Abstract
Lyme disease (LD), the most common tick-borne disease of canines and humans in N. America, is caused by the spirochete Borreliella burgdorferi. Subunit and bacterin vaccines are available for the prevention of LD in dogs. LD bacterin vaccines, which are comprised of cell lysates of two strains of B. burgdorferi, contain over 1000 different proteins and cellular constituents. In contrast, subunit vaccines are defined in composition and consist of either outer surface protein (Osp)A or OspA and an OspC chimeritope. In this study, we comparatively assessed antibody responses to OspA and OspC induced by vaccination with all canine bacterin and subunit LD vaccines that are commercially available in North America.
Dogs were administered a two-dose series of the vaccine to which they were assigned (3 weeks apart): Subunit-AC, Subunit-A, Bacterin-1, and Bacterin-2. Antibody titers to OspA and OspC were determined by ELISA and the ability of each vaccine to elicit antibodies that recognize diverse OspC proteins (referred to as OspC types) assessed by immunoblot. While all of the vaccines elicited similar OspA antibody responses, only Subunit-AC triggered a robust and broadly cross-reactive antibody response to divergent OspC proteins. The data presented within provide new information regarding vaccination-induced antibody responses to key tick and mammalian phase antigens by both subunit and bacterin LD canine vaccine formulations.
Keywords: Borrelia, Borreliella, Chimeritope, Ixodes scapularis, Tick-borne diseases
Introduction
Lyme disease (LD) is a significant health concern that affects companion animals (Littman et al., 2018) and humans (Burgdorfer et al., 1982; Benach et al., 1983). In North America, Borreliella burgdorferi is the primary causative agent of LD; in Europe, B. burgdorferi, Borreliella bavariensis, Borreliella garinii, and Borreliella afzelii are associated with disease (Adeolu and Gupta, 2014; Eisen, 2020). The LD spirochetes are transmitted amongst animals by Ixodes spp. ticks (Steere et al., 1978; Burgdorfer et al., 1982). LD is the most prevalent tick-borne disease in North America and Europe. It has been reported by the CDC that there are approximately 476,000 clinician-diagnosed cases of LD each year in humans (Kugeler et al., 2021). In dogs, 398,392 positive B. burgdorferi antibody (Ab) tests were catalogued by the Companion Animal Parasite Council (CAPC) in 2020 in the US.1 The actual number of B. burgdorferi-positive antibody tests is assumed to be much higher since only 30% of test data are collected by CAPC each year. The precise number of antibody-positive tests in European dogs is more difficult to determine due to differences in data collection. While a positive antibody test does not in all cases indicate active infection, it is clear from recent studies that geographic distribution of ixodid ticks is expanding (Eisen et al., 2016) and the risk of LD is increasing across the northern hemisphere (Sykes and Makiello, 2017; Vandekerckhove et al., 2019; Kugeler et al., 2021).
Clinical manifestations of canine LD are initially non-descript and, in most cases, develop slowly (Krupka and Straubinger, 2010; Little et al., 2010). Intermittent lameness due to polyarthritis is common (Levy and Magnarelli, 1992) and chronic infection can result in cardiac conduction disorders (Levy and Duray, 1988), neurological complications (Lesca et al., 2002), and protein-losing glomerulopathy leading to renal failure (Dambach et al., 1997). In experimentally-infected dogs, histological changes have been demonstrated even in dogs with sub-clinical LD. Inflammation of the tissues and joint capsules in B. burgdorferi infected dogs is common (Straubinger et al., 1998). Hyperkeratosis, lymphoplas-macytic vasculitis, arteritis, perineuritis, and meningitis may also develop.
Preventative strategies for LD in dogs include vaccination and the use of tick repellants and acaricides (Littman et al., 2018). In North America, subunit and bacterin LD vaccines are available (reviewed in Izac and Marconi, 2019). Subunit vaccines are defined in their composition and consist of recombinant lipidated outer surface protein A (OspA) or recombinant non-lipidated OspA in combination with a multivalent outer surface protein C (OspC) epitope-based recombinant protein (reviewed in O’Bier et al., 2020) referred to as a chimeritope (Izac et al., 2020b). In contrast to the defined antigenic composition of subunit vaccines, LD bacterin vaccines consist of more than 1000 different proteins, the overwhelming majority of which have not been demonstrated to elicit protective antibody (reviewed in O’Bier et al., 2020). All LD bacterin vaccines available in North America are a mix of cell lysates of two laboratory strains of B. burgdorferi (discussed in detail below).
Since the discovery of the causative agents of LD (Benach et al., 1983), OspA and OspC have been among the most intensively studied outer surface proteins produced by these pathogens. OspA and OspC are produced during distinctly different stages of the enzootic cycle (Schwan and Piesman, 2000; Schwan, 2003). OspC production is initiated within the tick midgut upon exposure to a bloodmeal and remains highly expressed during early stage infection in mammals (Caimano et al., 2019). In mammals OspC is one of the most abundant LD spirochete surface antigens (Iyer et al., 2015; Caimano et al., 2019) and it is required for the LD spirochetes to establish infection (Tilly et al., 2007; Earnhart et al., 2010). OspA is produced at high levels by spirochetes residing within the midguts of unfed ticks but it is not produced after the LD spirochetes enter into a mammal (Iyer et al., 2015; Caimano et al., 2019). While OspA plays an essential role in spirochete survival in unfed ticks, gene deletion studies have demonstrated that it is not required for survival in mammals (Pal et al., 2000). Consistent with the stages of infection during which each outer surface protein is expressed, anti-OspC antibodies can target LD spirochetes infecting both ticks and mammals, whilst anti-OspA antibodies only target LD spirochetes in the tick. The combined use of OspA and OspC as vaccine antigens (Marconi et al., 2020) elicits antibody responses that can target LD spirochetes during both stage of their enzootic cycle providing two independent and synergistic mechanisms of protection.
While the specific antigens in bacterin vaccines that contribute to protective immunity have not been defined, it has been suggested that OspA and OspC are key contributors (LaFleur et al., 2009). Due to the high level of OspA expression by laboratory-cultured LD spirochetes (Oliver Jr et al., 2016), it is likely that the OspA present in bacterin formulations elicits antibody that specifically targets spirochetes in ticks (Fikrig et al., 1992). In contrast to OspA, the low-level expression of OspC in laboratory cultured strains suggests that, in the context of a bacterin formulation, its contribution to inducing protective immunity is minimal (O’Bier et al., 2020). In addition, the expression of OspC during cultivation is limited to a subset of cells in the population (Oliver Jr et al., 2016; Xiang et al., 2017). OspC is genetically and antigenically diverse among LD isolates (Lagal et al., 2002; Earnhart and Marconi, 2007c). Distinct variants of OspC have been delineated and are referred to as OspC “types” with each assigned a letter or isolate of origin designation (OspC type A, OspC type B, OspC type PHoe, etc.) (Lagal et al., 2003; Brisson and Dykhuizen, 2004; Earnhart and Marconi, 2007c). It has been demonstrated in mice, rats, rabbits, canids (domestic and wild), horses, humans, and non-human primates that antibody responses to OspC during infection are OspC type-specific (Earnhart et al., 2005; Buckles et al., 2006; Izac et al., 2019, 2020a; Oliver Jr et al., 2016). Vaccination with a single OspC protein does not elicit production of antibodies that recognize diverse OspC types (Oliver Jr et al., 2016; Izac and Marconi, 2019), providing protection only against strains expressing closely related OspC types (Bockenstedt et al., 1997). OspC is a single copy, plasmid-encoded gene; therefore, an individual LD strain produces only a single OspC type (Marconi et al., 1993; Sadziene et al., 1993). The low-level production of OspC during cultivation and the type-specific antibody responses that it elicits raise questions regarding its relative contribution to immune responses elicited by LD bacterin vaccines.
In this study, we compared antibody responses to OspA and OspC in dogs vaccinated with the subunit and bacterin-based canine LD vaccines available in North America. In addition to antigen specific IgG titer determination, we assessed the potential of each vaccine to induce antibodies that recognize diverse OspC types and therefore potentially target diverse strains of the LD spirochetes. We demonstrate that there are both quantitative and qualitative differences in the antibody responses to OspC elicited by each vaccine. This study addresses key questions surrounding the potential contributions of OspA and OspC to protective immunity induced by administration of canine LD vaccines.
Materials and methods
Study inclusion/exclusion criteria
Purpose-bred beagles (21 males and 19 females; 8–9 weeks of age) were obtained from Ridglan Farms and acclimated for 7 days prior to initiating the study. Animals were sorted by date of birth and litter (dam) to form blocks of four dogs. Half of the blocks were randomly assigned to each of two rooms. Within rooms, blocks were randomly assigned to pens. The randomization was performed using a SAS program that utilizes a random number generator function (ranuni). Animals were observed at least once daily for general health and potential adverse health events. Dogs were maintained at research sites in accordance with USDA Animal Welfare Regulations (Code of Federal Regulations, Chapter 1, Subchapter A – Animal Welfare). The Zoetis Institutional Animal Care and Use Committee (IACUC) approved all protocols (Approval number, AUP # KZ-3081d-2015-06-mtw; Approval date, June 2015). Inclusion criteria consisted of negative antibody tests for OspA, OspC, and VlsE (C6 peptide) and good overall health. Screening for antibodies to OspA and OspC was done by ELISA as detailed below. To screen for antibodies to VlsE the SNAP 4Dx Plus lateral flow test was employed (IDEXX). Vaccines Subunit-AC (VANGUARD crLyme, Zoetis), Bacterin-1 (NOBIVAC Lyme, Merck), Subunit-A (RECOMBITEK Lyme, Boehringer-Ingelheim), and Bacterin-2 (Duramune Lyme, Elanco US) were randomly assigned to each treatment group and administered per the manufacturers’ label instructions on days 0 and 21. Blood was collected and serum harvested on Days 0, 21, and 35 using standard protocols.
Ligase independent cloning (LIC) and production of recombinant proteins
The genes encoding 23 full-length OspC proteins (indicated in Fig. 2) were PCR amplified from strains of known ospC genotype with PCR primers that possess tail sequences that allow for ligase independent cloning (LIC). PCR was performed using Pfu DNA polymerase according to the manufacturers protocol (Promega). The amplicons were purified using QIAquick PCR purification kits (QIAgen), annealed with pET46 Ek/LIC or pET45b+ (Novagen), transformed into Escherichia coli NovaBlue(DE3) cells (Novagen), recovered, purified, and transformed into E. coli BL21(DE3) cells (Novagen). Protein expression was induced with IPTG (1 mM) using standard methods. The cells were recovered by centrifugation (5000 × g; 15 min; 4 °C), suspended in lysis buffer (50 mM NaH2PO4; 300 mM NaCl; 40 mM imidazole; lysozyme, 1 mg/mL; 30 min), sonicated, and centrifuged (15,500 × g; 30 min; 4 °C). The N-terminal hexahistidine tagged OspC proteins were purified from the soluble fraction by nickel affinity chromatography using a Fast Protein Liquid Chromatography system (ÄKTA; Cytiva) with a 1 mL HisTrap FF column (Cytvia). Samples were loaded into a 10 ml Superloop (Cytvia) in running buffer (50 mM NaH2PO4; 300 mM NaCl; 40 mM imidazole) followed by washing with 10 mL of running buffer. Proteins were eluted with elution buffer (50 mM NaH2PO4; 300 mM NaCl; 500 mM imidazole). One mL fractions were collected from under the peak and dialyzed into phosphate buffered saline (PBS) overnight using Spectra/Por 1 (6–8 kDa cutoff) dialysis membranes (Spectrum Laboratories). Purified OspA and the OspC chimeritope, Ch14, that are the antigens contained in the Subunit-AC vaccine, were provided by Zoetis. The concentrations of the recombinant proteins were determined using the BCA assay.
Fig. 2.
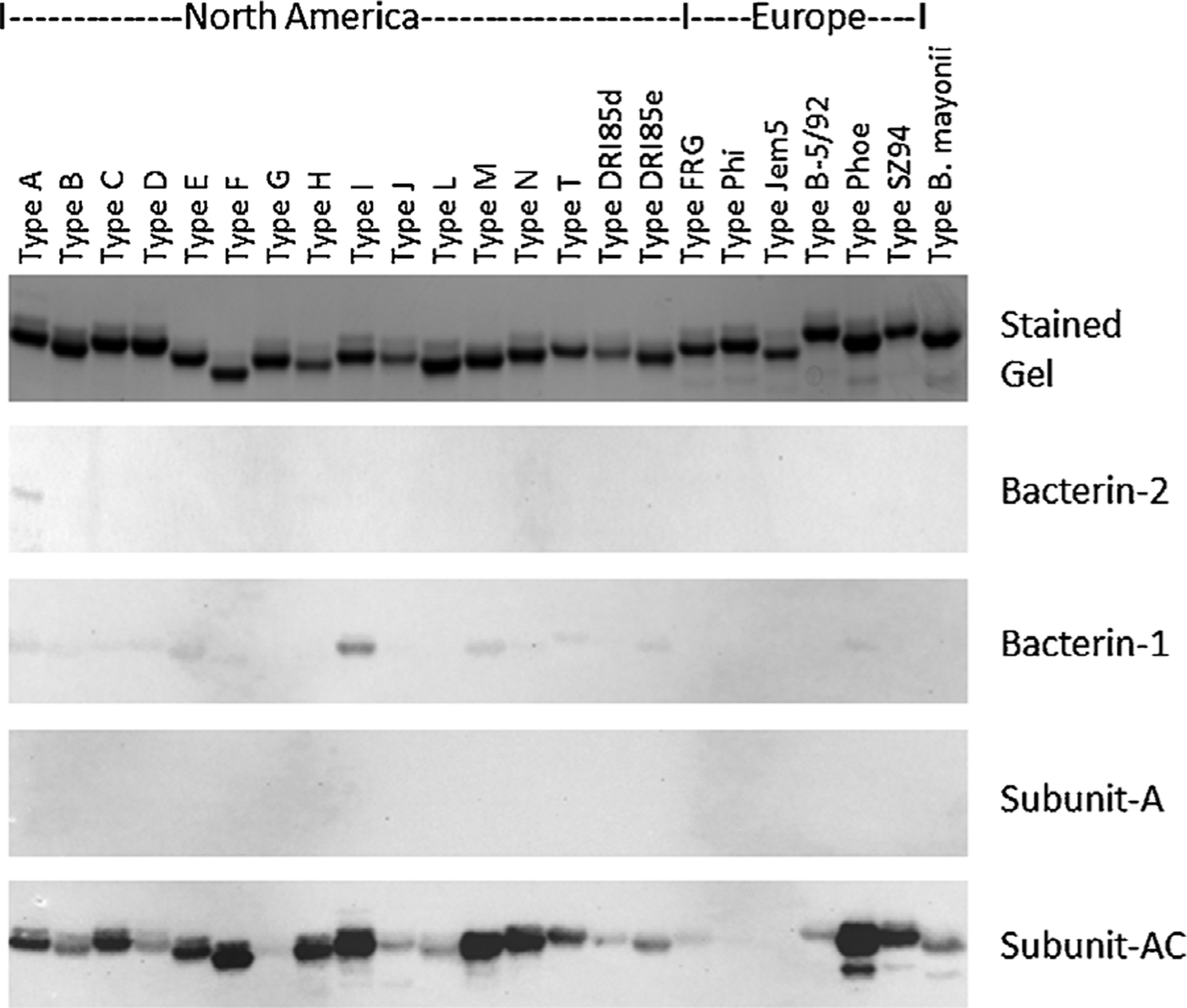
Comparative immunoblot analysis of outer surface protein (Osp) C antibody specificity. Twenty-three different OspC type proteins derived from North American or European Lyme disease isolates (as indicated) were generated as His-tagged recombinant proteins and purified using Fast Protein Liquid Chromatography. The recombinant proteins were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis. One gel was stained to visualize the proteins and others were immunoblotted. The blots were screened with pooled sera collected from three dogs within each vaccine group 2 weeks after the second vaccine dose on Day 35. The blots were imaged together for the same amount of time (148 s). The images were cropped to generate the figure.
Anti-OspA and OspC IgG titer determination
IgG titers to OspA and OspC were determined by ELISA using recombinant proteins as the immobilized antigens (250 ng per well; 96 well plates; 0.01 M borate buffer; overnight; 4 °C). Recombinant serotype 1 OspA, the most dominant OspA serotype in North America (Wilske et al., 1993), served as the detection antigen for anti-OspA antibodies and the OspC chimeritope, Ch14 (Marconi et al., 2020), served as the detection antigen for antibodies to OspC. Non-specific antibody binding was blocked by washing with blocking buffer (1% casein in PBS with 0.1% Tween 20; 300 μL per well). Primary sera, serially diluted in blocking buffer, were added to the ELISA plate wells (100 μL per well). The assay positive control for OspA and Ch14 was initially diluted to 1:25,600 and 1:6400, respectively. Plates were read when the initial dilution of the positive control reached an optical density of 1.6–2.1 at 405/490 nm. The assay negative control preimmune sera was diluted 1:200. For endpoint titer determination the serum samples were serially diluted. The minimum start dilutions were 1:25,600 and 1:6400 for OspA and Ch14, respectively. The plates were incubated (1 h; 37 °C) then horseradish peroxidase conjugated goat anti-dog IgG (H + L Chains) (Pierce) was added (1:20,000 dilution), the plates were washed and peroxidase substrate (ABTS, Sigma-Aldrich) was added (room temperature; 10–15 min). The plates were read as above and the test sample titers calculated from the average plus three standard deviations of the optical density values of negative control preimmune sera. All assays were done in duplicate.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analyses
The recombinant OspC types (500 ng) were assessed by SDS-PAGE, visualized by staining with Coomassie brilliant blue, immunoblotted, and screened as previously described with sera collected on Day 0 and 35 at a 1:1000 dilution (Izac et al., 2019). Due to limited serum volumes, the three serum samples from each treatment group that had the highest anti-OspC IgG titers were pooled and used to screen the immunoblots of recombinant OspC types. IgG binding was detected using horseradish peroxidase-conjugated rabbit anti-dog IgG secondary antibody (1:40,000) and chemiluminescence (Clarity Western ECL; Biorad). Images were captured using the ChemiDoc imaging system (Biorad). All blots were imaged together for 148 s using the auto-optimize function. Images were cropped to remove blank spaces in order to generate a multi-panel figure.
Statistical analyses
Antibody titers were logarithmically transformed. The transformed titers were analyzed with a general linear-mixed model for repeated measures. Pairwise treatment comparisons were made at each time point. Least square means at each time point, standard errors, and 95% confidence intervals were back-transformed to obtain the geometric mean titers (GMTs), standard errors, and their confidence intervals. In addition, minimums and maximums were calculated for each treatment and time point. All analyses were performed using the SAS software suite and all hypothesis tests were carried out at the 0.05 level of significance (two-sided, P < 0.05).
Results
Analysis of vaccination-induced antibody responses to OspA
To compare OspA antibody responses elicited by each canine LD vaccine, serum harvested from vaccinated purposed bred dogs were screened by ELISA and antigen specific IgG titers were determined for each individual animal and for each treatment group. All individual dogs, regardless of the vaccine administered, developed a robust antibody response to OspA by 2 weeks post-administration of the second vaccine dose (Day 35 sera; Fig. 1; Table 1). Differences in the OspA GMTs were noted between the Bacterin-1 and Bacterin-2 study groups. All other differences were not significant (Table 3).
Fig. 1.
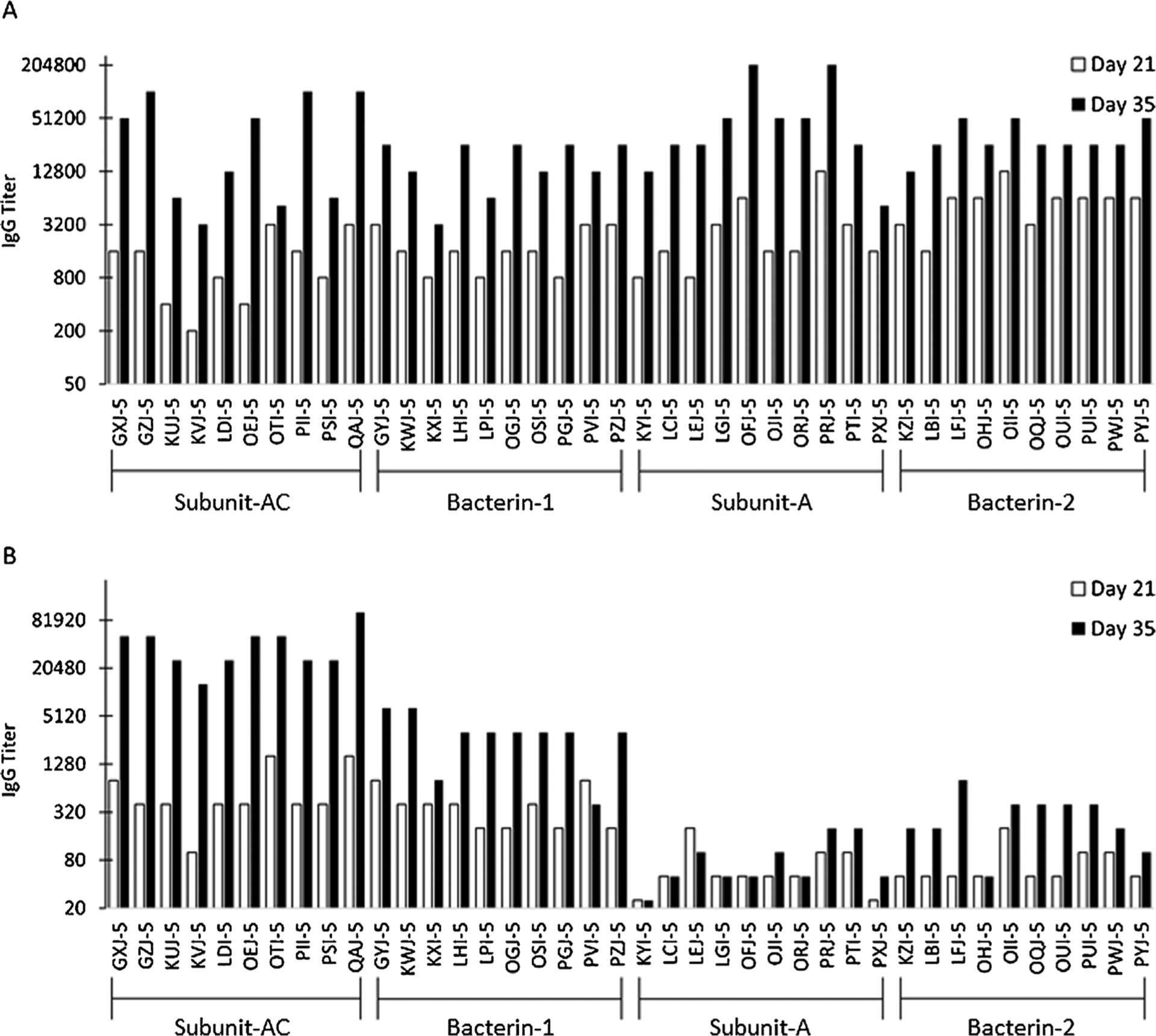
Comparative analysis of vaccination induced antibody titers to outer surface protein (Osp) A and OspC. Dogs were vaccinated with Subunit-AC, Bacterin-1, Subunit-A, and Bacterin-2. Antigen-specific IgG titers were determined in duplicate for each individual dog. Individual dog identifiers are indicated along the x axis. Panels A and B present antigen specific IgG titers for OspA (serotype 1) and OspC (Ch14), respectively.
Table 1.
Vaccination-induced anti-OspA antibody titers.
| Group: Vaccine treatment | Day | Back-transformed LSMa | Back-transformed SE | Range | Back-transformed 95% CI |
|---|---|---|---|---|---|
| Subunit-AC | 0 | 62 | 23 | 13–200 | 29–130 |
| 21 | 985 | 236 | 200–3200 | 605–1602 | |
| 35 | 27,437 | 7668 | 3200–102,400 | 15,553–48,404 | |
| Bacterin-1 | 0 | 71 | 26 | 50–200 | 34–149 |
| 21 | 1600 | 383 | 800–3200 | 983–2.603 | |
| 35 | 14,703 | 4109 | 3200–25,600 | 8334–25,939 | |
| Subunit-A | 0 | 87 | 32 | 13–400 | 41–184 |
| 21 | 22,263 | 542 | 800–12,800 | 1391–3681 | |
| 35 | 47,771 | 13,350 | 12,800–204,800 | 27,079–84,277 | |
| Bacterin-2 | 0 | 62 | 23 | 13–400 | 29–130 |
| 21 | 5198 | 1244 | 1600–12,800 | 3195–8458 | |
| 35 | 29,407 | 8218 | 12,800–51,200 | 16,669–51,879 |
Osp, outer surface protein; LSM, least squares mean; SE, standard error; 95% CI, 95% confidence intervals.
Due to the standard errors, the differences in OspA specific antibody titers between any vaccine were not significant.
Table 3.
Statistical significance of treatment pairwise comparisons of outer surface protein (Osp) A and OspC antibody titers.
| Comparison | Day | OspA antibody titer P |
OspC antibody titer P |
|---|---|---|---|
| Subunit-AC vs. Bacterin-1 | 0 | 0.7855 | 0.1327 |
| 21 | 0.1330 | 0.2814 | |
| 35 | 0.1079 | <0.0001 | |
| Subunit-AC vs. Subunit-A | 0 | 0.4975 | 0.6657 |
| 21 | 0.0130 | <0.0001 | |
| 35 | 0.1508 | <0.0001 | |
| Subunit-AC vs. Bacterin-2 | 0 | 1.0000 | 1.0000 |
| 21 | <0.0001 | <0.0001 | |
| 35 | 0.8545 | <0.0001 | |
| Bacterin-1 vs. Subunit-A | 0 | 0.6833 | 0.2814 |
| 21 | 0.2785 | <0.0001 | |
| 35 | 0.0042 | <0.0001 | |
| Bacterin-1 vs. Bacterin-2 | 0 | 0.7855 | 0.1327 |
| 21 | 0.0008 | <0.0001 | |
| 35 | 0.0758 | <0.0001 | |
| Subunit-A vs. Bacterin-2 | 0 | 0.4975 | 0.6657 |
| 21 | 0.0130 | 0.6657 | |
| 35 | 0.2065 | 0.0002 |
Analysis of vaccination-induced antibody responses to OspC
To compare OspC antibody responses elicited by each vaccine, the sera were screened by ELISA and individual titers and GMTS for each study group were determined. The antibody response to OspC differed among the study groups (Fig. 1; Table 2). Dogs vaccinated with Subunit-AC developed a high titer OspC-directed antibody response (GMT = 36,204) whereas the OspC GMTs for Bacterin-1 and Bacterin-2 were 2599 and 246, respectively. As expected, Subunit-A, which lacks OspC, did not elicit an anti-OspC antibody response. The difference in the GMT of study group Subunit-AC versus all other study groups at Day 35 was significant (P < 0.0001). Similarly, the difference in the GMT of study group Bacterin-1 versus study groups Subunit-A and Bacterin-2 was also significant (P = 0.0002). Table 3 presents significance of treatment pairwise comparisons of OspA and OspC antibody titers at each timepoint in the study.
Table 2.
Vaccine-induced anti-OspC antibody titers.
| Group: Vaccine treatment | Day | Back-trans-formed LSM | Back-transformed SE | Range | Back-transformed 95% CI |
|---|---|---|---|---|---|
| Subunit-AC | 0 | 33 | 8 | 13–200 | 21–52 |
| 21 | 493 | 111 | 100–1600 | 314–772 | |
| 35 | 36,205 | 8189 | 12,800–102,400 | 23,102–56,735 | |
| Bacterin-1 | 0 | 54 | 12 | 25–200 | 34–84 |
| 21 | 348 | 79 | 200–800 | 222–546 | |
| 35 | 2599 | 588 | 400–6400 | 1659–4073 | |
| Subunit-A | 0 | 38 | 9 | 13–200 | 24–59 |
| 21 | 57 | 13 | 25–200 | 37–90 | |
| 35 | 71 | 16 | 25–200 | 45–111 | |
| Bacterin-2 | 0 | 33 | 8 | 13–100 | 21–52 |
| 21 | 66 | 15 | 50–200 | 42–103 | |
| 35 | 246 | 56 | 50–800 | 157–386 |
Osp, outer surface protein; LSM, least squares mean; SE, standard error; 95% CI, 95% confidence intervals.
Analysis of the ability of vaccination induced anti-OspC antibody to bind to diverse OspC type proteins
To assess the breadth or conversely the specificity of the IgG response to OspC in dogs administered each vaccine, pooled sera from each treatment group were screened against 23 different recombinant OspC type proteins using an immunoblot format (Fig. 2). Consistent with the high anti-OspC IgG titers elicited by Subunit-AC, sera from this study group reacted strongly with diverse OspC proteins (Fig. 2). Sera from Bacterin-1 and Bacterin-2 study groups displayed weak binding to OspC with preferential binding to types I and A, respectively (Fig. 2).
Discussion
In this study, antibody responses to OspA and OspC in dogs administered commercially available bacterin and subunit LD vaccines were compared. After completion of the vaccine series, the anti-OspA GMTs were robust and similar for all vaccines assessed. An independent study, similarly reported that Subunit-AC and Subunit-A vaccines elicited equivalent anti-OspA antibody titers in dogs after the administration of two doses (Grosenbaugh et al., 2018). However, the anti-OspC GMTs differed significantly between vaccines. The highest OspC antibody titers were associated with Subunit-AC vaccine. The OspC antibody titers elicited by Bacterin-1 and Bacterin-2 were orders of magnitude lower (10–12x) than Subunit-AC. The low levels of OspC antibody induced by these bacterin vaccines is consistent with previous studies that have demonstrated low levels of OspC expression by B. burgdorferi during its cultivation (Oliver Jr et al., 2016; Xiang et al., 2017). One study reported that only 10% of the cells in a laboratory-cultured B. burgdorferi B31 type strain population express detectable levels of OspC (Oliver Jr et al., 2016). However, early studies detailing the development of Bacterin-1 indicate that the vaccine is derived from one B. burgdorferi strain that expresses OspA and a second strain that, because it is OspA-deficient (Rousselle et al., 1998), expresses increased levels of OspC (LaFleur et al., 2009). While the expression of OspA and OspC have been reported to be inversely regulated (Schwan, 2003), to our knowledge direct evidence for enhanced OspC expression by the OspA-deficient strain included in Bacterin-1 relative to any other strain has not been published. The OspC antibody titers induced by Bacterin-1 were 12 times lower than the titers induced by Subunit-AC, but the Bacterin-1 titers were 10 times higher than that induced by Bacterin-2. The identities of the strains used to formulate Bacterin-2 have not been reported and to our knowledge, the levels of OspA and OspC production by the strains that comprise this vaccine, have not been published.
Serum from dogs administered Subunit-AC was strongly immunoreactive with a diverse array of recombinant OspC proteins including OspC types associated with strains from both North America and Europe. In addition, the antibodies induced by Subunit-AC also bound to the OspC protein from the recently identified species Borrelia mayonii (Pritt et al., 2016a,b), suggesting a potential for cross-protection. The broad immunoreactivity of sera from dogs and other mammals vaccinated with OspC chimeritope proteins is consistent with their polyvalent epitope composition (Earnhart et al., 2007; Earnhart and Marconi, 2007a, b, c; Izac et al., 2020b). The LD bacterin vaccines assessed here elicited lower IgG titers to OspC with minimal cross reactivity with diverse OspC types. The ability of a LD vaccine to elicit robust antibody responses to both OspA and OspC is important in view of differential spirochete expression of these proteins in ticks and ticks/mammals, respectively. Since antibody targeting OspA can only bind to spirochetes within the tick mid-gut (Fikrig et al., 1992), protective efficacy of OspA alone vaccines (e.g. Subunit-A) is dependent on circulating antibody titer. While the minimal protective OspA antibody titer has not been determined in dogs, in humans it is 1200 U/mL (reviewed in O’Bier et al., 2020). Because an anamnestic response to OspA is not triggered by natural exposure to B. burgdorferi, repeated vaccine administration is required to maintain the minimal protective titer. The high OspA and OspC antibody titers induced by Subunit-AC allow for antibody-mediated killing to occur in both the tick and mammal with the potential for an OspC-induced anamnestic response (Hatke et al., 2020). In summary, this study provides important new information that contributes to our understanding of the antibody responses to OspA and OspC elicited by available canine LD vaccines.
Acknowledgements
This study was supported, in part, by grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Stephen and Alexandra Cohen Foundation, and Zoetis. We thank members of the Marconi lab for their assistance and invaluable advice and discussions.
Footnotes
Conflict of interest statement
RTM is an inventor of VANGUARD crLyme and collects royalties from the Virginia Commonwealth University Intellectual Property Foundation. RTM is a paid consultant and Key Opinion Leader in the field of Lyme disease and receives compensation for some presentations from Zoetis and VetGirl. VK, JM, NS, and AW are paid employees of Zoetis. None of the authors has any other financial or personal relationships that could inappropriately influence or bias the content of the paper.
See: Companion Animal Parasite Council, Parasite Prevalence Maps. https://capcvet.org/maps/#2019/all/lyme-disease/dog/united-states/ (accessed 6 April 2021)
References
- Adeolu M, Gupta RS, 2014. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: the emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek 105, 1049–1072. [DOI] [PubMed] [Google Scholar]
- Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, Cameron DJ, Ziegler JL, Barbour AG, Burgdorfer W, et al. , 1983. Spirochetes isolated from the blood of two patients with Lyme disease. New England Journal of Medicine 308, 740–742. [DOI] [PubMed] [Google Scholar]
- Bockenstedt LK, Hodzic E, Feng S, Bourrel KW, de Silva A, Montgomery RR, Fikrig E, Radolf JD, Barthold SW, 1997. Borrelia burgdorferi strain-specific OspC mediated immunity in mice. Infection and Immunity 65, 4661–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Dykhuizen DE, 2004. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168, 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckles EL, Earnhart CG, Marconi RT, 2006. Analysis of antibody response in humans to the type A OspC loop 5 domain and assessment of the potential utility of the loop 5 epitope in Lyme disease vaccine development. Clinical and Vaccine Immunology 13, 1162–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP, 1982. Lyme disease-a tick-borne spirochetosis? Science 216, 1317–1319. [DOI] [PubMed] [Google Scholar]
- Caimano MJ, Groshong AM, Belperron A, Mao J, Hawley KL, Luthra A, Graham DE, Earnhart CG, Marconi RT, Bockenstedt LK, et al. , 2019. The RpoS gatekeeper in Borrelia burgdorferi: an invariant regulatory scheme that promotes spirochete persistence in reservoir hosts and niche diversity. Frontiers in Microbiology 10, 1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambach DM, Smith CA, Lewis RM, Van Winkle TJ, 1997. Morphologic, immunohistochemical, and ultrastructural characterization of a distinctive renal lesion in dogs putatively associated with Borrelia burgdorferi infection: 49 cases (1987–1992). Veterinary Pathology 34, 85–96. [DOI] [PubMed] [Google Scholar]
- Earnhart CG, Marconi RT, 2007a. Construction and analysis of variants of a polyvalent Lyme disease vaccine: approaches for improving the immune response to chimeric vaccinogens. Vaccine 25, 3419–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Marconi RT, 2007b. An octavalent lyme disease vaccine induces antibodies that recognize all incorporated OspC type-specific sequences. Human Vaccines 3, 281–289. [DOI] [PubMed] [Google Scholar]
- Earnhart CG, Marconi RT, 2007c. OspC phylogenetic analyses support the feasibility of a broadly protective polyvalent chimeric Lyme disease vaccine. Clinical and Vaccine Immunology 14, 628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Buckles EL, Dumler JS, Marconi RT, 2005. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC murine antibody response. Infection and Immunity 73, 7869–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Buckles EL, Marconi RT, 2007. Development of an OspC-based tetravalent, recombinant, chimeric vaccinogen that elicits bactericidal antibody against diverse Lyme disease spirochete strains. Vaccine 25, 466–480. [DOI] [PubMed] [Google Scholar]
- Earnhart CG, Leblanc DV, Alix KE, Desrosiers DC, Radolf JD, Marconi RT, 2010. Identification of residues within ligand-binding domain 1 (LBD1) of the Borrelia burgdorferi OspC protein required for function in the mammalian environment. Molecular Microbiology 76, 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, 2020. Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: a review. Ticks and Tick Borne Diseases 11, 101359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB, 2016. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. Journal of Medicine Entomology 53, 349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E, Telford SR 3rd, Barthold SW, Kantor FS, Spielman A, Flavell RA,1992. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proceedings of the National Academy of Sciences of the United States of America 89, 5418–5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenbaugh DA, De Luca K, Durand PY, Feilmeier B, DeWitt K, Sigoillot-Claude C, Sajous ML, Day MJ, David F, 2018. Characterization of recombinant OspA in two different Borrelia vaccines with respect to immunological response and its relationship to functional parameters. BMC Veterinary Research 14, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatke AL, Green DR, Stasiak K, Marconi RT, 2020. Antibody profiling of a Borreliella burgdorferi (Lyme disease) C6 antibody positive, symptomatic Rottweiler and her pups. The Veterinary Journal 262, 105504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Caimano MJ, Luthra A, Axline D Jr., Corona A, Iacobas DA, Radolf JD, Schwartz I, 2015. Stage-specific global alterations in the transcriptomes of Lyme disease spirochetes during tick feeding and following mammalian host adaptation. Molecular Microbiology 95, 509–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izac JR, Marconi RT, 2019. Diversity of the Lyme disease spirochetes and its influence on immune responses to infection and vaccination. Veterinary Clinics of North America Small Animal Practice 49, 671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izac JR, Camire AC, Earnhart CG, Embers ME, Funk RA, Breitschwerdt EB, Marconi RT, 2019. Analysis of the antigenic determinants of the OspC protein of the Lyme disease spirochetes: evidence that the C10 motif is not immunodominant or required to elicit bactericidal antibody responses. Vaccine 37, 2401–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izac JR, Camire AC, Schuler EJA, Hatke AL, O’Bier NS, Oliver LD Jr., Corondi A, Plocinski OC, Desmond RP, Naimi WA, et al. , 2020a. Serologic evidence for the exposure of Eastern coyotes (Canis latrans) in Pennsylvania to the tick-borne pathogens Borreliella burgdorferi and Anaplasma phagocytophilum. mSphere 5, e00544–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izac JR, O’Bier NS, Oliver LD Jr., Camire AC, Earnhart CG, LeBlanc Rhodes DV, Young BF, Parnham SR, Davies C, Marconi RT, 2020b. Development and optimization of OspC chimeritope vaccinogens for Lyme disease. Vaccine 38, 1915–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupka I, Straubinger RK, 2010. Lyme borreliosis in dogs and cats: background, diagnosis, treatment and prevention of infections with Borrelia burgdorferi sensu stricto. Veterinary Clinics of North America Small Animal Practice 40, 1103–1119. [DOI] [PubMed] [Google Scholar]
- Kugeler KJ, Schwartz AM, Delorey MJ, Mead PS, Hinckley AF, 2021. Estimating the frequency of Lyme disease diagnoses, United States, 2010–2018. Emerging Infectious Diseases 27, 616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFleur RL, Dant JC, Wasmoen TL, Callister SM, Jobe DA, Lovrich SD, Warner TF, Abdelmagid O, Schell RF, 2009. Bacterin that induces anti-OspA and anti-OspC borreliacidal antibodies provides a high level of protection against canine Lyme disease. Clinical and Vaccine Immunology 16, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagal V, Postic D, Baranton G, 2002. Molecular diversity of the ospC gene in Borrelia. Impact on phylogeny, epidemiology and pathology. Wiener Klinische Wochenschrift 114, 562–567. [PubMed] [Google Scholar]
- Lagal V, Postic D, Ruzic-Sabljic E, Baranton G, 2003. Genetic diversity among Borrelia strains determined by single-strand conformation polymorphism analysis of the ospC gene and its association with invasiveness. Journal of Clinical Microbiology 41, 5059–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesca G, Deschamps R, Lubetzki C, Levy R, Assous M, 2002. Acute myelitis in early Borrelia burgdorferi infection. Journal of Neurology 249, 1472–1474. [DOI] [PubMed] [Google Scholar]
- Levy SA, Duray PH, 1988. Complete heart block in a dog seropositive for Borrelia burgdorferi. Similarity to human Lyme carditis. Journal of Veterinary Internal Medicine 2, 138–144. [DOI] [PubMed] [Google Scholar]
- Levy SA, Magnarelli LA, 1992. Relationship between development of antibodies to Borrelia burgdorferi in dogs and the subsequent development of limb/joint borreliosis. Journal of the American Veterinary Medicine Association 200, 344–347. [PubMed] [Google Scholar]
- Little SE, Heise SR, Blagburn BL, Callister SM, Mead PS, 2010. Lyme borreliosis in dogs and humans in the USA. Trends in Parasitology 26, 213–218. [DOI] [PubMed] [Google Scholar]
- Littman MP, Gerber B, Goldstein RE, Labato MA, Lappin MR, Moore GE, 2018. ACVIM consensus update on Lyme borreliosis in dogs and cats. Journal of Veterinary Internal Medicine 32, 887–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi RT, Samuels DS, Garon CF, 1993. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. Journal of Bacteriology 175, 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi RT, Garcia-Tapia D, Hoevers J, Honsberger N, King VL, Ritter D, Schwahn DJ, Swearingin L, Weber A, Teresa C, Winkler M, et al. , 2020. VANGUARD crLyme: a next generation Lyme disease vaccine that prevents B. burgdorferi infection in dogs. Vaccine X 6, 100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bier NS, Hatke AL, Camire AC, Marconi RT, 2020. Human and veterinary vaccines for Lyme disease. Current Issues in Molecular Biology 42, 191–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver LD Jr, Earnhart CG, Virgina-Rhodes D, Theisen M, Marconi R, 2016. Antibody profiling of canine IgG responses to the OspC protein of the Lyme disease spirochetes supports a multivalent approach in vaccine and diagnostic assay development. The Veterinary Journal 218, 27–33. [DOI] [PubMed] [Google Scholar]
- Pal U, de Silva AM, Montgomery RR, Fish D, Anguita J, Anderson JF, Lobet Y, Fikrig E, 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. Journal of Clinical Investigation 106, 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Mead PS, Johnson DK, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, et al. , 2016a. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. The Lancet Infectious Diseases 16, 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Respicio-Kingry LB, Sloan LM, Schriefer ME, Replogle AJ, Bjork J, Liu G, Kingry LC, Mead PS, Neitzel DF, et al. , 2016b. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. International Journal of Systematic and Evolutionary Microbiology 66, 4878–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle JC, Callister SM, Schell RF, Lovrich SD, Jobe DA, Marks JA, Wieneke CA, 1998. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. Journal of Infectious Diseases 178, 733–741. [DOI] [PubMed] [Google Scholar]
- Sadziene A, Wilske B, Ferdows MS, Barbour AG, 1993. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infection and Immunity 61, 2192–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, 2003. Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochemical Society Transactions 31, 108–112. [DOI] [PubMed] [Google Scholar]
- Schwan TG, Piesman J, 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. Journal of Clinical Microbiology 38, 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, Broderick TF, Malawista SE, 1978. Erythema chronicum migrans and Lyme arthritis: epidemiologic evidence for a tick vector. American Journal of Epidemiology 108, 312–321. [DOI] [PubMed] [Google Scholar]
- Straubinger RK, Straubinger AF, Summers BA, Jacobson RH, Erb HN, 1998. Clinical manifestations, pathogenesis, and effect of antibiotic treatment on Lyme borreliosis in dogs. Wiener Klinische Wochenschrift 110, 874–881. [PubMed] [Google Scholar]
- Sykes RA, Makiello P, 2017. An estimate of Lyme borreliosis incidence in Western Europe. Journal of Public Health (Oxford) 39, 74–81. [DOI] [PubMed] [Google Scholar]
- Tilly K, Bestor A, Jewett MW, Rosa P, 2007. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infection and Immunity 75, 1517–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove O, De Buck E, Van Wijngaerden E, 2019. Lyme disease in Western Europe: an emerging problem? A systematic review. Acta Clinica Belgica 1–9. [DOI] [PubMed] [Google Scholar]
- Wilske B, Preac-Mursic V, Göbel UB, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G, 1993. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. Journal of Clinical Microbiology 31, 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Yang Y, Du J, Lin T, Chen T, Yang XF, Lou Y, 2017. Investigation of ospC expression variation among Borrelia burgdorferi strains. Frontiers in Cellular and Infection Microbiology 7, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]


