infection-related causes of adverse pregnancy outcomes. Some of the consequences of GBS infection may include chorioamnionitis, preterm premature rupture of membranes (PPROM), preterm birth (PTB), stillbirths, early-onset and late-onset sepsis, neonatal meningitis 1,2. For most healthy adults, GBS is part of the commensal microflora of the digestive tract but may traverse to the vaginal tract to infection the newborn through ascending infection or ingestion/inhalation of infectious fluids through vertical transmission during childbirth 3. The leading risk factor of GBS disease is maternal vaginal colonization 4 and one longitudinal study revealed that up to 50% of the cohort transient GBS colonization at some point during the pregnancy 5. Currently, no commercial vaccine is available for GBS. Treatment includes antibiotic prophylaxis for mothers who screened positive for GBS prior to delivery. While treatment is available, hypersensitivities to first-line antibiotics 6, the lack of improvement of late-onset neonatal sepsis 7, and the rise of antibiotic resistant strains of GBS 8 call for novel treatments.
There is a wealth of evidence supporting the benefits of breastfeeding in improving infant health, including combating pathogenic bacterial infections. Laboratory and clinical studies have supported that human breast milk is able to decrease bacterial-related diarrhea 9, urinary tract infection 10,11, and many other diseases associated with bacterial infection 12. More studies have revealed that components of human breast milk, including maternal immunoglobulins 13, TGF-β 14, and milk oligosaccharides 15 aid in improve infant health. Components of human breast milk also influence the infant microbiome to protect against invading pathogens 16, and many of these components are able to directly interact with pathogenic bacteria. In fact, studies have shown that human oligosaccharides are able to suppress GBS growth and biofilm formation 17 and sensitize them to a wide range of antibiotics 18,19.
One antimicrobial glycoprotein secreted in high concentrations in human breast milk is lactoferrin. This antimicrobial protein can comprise of up to 20% of human breast milk. However, the concentration of lactoferrin in milk varies with time since childbirth. Generally, there are higher concentrations of lactoferrin in colostrum, which decreases as the infant matures 20. On average, lactoferrin concentrations in the colostrum are 7-9 g/L and decrease to 1-3 g/L in mature milk 21,22.
Lactoferrin is a glycoprotein that contains two iron binding sites 23 which participate in the host defense strategy known as “nutritional immunity”, the biological phenomenon of starving bacteria of essential metals 24. Iron, in particular, is involved in many crucial bacterial processes by acting as a cofactor for enzymes driving DNA replication, transcription, and central metabolism 25. As such, the access to iron is crucial for bacterial survival within the host niche. Lactoferrin has shown to have antimicrobial activity against a wide range of bacterial, viral, and fungal pathogens 26. Due to its potent antimicrobial activity, several groups have studied lactoferrin for therapeutic use. In one study, supplementation with lactoferrin decreased instances of late-onset neonatal sepsis and necrotizing enterocolitis in pre-term infants 27. Lactoferrin also shows promise as an agent against streptococcal infections 28, including a study demonstrating lactoferrin decreases biofilm formation in Streptococcus mutans 29. Biofilms are multicellular structures that are critical for many bacterial pathogens, including GBS, to circumnavigate host defenses and persist in the hostile environment 30,31. We hypothesized that human milk lactoferrin would potentially decrease bacterial growth, survival, and biofilm formation in GBS, and that this important host glycoprotein could potentially influence bacterial-host interactions in the context of human pregnancy.
In this study, we purified lactoferrin from donor human breast milk and demonstrated that human milk lactoferrin is able to decrease bacterial growth, survival, and biofilm formation by GBS in vitro. Furthermore, our work demonstrates that human milk lactoferrin has the capacity to inhibit GBS adherence to human gestational membrane tissues ex vivo, and that the antimicrobial properties are enhanced when the glycoprotein is in the apo- form.
Materials and Methods
Bacterial strains and culture conditions
S. agalactiae strain GB112, a clinical isolate from the human reproductive tract 32, was cultured on tryptic soy agar plates supplemented with 5% sheep blood (blood agar plates) at 37°C in ambient air overnight. The following day, bacteria were sub-cultured from blood agar plates into liquid medium (Todd Hewitt Broth - THB) and incubated in aerobic conditions (ambient air, shaking at 200 RPM) at 37°C overnight. The following day, bacterial density was measured spectrophotometrically to determine the optical density at 600 nm (OD600) and these bacterial cultures were utilized for growth, viability, biofilm, and co-culture assays.
Purification of lactoferrin from human breast milk
Expressed human breast milk was gathered from 17 healthy donors between 3 days and 3 months post-partum and stored between −80 and −20°C. De-identified human milk samples were provided by Dr. J. Hendrik Weitkamp from the Vanderbilt Department of Pediatrics, under a collection protocol approved by the Vanderbilt University Institutional Review Board (IRB #100897). Milk samples were thawed and centrifuged at 8000 g for 45 minutes to separate milk fats from the soluble fraction. Following centrifugation, the resultant top lipid layer was removed. Subsequently, proteins were precipitated from the soluble fraction by the addition of an equal volume of 100% ethanol to the soluble fraction and incubation at 4°C overnight. Precipitated proteins were fractionated by high performance liquid chromatography. Identity of the sample was determined by mass spectrometry analyses and protein folding was validated by circular dichroism.
Preparation of holo- or apo-lactoferrin
Iron-bound (holo-) or unbound (apo-) lactoferrin was prepared as previously described 33,34. Briefly, 10 mg/mL stock of purified lactoferrin was dialyzed against either 0.1 M sodium citrate-bicarbonate buffer pH 8.2 alone to generate apo-lactoferrin, or buffer containing 70 mM ferric chloride to generate holo-lactoferrin. Both apo- and holo-lactoferrin were dialyzed against 1X phosphate buffered saline (PBS) containing Chelex Resin (Sigma Aldrich) to remove any unbound iron content.
Evaluation of bacterial growth and viability
To determine bacterial growth or viability, optical density measurements at 600 nm (OD600) were recorded. GBS cultures were grown to stationary phase (OD600 between 0.2-0.3) and diluted at 1:10 in metal-limited THB medium (50% THB with 50% calprotectin buffer (100 mM NaCl, 3 mM CaCl2, 20 mM Tris pH 7.5 33,35)). 100 μL of 1:10 diluted cultures were added to each well in a 96-well plate. The appropriate concentration of purified lactoferrin was added into its corresponding well. The plates were left to grow at 37°C and OD600 was measured every hour for the first ten hours. The following day, bacterial density was determined by measuring OD600. For the viability assay, each well was serial-diluted at 1:10 up to 10−8 with PBS or liquid medium. Each dilution series was plated on blood agar plates and left to grow at 37°C in ambient air overnight. The following day, colonies were counted to determine colony forming units (CFU).
Quantification of bacterial biofilms
To evaluate bacterial biofilms, a crystal violet assay was utilized as previously described 36. Briefly, overnight GBS cultures were diluted 1:10 in THB medium in 96-well plates. To analyze the effect of lactoferrin on biofilm inhibition, lactoferrin was applied in increasing concentrations at the time of inoculation. To analyze the effect of lactoferrin on disrupting existing biofilms, lactoferrin was applied in increasing concentrations after biofilms were established for 24 hours. Biofilms were allowed to form at 37°C in ambient air overnight. OD600 was determined using a spectrophotometer and supernatant was removed and replaced with 0.1% crystal violet stain for thirty minutes. Wells were washed with deionized water three times and dried. The retained crystal violet was resolubilized with a solution of 80% ethanol and 20% acetone. Plates were incubated for at least 30 minutes and optical density was determined at 560 nm (OD560). Quantification was determined by using a ratio of OD560/OD600.
Gestational membrane-GBS co-culture assays
De-identified gestational membrane tissue samples were procured from term, non-laboring caesarean section-delivery live births at Vanderbilt University Medical Center with approval from the Vanderbilt University Medical Center Institutional Review Board (VUMC IRB #170777). 12-mm gestational membranes biopsy punches were isolated and cultured in RPMI 1640 medium (ThermoFisher, Waltham, MA) with 10% charcoal stripped fetal bovine serum (ThermoFisher) and 1% antibiotic/antimycotic solution (ThermoFisher) overnight at 37°C in room air supplemented with 5% carbon dioxide. The membranes were infected with 106 CFU/mL of GBS at in RMPI 1640 medium without antibiotics in the absence of lactoferrin treatment or supplemented with either apo- or holo-lactoferrin at a concentration of 250 μg/mL. Co-cultured tissues were incubated at 37°C in air supplemented with 5% carbon dioxide overnight.
Human fetal membrane immunohistochemistry (IHC) staining
Twenty-four hours post infection, membranes were transferred to histological cassettes and fixed in 4% formalin (buffered). Tissues were cut into 5-μm sections, and multiple sections were placed on each slide for analysis. For immunohistochemistry, slides were de-paraffinized, and heat-induced antigen retrieval was performed on the Bond Max automated IHC stainer (Leica Biosystems, Buffalo Grove, IL) using their Epitope Retrieval 2 solution for 5 to 20 min. Slides were incubated with a rabbit polyclonal anti-GBS antibody (ab78846; Abcam) for 1 h. The Bond Polymer Refine detection system (Leica Biosystems) was used for visualization. Slides were dehydrated and cleared, and coverslips were added before light microscopy analysis was performed.
High resolution electron microscopy analyses
Samples were fixed in 2.0% paraformaldehyde and 2.5% glutaraldehyde in 0.05 M sodium cacodylate buffer for at least 12 h prior to sequential dehydration with increasing concentrations of ethanol. Samples were subsequently dried at the critical point, using a CO2 drier (Tousimis, Rockville, MD), mounted onto an aluminum stub, and sputter coated with 80/20 gold-palladium. A thin strip of colloidal silver was painted at the sample edge to dissipate sample charging. Samples were imaged with a FEI Quanta 250 field-emission gun scanning electron microscope.
Statistical analyses
Statistical analyses of biofilm formation and bacterial growth were performed using a one-way ANOVA with either Tukey’s or Dunnett’s post hoc correction for multiple comparisons. All reported P values are adjusted to account for multiple comparisons. Analysis of bacterial viability was performed using log transformation of CFU data and either Mann-Whitney U or one-way ANOVA with either Tukey’s or Dunnett’s post hoc correction for multiple comparisons analyses. P values of ≤0.05 were considered significant. All data analyzed in this work were derived from at least three biological replicates. Statistical analyses were performed using GraphPad Prism 6 software (GraphPad Prism Software Inc., La Jolla, California).
Results
Purification of the glycoprotein, lactoferrin, from human breast milk
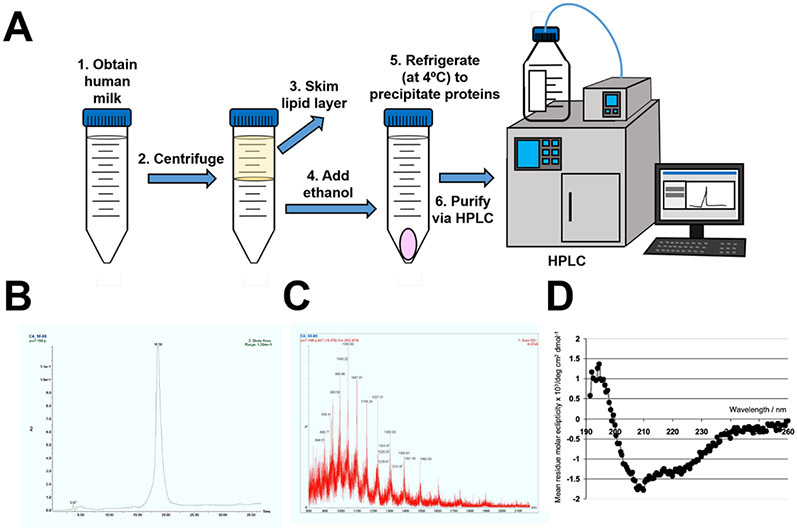
Previous reports have indicated that lactoferrin is a dominant glycoprotein in human breast milk with antimicrobial and immunomodulatory functions. In order to study the activity of human milk-derived lactoferrin against GBS, lactoferrin was isolated from donor milk and purified via HPLC (Figure 1). High-resolution mass fingerprinting technique (MALDI-TOF MS/MS fragmentation) was employed in conjunction with trypsin digestion and m/z peaks revealed a fragmentation pattern and molecular mass that was consistent with that of human lactoferrin (Figure 1C). Additionally, circular dichroism (CD) spectra was collected in the far-UV region (200-250 nm) to monitor conformational changes to the polypeptide backbone of the protein. The peaks observed at 208, 216, and 222 nm are consistent with a dominant isoform of apo-lactoferrin derived from the human milk sample when compared with published CD spectra of lactoferrin 37.
Figure 1. Purification of the innate immune antimicrobial glycoprotein, lactoferrin.
A) Conceptual diagram of the protocol for purification of lactoferrin from human breast milk. Milk is centrifuged, lipid layer is removed, ethanol is added to precipitate milk proteins under refrigeration, and lactoferrin is purified by chromatography techniques. B) Chromatograph indicating a single peak, demonstrating purity of lactoferrin sample. C) Mass spectrometry results demonstrating the identity of lactoferrin within the sample. D) Circular dichroism graph results demonstrating the proper folding of lactoferrin, and that the bulk of the sample is present in the apo-form.
Lactoferrin inhibits GBS growth
Previous studies have shown that lactoferrin possesses antimicrobial activity against GBS 34. In order to verify the ability of our purified lactoferrin to inhibit bacterial growth, GBS growth was measured for the first ten hours and overnight across increasing concentration of apo- and holo- lactoferrin. Spectrophotometric analysis of bacterial growth revealed that lactoferrin at concentrations at or above 500 μg/mL significance dampened bacterial growth between 3 to 10 hours post-inoculation, compared to the respective timepoint grown without lactoferrin treatment (Figure 2A) (P < 0.05, Student’s T-test). However, this phenotype was not observed when treated with holo-lactoferrin (Figure 2B), suggesting that lactoferrin purified from breast milk can possess antimicrobial activity against GBS, likely through an iron binding-dependent mechanism.
Figure 2. Analysis of bacterial growth in increasing concentrations of lactoferrin.
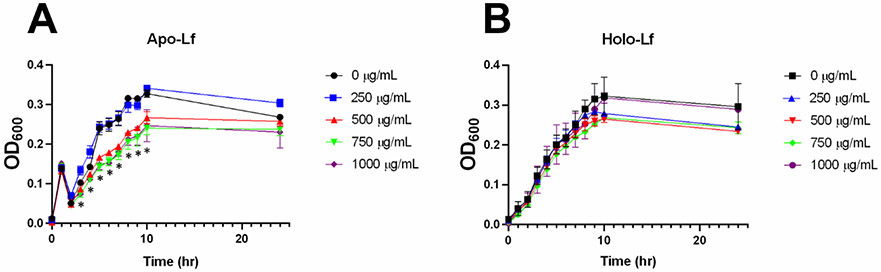
GBS was grown in increasing concentrations (0, 250, 500, 750, 1000 μg/mL) of A) apo-lactoferrin (apo-Lf) or B) holo-lactoferrin (holo-Lf) over a 24-hour period. Bacterial growth was determined by measuring cellular density (optical density at 600 nm or OD600). Points equal a mean +/− SEM, n=3-5. *P<0.05, Student’s t test comparison to bacteria grown in medium alone at the same time point. Apo-lactoferrin significantly inhibits GBS growth at concentrations of 500, 750, and 1000 μg/mL at 3, 4, 5, 6, 7, 8, 9, and 10 hours post-inoculation, a result that was ablated by saturation of the glycoprotein with iron.
To determine the effect of lactoferrin on cell viability, we plated serial dilutions of the culture at 24 hours post-inoculation to determine differences in bacterial viability across increasing concentrations of lactoferrin. Both apo- and holo- lactoferrin were able to inhibit bacterial viability at concentrations at or above 250 μg/mL, when compared to cultures grown without lactoferrin (Supplemental Figure 1) (P < 0.05, Student’s t test). In summary, apo-lactoferrin purified from human breast milk was able to inhibit bacterial growth, while both isoforms were able to inhibit bacterial viability.
Lactoferrin inhibits GBS biofilm in vitro
To determine if lactoferrin possesses anti-biofilm activity, GBS was treated with a concentration of lactoferrin (250 μg/mL) that was sub-inhibitory to bacterial growth and biofilm formation was quantified using a crystal violet assay. At this concentration, biofilm formation, as measured by optical density at 560 nm and normalized by optical density at 600 nm, was significantly reduced by 50% when cultured in the presence of apo-lactoferrin (Figure 4) (One-tailed Student’s t test, P=0.0201). Culture in the presence of holo-lactoferrin resulted in a 42% decrease in biofilm formation, though this was statistically indistinguishable from the negative controls grown in medium alone (One-tailed Student’s t test, P=0.0918). High resolution scanning electron microscopy (SEM) revealed results congruent with the quantitative analyses. GBS grown in medium alone formed robust biofilms on the abiotic substrate (glass coverslips), and culture in the presence of 250 μg/mL of apo-lactoferrin resulted in diminished GBS biofilm tertiary architectural structure. GBS grown in the presence of 250 μg/mL of holo-lactoferrin resulted in diminished GBS biofilm tertiary architectural structure, however, this phenotype was observed to a lesser extent than results obtained from cultures treated with apo-lactoferrin. Taken together, GBS exposure to lactoferrin inhibits biofilm formation as a consequence of iron chelation.
Figure 4. Analysis of ex vivo bacterial biofilm formation on an abiotic surface in the presence or absence of lactoferrin.
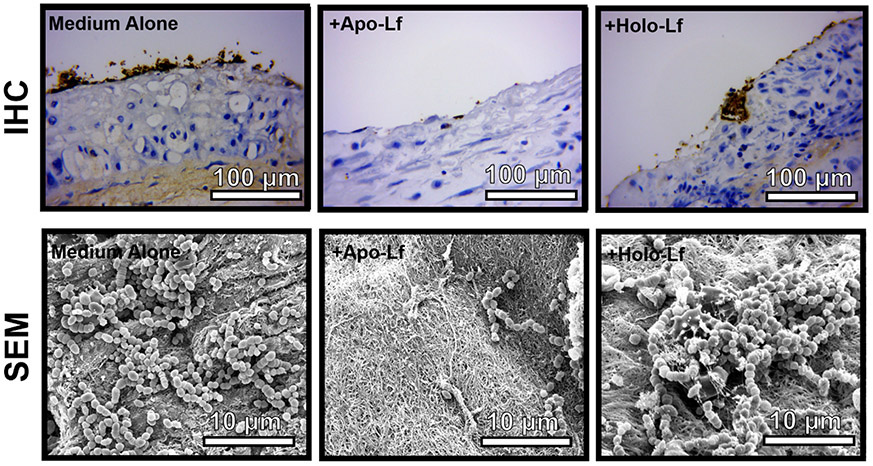
GBS was grown in co-culture with gestational membrane tissue in medium alone or medium supplemented with 250 μg/mL of either apo-lactoferrin (apo-Lf) or holo-lactoferrin (holo-Lf). Bacterial biofilm was analyzed by either immunohistochemical (IHC) staining with an antibody specifically to GBS (brown staining) and high-resolution scanning electron microscopy (SEM) at high magnification (10,000x, magnification bar indicates 10 μm). Apo-lactoferrin significantly inhibits GBS adherence to gestational membrane tissue, while holo-lactoferrin has an intermediate phenotype that is comparable to the control sample cultured in medium alone.
Lactoferrin inhibits GBS adherence to gestational membrane tissues ex vivo
In order to investigate if purified human milk lactoferrin can disrupt GBS biofilm formation on human tissues, gestational membrane biopsies were acquired and co-cultured with GBS in medium alone or medium supplemented with either apo- or holo- isoforms of lactoferrin at a concentration of 250 μg/mL. Co-cultures were incubated for 24 hours to promote adherence of bacteria and interactions within the gestational membranes. The tissues were then fixed and processed for immunohistochemistry staining for GBS. IHC revealed that GBS is able to adhere to, and form, microcolonies on the maternal decidual side of the membrane (Figure 5). Treatment with apo-lactoferrin inhibited this phenotype while GBS was able to form biofilm colonies when treated with the same concentration of holo-lactoferrin. In conclusion, lactoferrin is able to suppress GBS biofilm formation on human gestation membrane tissues.
Figure 5. Model of lactoferrin iron binding-dependent inhibition of biofilm and growth.
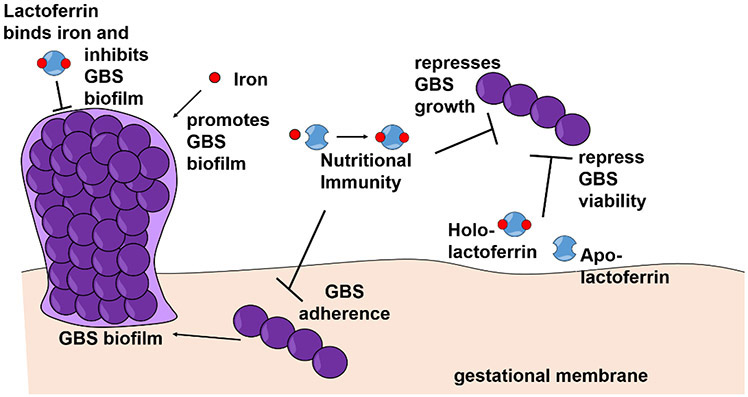
Free iron ions promote GBS biofilm formation. Apo-lactoferrin binds iron and inhibits biofilm formation and GBS growth via iron sequestration in a process termed, “nutritional immunity”. Apo-lactoferrin binds iron and inhibits GBS adherence to gestational membranes. Both holo- and apo-lactoferrin have the capacity to repress GBS viability.
Discussion
In our study, we were successful in purifying human lactoferrin from donor breastmilk using ion-exchange chromatography. This technique has been utilized to purify lactoferrin from a host of mammalian breast milk 38 and we were able to assess the quality of the purification by chromatography, mass spectrometry, and circular dichroism analysis (Figure 1). At of the time of this study, lactoferrin utility has been studied for a wild range of human diseases, many of which are particularly detrimental the infants. However, the majority of these studies focus on the utility of bovine lactoferrin.
One study assessed the use of bovine lactoferrin in preventing sepsis and necrotizing enterocolitis found that lactoferrin supplementation was able to reduce late-onset neonatal sepsis but not necrotizing enterocolitis 27. Interestingly, GBS infection leads to preterm birth and late-onset neonatal sepsis. In another randomized placebo-controlled clinical trial with bovine lactoferrin supplementation, neonates with low-birth rate exhibited less incidences of late-onset sepsis 39. Meanwhile, another group sought to determine the impact of bovine lactoferrin in preventing diarrhea in children found that supplementation did not reduced the incidences of occurrence but showed that across time, the prevalence and severity of diarrhea was decreased 40. While there is some encouraging evidence of the benefits of bovine lactoferrin, another study didn’t observe any differences in incidences of infant sepsis with birth weight of under 2000 grams 41. Similarly, another cohort observed no improvement in death or major comorbidities but consistent with other findings, found a reduction in late-onset sepsis with bovine lactoferrin supplementation 42.
While some benefits have been observed with bovine lactoferrin supplementation, even more success have been shown when lactoferrin is used in combination with other biological agents. For instance, children supplemented with lactoferrin and lysozyme, another component of breast milk observed to improve gut health, experienced decreased hospitalization and reduced development of acute malnutrition 43. In another study with lactoferrin in combination with Lactobacillus GG, infants with intervention showed a decreased incidence of severe necrotizing enterocolitis 44. Both trials with bovine lactoferrin showed improvement in diseases; however, it remains to be seen how human lactoferrin may affect outcomes.
With pertinence to lactoferrin and GBS disease, one study found that vaginal supplementation in pregnant women with bacterial vaginosis reduced the rates of preterm births 45. As GBS can infect the fetus through vaginal ascending infections, there is merit in studying the use of human lactoferrin in the prevention of GBS-mediated pre-term births and adverse pregnancy outcomes.
The antimicrobial activity of lactoferrin has been implicated in many microbial species, as well as viruses, and fungi 28. Our work shows that lactoferrin inhibits GBS bacterial growth. In addition to GBS, lactoferrin also displays antimicrobial effects on other neonatal pathogens including Staphylococcus epidermidis and Escherichia coli, and the probiotic Bifidobacterium breve 46. One mechanism lactoferrin, and several peptides derived from the protein, is able to kill bacterial cells, in particular Staphylococcus aureus and E. coli, is through permeabilizing of the bacterial membrane 47. Other similar studies of lactoferrin and its peptide-derivative demonstrated inhibition of bacterial growth of Vibrio cholerae and Other Vibrio species, attributing to its ability to disrupt bacterial membranes 48,49. Lactoferrin has also shown to inhibit the growth of intracellular pathogens Salmonella typhimurium. In particular, lactoferrin and other milk glycoproteins are able to inhibit the bacterium from adhering to mammalian cells, preventing them from entering the cell and replicating intracellular. This reveals another mechanism by with lactoferrin is able to disrupt bacterial growth 50.
Lactoferrin is able to bind two irons and starve microbes of the essential metal 51. We were able to show that lactoferrin’s antimicrobial activity against GBS is in largely due to the chelation of nutrient iron, a result that was attenuated when the molecule was pre-saturated with iron to form the holo-isoform. This is consistent with previous studies with recombinant lactoferrin inhibiting GBS growth through an iron-dependent mechanism 34. However, our studies also reveal that holo-lactoferrin is able to inhibit bacterial viability, suggesting that both iron-dependent and independent mechanisms play a role in its antimicrobial activity against GBS.
Majority of the anti-biofilm properties of lactoferrin have been studied with Pseudomonas aeruginosa, a pathogen known for its biofilm forming capabilities and its detrimental effects to human health. A group studying the effects of lactoferrin and peptide-derivatives showed that the protein is able to downregulate virulence factors (pyocyanin and elastase) and reduce biofilm formation in a type strain of P. aeruginosa 52. Meanwhile, another group demonstrated that lactoferrin inhibits P. aeruginosa biofilm formation, especially under anaerobic conditions 53. Beyond laboratory strains, lactoferrin also exhibit antibiofilm effects on clinical strains of P. aeruginosa and pretreatment with ferric iron ablated some of the phenotype, suggesting that iron chelation is involved 54. This iron-dependent mechanism is consistent with our finding in which apo-lactoferrin decreased GBS biofilm formation at a sub-inhibitory concentration whereas holo-lactoferrin did decrease biofilm formation, though it did not achieve statistical significance.
There have also been studies assessing the anti-biofilm effects of lactoferrin against a range of Streptococcal species. Several studies have demonstrated that coating titanium surfaces inhibited bacterial adhesion and subsequent biofilm formation in Streptococcus gordonii 55 and Streptococcus sanguinis 56. Coating of lactoferrin to other surfaces also resulted in limited adhesion of Streptococcus mutans 57. Finally, lactoferrin has been shown to inhibit biofilm formation in S. mutans 29. To our knowledge, our study is amongst the first to investigate the antimicrobial and anti-biofilm properties of human lactoferrin against GBS.
One recent study with Streptococcus pneumoniae revealed that lactoferrin is able to decrease biofilm formation 58. Another study of S. pneumoniae revealed that iron increases biofilm formation while iron chelation lead to disruption of biofilm formation 59. The group further characterized the role of the mononuclear iron protein S-ribosylhomocysteine lyase (LuxS) and quorum sensing and biofilm formation. As we saw similar iron-chelation dependent disruption of biofilm formation through the activity of apo-lactoferrin, it is plausible that GBS possess similar iron sensing pathways that govern biofilm formation.
We observed that lactoferrin possess antimicrobial and biofilm properties against GBS in laboratory in vitro conditions on abiotic surfaces. In order to further our studies, we sought to investigate if the antibiofilm and antimicrobial properties of lactoferrin could be determined on a relevant biotic surface. To do this, we acquired human fetal membranes and performed co-cultures with GBS ex vivo to determine if lactoferrin could alter GBS adherence to relevant human tissues. Congruent with our in vitro studies, we observed that treatment with lactoferrin disrupted GBS biofilm formation, and that this phenotype was most pronounced with the apo-isoform of lactoferrin. Previous studies using primary human gestational tissue ex vivo models to investigate bacterial biofilm formation have shown that bacterial biofilm formation on gestational tissues can be correlated with enhanced production of pro-inflammatory cytokines such as IL-6, IL-1β, IL-2, IFN-γ, TNF-α, and GM-CSF 60. Thus, it is possible that lactoferrin could inhibit bacterial adherence and biofilm formation and could also influence immunological outcomes associated with inflammation.
Several studies have explored the protective properties of lactoferrin against a range of infectious bacterial diseases in vivo. Other groups have shown using lactoferrin knockout mice that the protein is important in the defense against Aggregatibacter actinomycetemcomitans 61 and Streptococcus mutans related bacteremia 62. Other studies have demonstrated the benefit of lactoferrin supplementation against a variety of bacterial diseases. Diseases outcomes of candidiasis 63, Uropathogenic E. coli 64, enterohaemorrhagic E. coli 65, Methicillin-resistant Staphylococcus aureus 66, and Mycobacterium tuberculosis 67 improved upon introduction of lactoferrin. Lactoferrin was also used in combination with antibiotics to improve outcomes of Helicobacter pylori disease 68. Future studies may involve investigating lactoferrin as a therapeutic against GBS infection in relevant animal models of disease.
In summary, this study revealed that lactoferrin is able to inhibit GBS growth and biofilm formation by binding to free iron ions in the environment, thus starving the pathogen of this essential metal by the process of “nutritional immunity” (Figure 5). Furthermore, lactoferrin inhibits GBS adherence to human gestational membranes by limiting the availability of iron for GBS.
Supplementary Material
Figure 3. Analysis of in vitro bacterial biofilm formation on an abiotic surface in the presence or absence of lactoferrin.
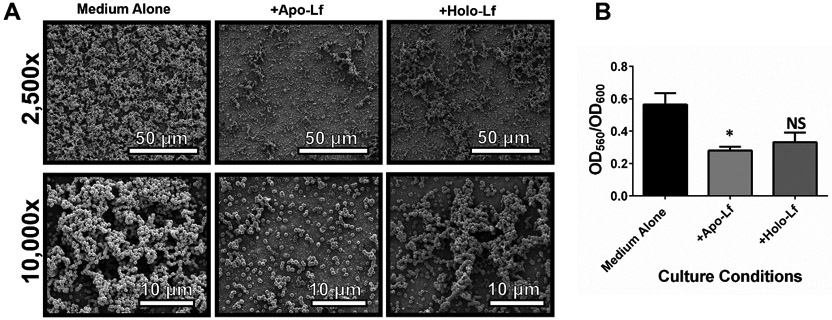
GBS was grown in medium alone or medium supplemented with 250 μg/mL of either apo-lactoferrin (apo-Lf) or holo-lactoferrin (holo-Lf). Bacterial biofilm was analyzed by A) high-resolution scanning electron microscopy (SEM) at low magnification (2,500x, magnification bar indicates 50 μm) and high magnification (10,000x, magnification bar indicates 10 μm) and by B) quantitative analysis of crystal violet staining at an optical density of 560 nm (OD560) normalized to bacterial cell density (OD600). Bars indicate mean values of at least three biological replicates +/−SEM. *P<0.05, Student’s t test comparison to bacteria grown in medium alone. Apo-lactoferrin significantly inhibits GBS biofilm formation, while holo-lactoferrin has an intermediate phenotype that is statistically indistinguishable from the negative control.
Acknowledgments
This work has been funded by the National Institutes of Health grant R01 HD090061 (to J.A.G.) and by NIH T32 HL007411-36S1 (supporting J.L.) 2T32AI112541-06 (supporting J.F.), K08AAI151100 (supporting R.S.D.), R35GM133602 (to S.D.T.), and F32HD100087 (to A.J.E.). Additional funding from the National Science Foundation Award Numbers 1547757 and 1400969, and NIH grant GM05551 (to S.M.D.) supported this work. Additional support was provided by NIH U01TR002398, NIH R01AI134036, and the March of Dimes (to D.M.A.), and from the Vanderbilt Institute for Clinical and Translational Research program supported by the National Center for Research Resources, Grant UL1 RR024975-01, and the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06.
References
- (1).Koumans EHA; Rosen J; Van Dyke MK; Zell E; Phares CR; Taylor A; Loft J; Schrag S Prevention of Mother-to-Child Transmission of Infections during Pregnancy: Implementation of Recommended Interventions, United States, 2003-2004. Am. J. Obstet. Gynecol 2012, 206 (2), 158.e1–158. 10.1016/j.ajog.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Goldenberg RL; Culhane JF; Iams JD; Romero R Epidemiology and Causes of Preterm Birth. Lancet 2008, 371 (9606), 75–84. 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Verani JR; McGee L; Schrag SJ Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines from CDC, 2010. Morb. Mortal. Wkly. Rep 2010, 59 (RR-10), 1–36. [PubMed] [Google Scholar]
- (4).Russell NJ; Seale AC; O’Driscoll M; O’Sullivan C; Bianchi-Jassir F; Gonzalez-Guarin J; Lawn JE; Baker CJ; Bartlett L; Cutland C; et al. Maternal Colonization with Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-Analyses. Clin. Infect. Dis 2017, 65 (suppl_2), S100–S111. 10.1093/cid/cix658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kwatra G; Adrian PV; Shiri T; Buchmann EJ; Cutland CL; Madhi SA Serotype-Specific Acquisition and Loss of Group B Streptococcus Recto-Vaginal Colonization in Late Pregnancy. PLoS One 2014, 9 (6), e98778. 10.1371/journal.pone.0098778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Matteson KA; Lievense SP; Catanzaro B; Phipps MG Intrapartum Group b Streptococci Prophylaxis in Patients Reporting a Penicillin Allergy. Obstet. Gynecol 2008. 10.1097/AOG.0b013e318160ff9d. [DOI] [PubMed] [Google Scholar]
- (7).Nanduri SA; Petit S; Smelser C; Apostol M; Alden NB; Harrison LH; Lynfield R; Vagnone PS; Burzlaff K; Spina NL; et al. Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance. JAMA Pediatrics. 2019. 10.1001/jamapediatrics.2018.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Le Doare K; O’Driscoll M; Turner K; Seedat F; Russell NJ; Seale AC; Heath PT; Lawn JE; Baker CJ; Bartlett L; et al. Intrapartum Antibiotic Chemoprophylaxis Policies for the Prevention of Group B Streptococcal Disease Worldwide: Systematic Review. Clinical Infectious Diseases. 2017. 10.1093/cid/cix654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Morrow AL; Ruiz-Palacios GM; Altaye M; Jiang X; Lourdes Guerrero M; Meinzen-Derr JK; Farkas T; Chaturvedi P; Pickering LK; Newburg DS Human Milk Oligosaccharides Are Associated with Protection against Diarrhea in Breast-Fed Infants. J. Pediatr 2004. 10.1016/j.jpeds.2004.04.054. [DOI] [PubMed] [Google Scholar]
- (10).Coppa GV; Gabrielli O; Giorgi P; Catassi C; Montanari MP; Varaldo PE; Nichols BL Preliminary Study of Breastfeeding and Bacterial Adhesion to Uroepithelial Cells. Lancet 1990. 10.1016/0140-6736(90)90350-E. [DOI] [PubMed] [Google Scholar]
- (11).Mårild S; Hansson S; Jodal U; Odén A; Svedberg K Protective Effect of Breastfeeding against Urinary Tract Infection. Acta Paediatr. Int. J. Paediatr 2004. 10.1080/08035250310007402. [DOI] [PubMed] [Google Scholar]
- (12).Oddy WH The Impact of Breastmilk on Infant and Child Health. Breastfeeding review : professional publication of the Nursing Mothers’ Association of Australia. 2002. [PubMed] [Google Scholar]
- (13).Rogier EW; Frantz AL; Bruno MEC; Wedlund L; Cohen DA; Stromberg AJ; Kaetzel CS Secretory Antibodies in Breast Milk Promote Long-Term Intestinal Homeostasis by Regulating the Gut Microbiota and Host Gene Expression. Proc. Natl. Acad. Sci. U. S. A 2014. 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Trend S; Strunk T; Lloyd ML; Kok CH; Metcalfe J; Geddes DT; Lai CT; Richmond P; Doherty DA; Simmer K; et al. Levels of Innate Immune Factors in Preterm and Term Mothers’ Breast Milk during the 1st Month Postpartum. Br. J. Nutr 2016. 10.1017/S0007114516000234. [DOI] [PubMed] [Google Scholar]
- (15).Newburg DS; Ruiz-Palacios GM; Altaye M; Chaturvedi P; Meinzen-Derr J; de Lourdes Guerrero M; Morrow AL Innate Protection Conferred by Fucosylated Oligosaccharides of Human Milk against Diarrhea in Breastfed Infants. Glycobiology 2004. 10.1093/glycob/cwh020. [DOI] [PubMed] [Google Scholar]
- (16).Pärnänen K; Karkman A; Hultman J; Lyra C; Bengtsson-Palme J; Larsson DGJ; Rautava S; Isolauri E; Salminen S; Kumar H; et al. Maternal Gut and Breast Milk Microbiota Affect Infant Gut Antibiotic Resistome and Mobile Genetic Elements. Nat. Commun 2018. 10.1038/s41467-018-06393-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Ackerman DL; Craft KM; Doster RS; Weitkamp JH; Aronoff DM; Gaddy JA; Townsend SD Antimicrobial and Antibiofilm Activity of Human Milk Oligosaccharides against Streptococcus Agalactiae, Staphylococcus Aureus, and Acinetobacter Baumannii. ACS Infect. Dis 2018. 10.1021/acsinfecdis.7b00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Craft KM; Gaddy JA; Townsend SD Human Milk Oligosaccharides (HMOs) Sensitize Group B Streptococcus to Clindamycin, Erythromycin, Gentamicin, and Minocycline on a Strain Specific Basis. ACS Chem. Biol 2018. 10.1021/acschembio.8b00661. [DOI] [PubMed] [Google Scholar]
- (19).Chambers SA; Moore RE; Craft KM; Thomas HC; Das R; Manning SD; Codreanu SG; Sherrod SD; Aronoff DM; McLean JA; et al. A Solution to Antifolate Resistance in Group B Streptococcus: Untargeted Metabolomics Identifies Human Milk Oligosaccharide-Induced Perturbations That Result in Potentiation of Trimethoprim. MBio 2020. 10.1128/mBio.00076-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Manzoni P; Dall’Agnola A; Tomé D; Kaufman DA; Tavella E; Pieretto M; Messina A; De Luca D; Bellaiche M; Mosca A; et al. Role of Lactoferrin in Neonates and Infants: An Update. American Journal of Perinatology. 2018. 10.1055/s-0038-1639359. [DOI] [PubMed] [Google Scholar]
- (21).Ronayne de Ferrer PA; Baroni A; Sambucetti ME; López NE; Ceriani Cernadas JM Lactoferrin Levels in Term and Preterm Milk. J. Am. Coll. Nutr 2000. 10.1080/07315724.2000.10718933. [DOI] [PubMed] [Google Scholar]
- (22).Rai D; Adelman AS; Zhuang W; Rai GP; Boettcher J; Lönnerdal B Longitudinal Changes in Lactoferrin Concentrations in Human Milk: A Global Systematic Review. Crit. Rev. Food Sci. Nutr 2014. 10.1080/10408398.2011.642422. [DOI] [PubMed] [Google Scholar]
- (23).Weinberg ED Nutritional Immunity. Host’s Attempt to Withold Iron from Microbial Invaders. JAMA 1975, 231 (1), 39–41. [DOI] [PubMed] [Google Scholar]
- (24).Becker KW; Skaar EP Metal Limitation and Toxicity at the Interface between Host and Pathogen. FEMS Microbiol. Rev 2014, 38 (6), 1235–1249. 10.1111/1574-6976.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Andreini C; Bertini I; Cavallaro G; Holliday GL; Thornton JM Metal Ions in Biological Catalysis: From Enzyme Databases to General Principles. J. Biol. Inorg. Chem 2008. 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- (26).Legrand D Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr 2016, 173 Suppl, S10–5. 10.1016/j.jpeds.2016.02.071. [DOI] [PubMed] [Google Scholar]
- (27).Pammi M; Suresh G Enteral Lactoferrin Supplementation for Prevention of Sepsis and Necrotizing Enterocolitis in Preterm Infants. Cochrane Database of Systematic Reviews. 2017. 10.1002/14651858.CD007137.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lu J; Francis J; Doster RS; Haley KP; Craft KM; Moore RE; Chambers SA; Aronoff DM; Osteen K; Damo SM; et al. Lactoferrin: A Critical Mediator of Both Host Immune Response and Antimicrobial Activity in Response to Streptococcal Infections. ACS Infect. Dis 2020. 10.1021/acsinfecdis.0c00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Allison LM; Walker LA; Sanders BJ; Yang Z; Eckert G; Gregory RL Effect of Human Milk and Its Components on Streptococcus Mutans Biofilm Formation. J. Clin. Pediatr. Dent 2015, 39 (3), 255–261. 10.17796/1053-4628-39.3.255. [DOI] [PubMed] [Google Scholar]
- (30).Kostakioti M; Hadjifrangiskou M; Hultgren SJ Bacterial Biofilms: Development, Dispersal, and Therapeutic Strategies in the Dawn of the Postantibiotic Era. Cold Spring Harb. Perspect. Med 2013. 10.1101/cshperspect.a010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Rosini R; Margarit I Biofilm Formation by Streptococcus Agalactiae: Influence of Environmental Conditions and Implicated Virulence Factor. Frontiers in Cellular and Infection Microbiology. 2015. 10.3389/fcimb.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Korir ML; Flaherty RA; Rogers LM; Gaddy JA; Aronoff DM; Manning SD Investigation of the Role That NADH Peroxidase Plays in Oxidative Stress Survival in Group B Streptococcus. Front. Microbiol 2018. 10.3389/fmicb.2018.02786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Senkovich O; Ceaser S; McGee DJ; Testerman TL Unique Host Iron Utilization Mechanisms of Helicobacter Pylori Revealed with Iron-Deficient Chemically Defined Media. Infect. Immun 2010. 10.1128/IAI.01258-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kothary V; Doster RS; Rogers LM; Kirk LA; Boyd KL; Romano-Keeler J; Haley KP; Manning SD; Aronoff DM; Gaddy JA Group B Streptococcus Induces Neutrophil Recruitment to Gestational Tissues and Elaboration of Extracellular Traps and Nutritional Immunity. Front. Cell. Infect. Microbiol 2017, 7, 19. 10.3389/fcimb.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Haley KP; Delgado AG; Piazuelo MB; Mortensen BL; Correa P; Damo SM; Chazin WJ; Skaar EP; Gaddy JA The Human Antimicrobial Protein Calgranulin C Participates in Control of Helicobacter Pylori Growth and Regulation of Virulence. Infect. Immun 2015. 10.1128/IAI.00544-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Gaddy JA; Tomaras AP; Actis LA The Acinetobacter Baumannii 19606 OmpA Protein Plays a Role in Biofilm Formation on Abiotic Surfaces and in the Interaction of This Pathogen with Eukaryotic Cells. Infect. Immun 2009. 10.1128/IAI.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wang XY; Guo HY; Zhang W; Wen PC; Zhang H; Guo ZR; Ren FZ Effect of Iron Saturation Level of Lactoferrin on Osteogenic Activity in Vitro and in Vivo. J. Dairy Sci 2013. 10.3168/jds.2012-5692. [DOI] [PubMed] [Google Scholar]
- (38).Conesa C; Sánchez L; Rota C; Pérez MD; Calvo M; Farnaud S; Evans RW Isolation of Lactoferrin from Milk of Different Species: Calorimetric and Antimicrobial Studies. Comp. Biochem. Physiol. - B Biochem. Mol. Biol 2008. 10.1016/j.cbpb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- (39).Kaur G; Gathwala G Efficacy of Bovine Lactoferrin Supplementation in Preventing Late-Onset Sepsis in Low Birth Weight Neonates: A Randomized Placebo-Controlled Clinical Trial. J. Trop. Pediatr 2015. 10.1093/tropej/fmv044. [DOI] [PubMed] [Google Scholar]
- (40).Ochoa TJ; Chea-Woo E; Baiocchi N; Pecho I; Campos M; Prada A; Valdiviezo G; Lluque A; Lai D; Cleary TG Randomized Double-Blind Controlled Trial of Bovine Lactoferrin for Prevention of Diarrhea in Children. J. Pediatr 2013. 10.1016/j.jpeds.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Ochoa TJ; Zegarra J; Bellomo S; Carcamo CP; Cam L; Castañeda A; Villavicencio A; Gonzales J; Rueda MS; Turin CG; et al. Randomized Controlled Trial of Bovine Lactoferrin for Prevention of Sepsis and Neurodevelopment Impairment in Infants Weighing Less Than 2000 Grams. In Journal of Pediatrics; 2020. 10.1016/j.jpeds.2019.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Tarnow-Mordi WO; Abdel-Latif ME; Martin A; Pammi M; Robledo K; Manzoni P; Osborn D; Lui K; Keech A; Hague W; et al. The Effect of Lactoferrin Supplementation on Death or Major Morbidity in Very Low Birthweight Infants (LIFT): A Multicentre, Double-Blind, Randomised Controlled Trial. Lancet Child Adolesc. Heal 2020. 10.1016/S2352-4642(20)30093-6. [DOI] [PubMed] [Google Scholar]
- (43).Cheng WD; Wold KJ; Bollinger LB; Ordiz MI; Shulman RJ; Maleta KM; Manary MJ; Trehan I Supplementation with Lactoferrin and Lysozyme Ameliorates Environmental Enteric Dysfunction: A Double-Blind, Randomized, Placebo-Controlled Trial. Am. J. Gastroenterol 2019. 10.14309/ajg.0000000000000170. [DOI] [PubMed] [Google Scholar]
- (44).Meyer MP; Alexander T Reduction in Necrotizing Enterocolitis and Improved Outcomes in Preterm Infants Following Routine Supplementation with Lactobacillus GG in Combination with Bovine Lactoferrin. J. Neonatal. Perinatal. Med 2017. 10.3233/NPM-16130. [DOI] [PubMed] [Google Scholar]
- (45).Miranda M; Saccone G; Ammendola A; Salzano E; Iannicelli M; De Rosa R; Nazzaro G; Locci M Vaginal Lactoferrin in Prevention of Preterm Birth in Women with Bacterial Vaginosis. J. Matern. Neonatal Med 2019, 1–5. 10.1080/14767058.2019.1690445. [DOI] [PubMed] [Google Scholar]
- (46).Woodman T; Strunk T; Patole S; Hartmann B; Simmer K; Currie A Effects of Lactoferrin on Neonatal Pathogens and Bifidobacterium Breve in Human Breast Milk. PLoS One 2018. 10.1371/journal.pone.0201819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Flores-Villaseñor H; Canizalez-Román A; Reyes-Lopez M; Nazmi K; De La Garza M; Zazueta-Beltrán J; León-Sicairos N; Bolscher JGM Bactericidal Effect of Bovine Lactoferrin, LFcin, LFampin and LFchimera on Antibiotic-Resistant Staphylococcus Aureus and Escherichia Coli. In BioMetals; 2010. 10.1007/s10534-010-9306-4. [DOI] [PubMed] [Google Scholar]
- (48).Leon-Sicairos N; Canizalez-Roman A; de la Garza M; Reyes-Lopez M; Zazueta-Beltran J; Nazmi K; Gomez-Gil B; Bolscher JG Bactericidal Effect of Lactoferrin and Lactoferrin Chimera against Halophilic Vibrio Parahaemolyticus. Biochimie 2009. 10.1016/j.biochi.2008.06.009. [DOI] [PubMed] [Google Scholar]
- (49).Acosta-Smith E; Viveros-Jiménez K; Canizalez-Román A; Reyes-Lopez M; Bolscher JGM; Nazmi K; Flores-Villaseñor H; Alapizco-Castro G; de la Garza M; Martínez-Garcia JJ; et al. Bovine Lactoferrin and Lactoferrin-Derived Peptides Inhibit the Growth of Vibrio Cholerae and Other Vibrio Species. Front. Microbiol 2018. 10.3389/fmicb.2017.02633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Bessler HC; De Oliveira IR; Giugliano LG Human Milk Glycoproteins Inhibit the Adherence of Salmonella Typhimurium to HeLa Cells. Microbiol. Immunol 2006. 10.1111/j.1348-0421.2006.tb03863.x. [DOI] [PubMed] [Google Scholar]
- (51).Hood MI; Skaar EP Nutritional Immunity: Transition Metals at the Pathogen-Host Interface. Nature Reviews Microbiology. 2012. 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Xu G; Xiong W; Hu Q; Zuo P; Shao B; Lan F; Lu X; Xu Y; Xiong S Lactoferrin-Derived Peptides and Lactoferricin Chimera Inhibit Virulence Factor Production and Biofilm Formation in Pseudomonas Aeruginosa. J. Appl. Microbiol 2010. 10.1111/j.1365-2672.2010.04751.x. [DOI] [PubMed] [Google Scholar]
- (53).O’May CY; Sanderson K; Roddam LF; Kirov SM; Reid DW Iron-Binding Compounds Impair Pseudomonas Aeruginosa Biofilm Formation, Especially under Anaerobic Conditions. J. Med. Microbiol 2009. 10.1099/jmm.0.004416-0. [DOI] [PubMed] [Google Scholar]
- (54).Kamiya H; Ehara T; Matsumoto T Inhibitory Effects of Lactoferrin on Biofilm Formation in Clinical Isolates of Pseudomonas Aeruginosa. J. Infect. Chemother 2012. 10.1007/s10156-011-0287-1. [DOI] [PubMed] [Google Scholar]
- (55).Nagano-Takebe F; Miyakawa H; Nakazawa F; Endo K Inhibition of Initial Bacterial Adhesion on Titanium Surfaces by Lactoferrin Coating. Biointerphases 2014. 10.1116/1.4867415. [DOI] [PubMed] [Google Scholar]
- (56).Godoy-Gallardo M; Mas-Moruno C; Fernández-Calderón MC; Pérez-Giraldo C; Manero JM; Albericio F; Gil FJ; Rodríguez D Covalent Immobilization of HLf1-11 Peptide on a Titanium Surface Reduces Bacterial Adhesion and Biofilm Formation. Acta Biomater. 2014. 10.1016/j.actbio.2014.03.026. [DOI] [PubMed] [Google Scholar]
- (57).Danielsson Niemi L; Hernell O; Johansson I Human Milk Compounds Inhibiting Adhesion of Mutans Streptococci to Host Ligand-Coated Hydroxyapatite in Vitro. Caries Res. 2009. 10.1159/000213888. [DOI] [PubMed] [Google Scholar]
- (58).Angulo-Zamudio UA; Vidal JE; Nazmi K; Bolscher JGM; Leon-Sicairos C; Antezana BS; Canizalez-Roman A; León-Sicairos N Lactoferrin Disaggregates Pneumococcal Biofilms and Inhibits Acquisition of Resistance Through Its DNase Activity. Front. Microbiol 2019. 10.3389/fmicb.2019.02386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Trappetti C; Potter AJ; Paton AW; Oggioni MR; Paton JC LuxS Mediates Iron-Dependent Biofilm Formation, Competence, and Fratricide in Streptococcus Pneumoniae. Infect. Immun 2011. 10.1128/IAI.05644-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Doster RS; Kirk LA; Tetz LM; Rogers LM; Aronoff DM; Gaddy JA Staphylococcus Aureus Infection of Human Gestational Membranes Induces Bacterial Biofilm Formation and Host Production of Cytokines. J. Infect. Dis 2017. 10.1093/infdis/jiw300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Velusamy SK; Poojary R; Ardeshna R; Alabdulmohsen W; Fine DH; Velliyagounder K Protective Effects of Human Lactoferrin during Aggregatibacter Actinomycetemcomitans-Induced Bacteremia in Lactoferrin-Deficient Mice. Antimicrob. Agents Chemother 2014. 10.1128/AAC.00020-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Velusamy SK; Fine DH; Velliyagounder K Prophylactic Effect of Human Lactoferrin against Streptococcus Mutans Bacteremia in Lactoferrin Knockout Mice. Microbes Infect. 2014. 10.1016/j.micinf.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Velliyagounder K; Rozario SD; Fine DH The Effects of Human Lactoferrin in Experimentally Induced Systemic Candidiasis. J. Med. Microbiol 2019. 10.1099/jmm.0.001098. [DOI] [PubMed] [Google Scholar]
- (64).Patras KA; Ha AD; Rooholfada E; Olson J; Ramachandra Rao SP; Lin AE; Nizet V Augmentation of Urinary Lactoferrin Enhances Host Innate Immune Clearance of Uropathogenic Escherichia Coli. J. Innate Immun 2019. 10.1159/000499342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Flores-Villaseñor H; Canizalez-Román A; Velazquez-Roman J; Nazmi K; Bolscher JGM; Leon-Sicairos N Protective Effects of Lactoferrin Chimera and Bovine Lactoferrin in a Mouse Model of Enterohaemorrhagic Escherichia Coli O157:H7 Infection. Biochem. Cell Biol 2012. 10.1139/o11-089. [DOI] [PubMed] [Google Scholar]
- (66).Hwang SA; Kruzel ML; Actor JK Immunomodulatory Effects of Recombinant Lactoferrin during MRSA Infection. Int. Immunopharmacol 2014. 10.1016/j.intimp.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Hwang SA; Kruzel ML; Actor JK Oral Recombinant Human or Mouse Lactoferrin Reduces Mycobacterium Tuberculosis TDM Induced Granulomatous Lung Pathology1. Biochem. Cell Biol 2017. 10.1139/bcb-2016-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Yuan Y; Wu Q; Cheng G; Liu X; Liu S; Luo J; Zhang A; Bian L; Chen J; Lv J; et al. Recombinant Human Lactoferrin Enhances the Efficacy of Triple Therapy in Mice Infected with Helicobacter Pylori. Int. J. Mol. Med 2015. 10.3892/ijmm.2015.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.