Abstract
Intestinal parasitic infection (IPI) constitute a global health burden causing clinical morbidity in 450 million people. Many of these are women of reproductive age and children in developing countries. Mass deworming programmes with improvement in lifestyle are likely to reduce the intensity and prevalence of infection over the years. Hence, we aimed to assess the prevalence of intestinal parasitic infections among patients in a tertiary healthcare setting and to examine its time trends. A descriptive cross-sectional study was done using routinely collected data in a tertiary care hospital in South India. Details of examination of stool samples for the presence of intestinal helminth and protozoan ova/cysts, over the period of 5 years (2014–2019) were extracted from laboratory register and hospital information system. The presence of intestinal parasitic infection was determined by stool microscopy (direct wet mount and concentration techniques). Of the total 3267 stool samples, 303 (9.3%) had at least one parasite; 3.9% (93/3267) with helminths and 2.5% (81/3267) Entamoeba and multi-parasitism was seen in 0.14%. Stool samples from more than 18 years age had high positivity rate than others. Majority of the helminth infections were caused by Ascaris (57%) followed by hookworm (42%). Initially IPI which was 10.9% in 2014 declined to 10% in 2016 and attained a peak of 12.4% in 2017 then decreased to 6.7% in 2018. Nearly one out of ten patients had a parasitic infection. Prevalence surveys in the community followed by strengthening the deworming procedures will reduce the burden of IPIs.
Keywords: Intestinal parasitic infections, Trends, Helminths, Protozoa, South India
Introduction
Burden
Intestinal parasitic infection (IPI) affects around 3.5 billion people and causes illness in 450 million and many of these are women of reproductive age and children in developing countries. The annual death of 2 lakhs has been attributed to parasitic infections in developing countries (Saurabh et al. 2017). Soil transmitted helminths like Ascaris lumbricoides, Trichuris trichiura and the hookworms (Necator americanus and Ancylostoma duodenale) are the most common and important in terms of child health (Hall et al. 2008). Globally, more than 2 billion people are infected with soil-transmitted helminths (STH) and out of that 1 billion infected with Ascaris, 740 million with hookworm and 795 million with whipworm (Kaliappan et al. 2013). The global disease burden attributed to intestinal helminths is 39 million disability adjusted life years (Pullan et al. 2014).
Mitigation measures
School-based biannual National deworming programme was launched by the Government of India in 2015 to control the STH infection with the target of reaching 75% of children at risk (Clarke et al. 2017). After the introduction of mass deworming, though the burden of STH reduced to a level where it will not be a public health problem, periodic surveys are required to monitor the trends in the prevalence of STH (Adriko et al. 2018). Most of the published studies have focussed on high-risk groups. Community-based studies in other groups were less when compared to a specific target population. Hence, this hospital-based prevalence assessment will be an indirect method of estimating the parasite prevalence and its burden in this area which can help the control programmes in the community. With this background, we aimed to explore the burden of intestinal parasites and to examine its trends among the routinely collected hospital samples as most of these infestations can be silent in a healthy individual.
Materials and methods
A record-based study was conducted in the department of Microbiology of a tertiary care hospital in Puducherry, South India. We included all the stool samples sent to the parasitology laboratory for diagnostic screening from January 2014 to June 2019. Details regarding age, gender and residence were retrieved from Health management information System (HMIS) and results of stool microscopy were noted from the records maintained in the parasitology laboratory along with HMIS.
Source and target population
The tertiary care centre is located in Puducherry and also attracts various patients from 2 to 3 adjoining districts of Tamil Nadu. We have included all the participants, both rural and urban, whose stool sample we have received during this study period. Being a tertiary care facility, this institute caters to Tamil Nadu too besides other nearby states. Hence, the results can be used as a base to reference the control programmes besides those being done by the Tamil Nadu government itself. Patients’ socio-demographic data and results of stool microscopy for 5 years were retrieved from the electronic database maintained in the records section of the hospital and used for this study.
Inclusion and exclusion criteria
Physicians suspecting a patient of parasite infection based on the signs and symptoms and pre-existing conditions like patients on immunosuppressants were advised to submit a stool sample for screening of intestinal parasites. Patient whose samples were inadequate or with incomplete results or with confirmed non-infectious gastrointestinal problems etc. were excluded
Sample size and sampling technique
Since the study was an exploratory one, we have included all the participants who had given a sample for screening during the study period. All the consecutive samples whose patients fit into our inclusion criteria were taken for the study.
Identification of intestinal parasites
After receiving the fresh stool samples, saline (0.85%) and iodine (Lugol’s) wet mounts were prepared on the same slide within 1 h for the identification of trophozoites, cysts, larvae and helminth eggs. The microscopic slides were covered with coverslips and the whole area under the coverslip was screened under a microscope in low magnification (10×). If something suspicious was seen, it was checked under high magnification (40×) for confirmation. As some structures were not seen clearly in the wet mount, permanent staining methods like trichrome staining and modified acid-fast staining were done for those doubtful samples and visualized under oil immersion (100×). These were checked by 2 independent observers. The diagnosis was confirmed with the identification of internal structures (Garcia et al. 2016). All the samples were processed within 1–2 h of collection.
Quality assurance technique
All the smears were screened independently by the laboratory technician and a resident available in the parasitology laboratory of the department. To ensure the quality, all the positive slides and 10% of the negative slides were cross-checked by the consultant microbiologist who has completed master’s in Microbiology. Besides this, the laboratory is regularly participating in External quality assurance programmes conducted by Centre for Disease Control (CDC), the United States of America and also by Indian Academy of Tropical Parasitology, India.
Ethics and informed consent
Since it was a record-based study using the routinely collected data in a tertiary care centre, we did not take individual patient consent as it was not deemed necessary as per the guidelines of the Institute Ethics Committee of the tertiary care centre. The data were extracted and analysed in a de-identified manner.
Statistical analysis
The extracted data from records and HMIS were entered in Microsoft Excel and analyzed using STATA software, version 12.0. Categorical variables like gender, residence and presence of IPI were expressed in proportions with 95% confidence interval (CI). Total number of positive samples in each year was taken for trend analysis. Association between socio demographic variables and parasitic infection was assessed using Chi square test and a p value of < 0.05 was considered for statistical significance.
Results
Participant characteristics
A total of 3267 patients’ stool samples over 5-year period were included in the study. Mean (standard deviation) age of the participant was 37 (17) years. Figure 1 shows the selection of study participants for the study.Of the total, 13% (430/3267) were in paediatric age group (less than 18 years) and 57% of stool samples were provided by 19–45-year-old patients. More than half of our participants were females 57% (1859/3267) (Table 1).
Fig. 1.
Flowchart showing the data extraction and inflow of study participants in a tertiary care centre, Puducherry, South India
Table 1.
Presence of intestinal parasites in hospital sample of a tertiary care hospital, Puducherry, South India (N = 303)
| Parasite | Number | Proportion (%) |
|---|---|---|
| Entamoeba | 81 | 2.5 |
| Blastocystis | 52 | 1.6 |
| Ascaris | 53 | 1.6 |
| Hookworm | 39 | 1.2 |
| Stronglyoides | 32 | 1.0 |
| Giardia | 22 | 0.7 |
| Trichuris | 1 | 0.03 |
| Multi parasitism | 6 | 0.2 |
| Others | 18 | 0.6 |
Prevalence of intestinal parasites
The overall prevalence of IPI in the hospital sample was 9.3% (303/3267, 95% CI 8.3–10.3%). The yield of positivity by direct microscopy was 98% (297/303) and the additional detection by staining method was 2% (6/303). The most common parasite found was Entamoeba spp. 2.5% (81/3267) followed by Ascaris 1.6% (52/3267) and Blastocystis with 1.6% (53/3267). Multi-parasitism (the presence of more than one parasite) was seen in six (0.2%) patients (Table 2).
Table 2.
Association of parasitic infection with the other socio demographic factors in the enrolled participants in a tertiary care centre, Puducherry, South India
| Variable | Total N (%) | Infected with parasite n (%) | p value |
|---|---|---|---|
| Total | 3267 | 303 (9.7) | |
| Gender (n = 3264) | |||
| Male | 1405 (43.0) | 129 (9.1) | 0.85 |
| Female | 1859 (57.0) | 174 (9.4) | |
| Age category (years) | |||
| 0–18 | 430 (13.2) | 29 (6.7) | 0.15 |
| 19–45 | 1856 (56.8) | 181 (9.7) | |
| > 45 | 981 (30.3) | 93 (9.3) | |
| Residence | |||
| Puducherry | 747 (22.8) | 54 (7.2) | 0.02* |
| Neighbouring districts | 1914 (58.6) | 201 (10.5) | |
| Other districts and states | 606 (18.6) | 48 (7.9) | |
# Gender missing for three participants, *statistically significant p value
Of the total 303 positive samples, 42% were STHs (126/303). In STH, Ascaris (42.1%) was the major parasite followed by hookworm (31%) and Strongyloides spp (26.2%). In protozoa, nearly 50% was contributed by Entamoeba spp followed by Blastocystis (29.4%) and Giardia 12.4% (Fig. 2).
Fig. 2.
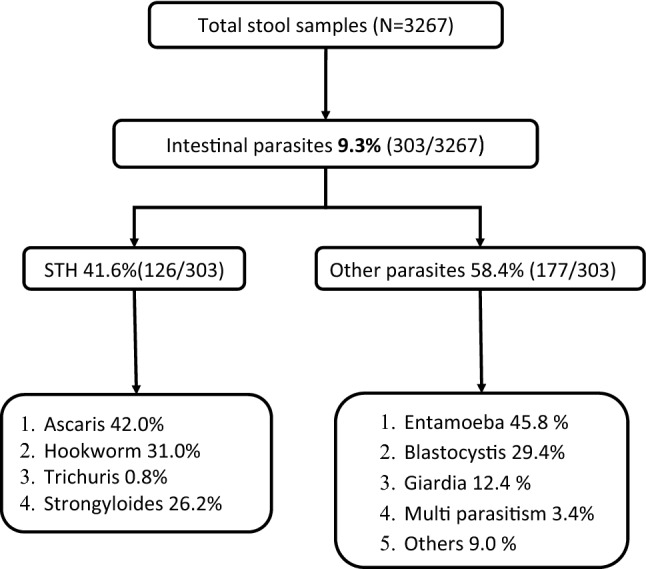
Flow diagram shows the positivity rate of each parasite among the patients in a tertiary care centre, Puducherry
Associated factors
The prevalence of IPI did not differ between males and females (9.1% vs. 9.3%; p = 0.85). The prevalence was lower in 0–18 years age group (6.7%) compared to 19–45 years (9.7%) and > 45 years (9.3%) age groups. However, this difference was not statistically significant (p = 0.15).
Temporal trend: In 2014, the prevalence of IPI was 10.9%, declined to 10% in 2016 and attained a peak of 12.4% in 2017 followed by a decline to 6.7% in 2018. Yearly prevalence of the major parasites (Entamoeba, hookworm and Ascaris) was included in the trend analysis. Our data did not show any linear trend over the years in any of the parasites. Proportion contributed by Ascaris was initially increasing from 2014 to 2016, then declined to 1.9% in 2017 and attained to zero in 2018 (Fig. 3) and there was a constant presence of hookworm infestations in our setting (Fig. 4).
Fig. 3.
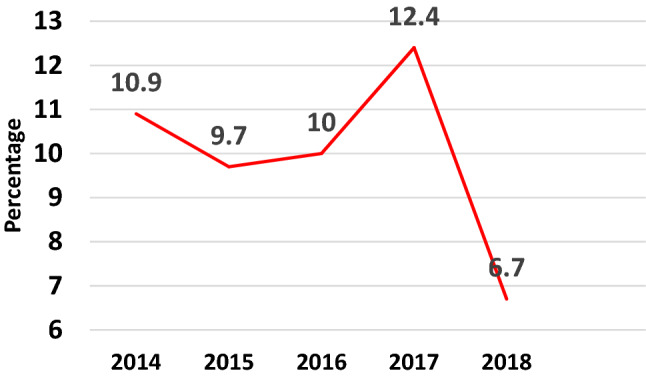
Pattern of overall trends in parasitic infections over the 5 years
Fig. 4.
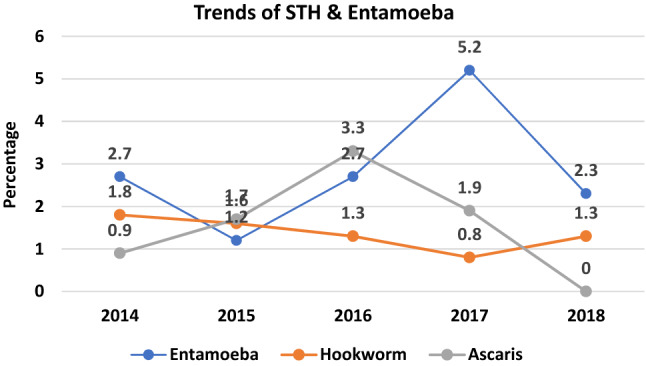
Pattern of the trends of some common parasites in this study
Discussion
The overall positivity rate of IPI in our study over the past 5 years was 9.3% and this is comparable (8.9%) to other studies conducted in other tertiary hospitals in India (Praharaj et al. 2017; Sethi et al. 2019). Our prevalence estimates were lower than studies from Haryana and Chandigarh which included high-risk groups (school children and pregnant women) and rural areas (Sehgal et al. 2010; Sangwan et al. 2017).
In our setting, Entamoeba spp was the major parasite (2.5%) over the 5 years followed by Ascaris lumbricoides (1.6%) and Blastocystis (1.6%). Other studies have found that Giardia was the most common parasite followed by Entamoeba and this variation might be due to geographical difference in the prevalence of intestinal parasites (Praharaj et al. 2017).
In our study, the prevalence of IPI in males and females were similar (9.1% and 9.4%). This is in contrast with other studies that reported males having a higher prevalence of parasitic infection compared to females (Sethi et al. 2019). In our setting, males and females were equally involved in the rearing of animals and agricultural works and hence the risk of infection may not be different.
In the trend analysis, there was a decrease from 10.9 to 10.0% then attained a peak of 12.4% in 2017 which later decreased to 6.7% in 2018 but other studies have shown the declining trend in parasitic infection over the years (Sethi et al. 2019). The exact reasons for the peak in 2017 was not known but the possible explanation could be one of the following. As a preventive strategy, the national deworming day was started in India in the year 2015 and scaled up to cover all the districts of the country in 2016. Still, the programme was in its nascent stage of implementation in the year 2017. The programme mainly covers the adolescent age group (0–19 years) and less coverage in the remaining ages might be the reason for the peak despite the improvement in sanitary and living conditions. It may be due to re-infection too as most people in rural areas have a habit of walking barefooted due to the hot and humid climate. Another possibility may be due to under coverage in the mass deworming programme or increasing drug resistance to these parasites esp STHs due to the drug pressure. This has been reported from various areas where mass and blanket deworming is being done using a single drug only. This has paved the way to look into these issues through various research studies. The decrease in 2018 to 6.7% is encouraging and this could be attributed to mass deworming and improvement in living conditions
Strengths and limitations
We have included 4 years of data from patients attending the tertiary institute for the study and we could see the distribution pattern and trends in the parasitic prevalence. Most of these patients may be symptomatic for a disease but may be silent for parasitic infections. This will help us to estimate how many are carrying the silent burden of this infection and regular screening can be done when a person visits the hospital irrespective of its symptomatology. It will help not only those who are the vulnerable groups in the community like children and pregnant women but also others who may act as a reservoir population.
We have used only microscopic techniques with a single stool sample for the identification of parasites and inclusion criteria depends on the physician and all the referring physicians may not follow the same referral criteria for parasite screening. A follow-up sample after treatment or deworming would have been very helpful. Microscopy itself has its drawbacks. The results might be underestimated because the diagnosis depends on the intensity of infection and microscopy is labor-intensive. We haven’t included other methods like serology or molecular methods like Polymerase Chain reaction etc. for these samples to supplement the microscopy during the study period, though these are now done routinely. The patient’s baseline intestinal parasitic infections (IPIs) and underlying disease characteristics for the area under study aren’t available previously and hence comparison was not possible. Also, this study, being restricted to a single site may be another limitation.
Conclusion
One out of ten patients were positive for intestinal parasitic infections. If we expand the screening procedures with the minimum of three stool samples to all the admitted patients irrespective of their symptoms, the yield will be more. Some parasites may be missed out in wet mounts, so molecular screening and staining methods should be added in routine for all. Prevalence surveys in the general population were encouraged to know the true parasitic prevalence.
Author contributions
RU, NR and GK conceptualised the study. RU, AGR, AG and DL extracted the data. RU performed the analysis.RU and NR wrote the first draft. GK, AGR, AG and DL provided critical comments and approved the final version of the manuscript.
Funding
The author received no financial support for the research or publication.
Compliance with ethical standards
Conflict of interest
The authors declared no conflict of interest with respect to research, authorship or publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adriko M, et al. Impact of a national deworming campaign on the prevalence of soil-transmitted helminthiasis in Uganda (2004–2016): Implications for national control programs. PLoS Negl Trop Dis. 2018;12(7):1–15. doi: 10.1371/journal.pntd.0006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke NE, et al. Differential effect of mass deworming and targeted deworming for soil-transmitted helminth control in children: a systematic review and meta-analysis. Lancet. 2017;389(10066):287–297. doi: 10.1016/S0140-6736(16)32123-7. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Microbiology AS. Diagnostic medical parasitology. Washington, DC: ASM Press; 2016. [Google Scholar]
- Hall A, et al. ‘A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Mater Child Nutr. 2008;4(Suppl 1(1)):118–236. doi: 10.1111/j.1740-8709.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliappan SP, et al. Prevalence and clustering of soil-transmitted helminth infections in a tribal area in southern India. Trop Med Int Health TM&IH Engl. 2013;18(12):1452–1462. doi: 10.1111/tmi.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praharaj I, et al. Temporal trends of intestinal parasites in patients attending a tertiary care hospital in South India: a seven-year retrospective analysis. Indian J Med Res. 2017;146(1):111–120. doi: 10.4103/ijmr.IJMR_1236_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan RL, et al. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vect. 2014;7(1):1–19. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan J, Mane P, Lathwal S. Burden of intestinal parasitic infection in patients attending tertiary care hospital in rural Haryana: a three year retro-spective study. Perspect Med Res. 2017;5:3–7. [Google Scholar]
- Saurabh K, et al. Spectrum of parasitic infections in patients with diarrhoea attending a tertiary care hospital in Western Rajasthan, India. J Clin Diagnost Res. 2017;11(8):01–04. doi: 10.7860/JCDR/2017/29001.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal R, et al. Prevalence of intestinal parasitic infections among school children and pregnant women in low socio-economic area, Chandigarh, North India. Rev Infect. 2010;2(1):100–105. [Google Scholar]
- Sethi S, et al. Changing trends of intestinal parasitic infections in Chandigarh (NG): hospital based study. Indian J Med Microbiol. 2019;18(3):106–109. [Google Scholar]


