Abstract
Background
Myelosuppression is a potential dose-limiting factor in radioligand therapy (RLT). This study aims to investigate occurrence, severity and reversibility of hematotoxic adverse events in patients undergoing RLT with 177Lu-PSMA-617 for metastatic castration-resistant prostate cancer (mCRPC). The contribution of pretreatment risk factors and cumulative treatment activity is taken into account specifically.
Methods
RLT was performed in 140 patients receiving a total of 497 cycles. A mean activity of 6.9 1.3 GBq 177Lu-PSMA-617 per cycle was administered, and mean cumulative activity was 24.6 15.9 GBq. Hematological parameters were measured at baseline, prior to each treatment course, 2 to 4 weeks thereafter and throughout follow-up. Toxicity was graded based on Common Terminology Criteria for Adverse Events v5.0.
Results
Significant (grade ≥ 3) hematologic adverse events occurred in 13 (9.3%) patients, with anemia in 10 (7.1%), leukopenia in 5 (3.6%) and thrombocytopenia in 6 (4.3%). Hematotoxicity was reversible to grade ≤ 2 through a median follow-up of 8 (IQR 9) months in all but two patients who died from disease progression within less than 3 months after RLT. Myelosuppression was significantly more frequent in patients with pre-existing grade 2 cytopenia (OR: 3.50, 95%CI 1.08–11.32, p = 0.04) or high bone tumor burden (disseminated or diffuse based on PROMISE miTNM, OR: 5.08, 95%CI 1.08–23.86, p = 0.04). Previous taxane-based chemotherapy was associated with an increased incidence of significant hematotoxicity (OR: 4.62, 95%CI 1.23–17.28, p = 0.02), while treatment with 223Ra-dichloride, cumulative RLT treatment activity and activity per cycle were not significantly correlated (p = 0.93, 0.33, 0.29).
Conclusion
Hematologic adverse events after RLT have an acceptable overall incidence and are frequently reversible. High bone tumor burden, previous taxane-based chemotherapy and pretreatment grade 2 cytopenia may be considered as risk factors for developing clinically relevant myelosuppression, whereas cumulative RLT activity and previous 223Ra-dichloride treatment show no significant contribution to incidence rates.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13550-021-00805-7.
Keywords: PSMA, 177Lu-PSMA-617, Hematologic adverse events, Hematotoxicity, Metastatic castration-resistant prostate cancer
Background
Metastatic castration-resistant prostate cancer (mCRPC) is associated with high disease-specific morbidity and mortality [1]. Therapeutic options prolonging overall survival are limited to second-generation antiandrogens (enzalutamide and abiraterone), sipuleucel-T and potentially myelotoxic treatments, including taxane-based chemotherapy and bone-seeking 223Ra-dichloride [2–7]. In recent years, radioligand therapy (RLT) directed at the type II transmembrane glycoprotein prostate-specific membrane antigen (PSMA) has been increasingly adopted as a novel treatment for mCRPC. Small-molecule PSMA inhibitors labeled with beta-emitting 177Lutetium, most notably the Glu-urea-based radioligand 177Lu-PSMA-617 and 177Lu-DOTAGA-(I-y)fk(Sub-KuE), briefly termed 177Lu-PSMA-I&T have yielded promising anti-tumoral activity with favorable overall tolerability [8, 9].
Hematological decline is a frequent occurrence in patients with progressive mCRPC and considered a risk factor for poor outcome. Based on evidence derived from peptide receptor radionuclide therapy (PRRT) in neuroendocrine neoplasias, the risk of myelosuppression has been taken into account as dose-limiting factor also in RLT. While descriptive assessment of myelotoxic events has been included in a number of prospective and retrospective trials [9–18], their association with potential predisposing factors remains to be elucidated.
Pretreatment factors implicated in the risk of myelosuppression during radionuclide therapy may include preexisting hematologic impairment, previous myelotoxic therapies and bone tumor burden [19]. Irradiation to the bone marrow during RLT can further add to deterioration of hematopoietic function [20]. However, the impact of RLT-specific variables, including administered treatment activity and cumulative activity, has so far not been investigated.
The aim of this study was to examine incidence, severity and reversibility of myelosuppression in patients undergoing RLT with 177Lu-PSMA-617 in a sizable and heterogenous cohort. Predisposing factors, including previous therapies, disease burden, as well as administered activity per cycle and treatment course, were then analyzed regarding their contribution to new onset hematologic adverse events.
Methods
Patients
A total of 140 patients were treated with 177Lu-PSMA-617 in this retrospective single-center series. Production and administration of 177Lu-PSMA-617 were performed in accordance with legal regulations set out in the German Drug Registration and Administration Act (AMG § 13 2b). Inclusion criteria for RLT mandated that patients have histologically proven, non-resectable, metastatic prostate cancer with disease progression under standard treatment. Indications were confirmed by an interdisciplinary team including board-certified nuclear medicine physicians, urologists, radiation oncologists, pathologists and oncologists. Sufficient PSMA expression in target lesions was defined as an uptake exceeding the liver uptake on 68 Ga-PSMA-11 PET/CT imaging, i.e., scores 2 and 3 according to EANM standardized reporting guidelines v1.0 [21]. An estimated glomerular filtration rate (eGFR [based on the Chronic Kidney Disease Epidemiology Collaboration equation]) of > 30 mL/min/1.73 m2, hemoglobin ≥ 8.0 g/dL, white blood cells (WBC) ≥ 2.00 × 109/L and platelets ≥ 75 × 109/L were required for treatment initiation. Extent of bone tumor burden on PET/CT imaging was classified based on the Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) initiative as well as previous reports and categorized into 1) uni-/oligo-/multifocal (1–20 lesions) or 2) disseminated/diffuse to obtain sufficient group sizes for subsequent analysis [22, 23].
Administration
PSMA-617 was obtained from ABX GmbH (Radeberg, Germany), and radiolabeling with 177LuCl3 was carried out as described in detail before [10, 16]. Quality control was overseen by experienced radiochemists and physicians with respective training in the field. 177Lu-PSMA-617 was administered by slow intravenous injection over 30–60 s. Infusion of 1000 mL of saline was initiated 30 min before application at a continuous rate of 300 mL/h. With the intention to limit the uptake to the parotid and submandibular, icepacks were locally applied 30 min before therapy and continued for 1 h [24]. All therapies were performed as in-patient procedures at our nuclear medicine therapy ward. As mandated by radiation protection legislation, patients remained hospitalized for a minimum 48 h; median hospitalization was 3 (range 2–5) days per cycle.
Toxicity assessment
Repeat blood tests of hematological parameters (hemoglobin, white blood cells and platelets) were undertaken at baseline, prior to each therapy cycle, 2–4 weeks after each cycle and in 6–12-week intervals throughout follow-up. Severity of hematologic adverse events was graded based on Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Grade ≥ 3 toxicities were termed significant.
Statistical analysis
Results are presented as median with interquartile range (IQR) and mean ± standard deviation for continuous variables. Categorical variables are reported as frequencies with respective percentages. The paired Student’s t-test was used to compare intraindividual changes in hematologic parameters. Logistic regression analyses were undertaken to explore risk factors relevant for hematological decline. Analysis was carried out per patient (patient-based) and per cycle (cycle-based). Significant hematologic toxicity was defined as an increase in toxicity to grade 3 or higher during the course of RLT and transformed into a dichotomized variable. First, logistic regression analysis was performed for each categorical risk factor. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. Association of hematologic toxicity with continuous baseline variables and administered activity was analyzed using nonparametric rank correlation (Spearman’s correlation coefficient denoted with rs). Statistical analyses were performed with SPSS (version 27.0, IBM, Armonk, NY), and GraphPad Prism (version 9.0.1, GraphPad Software, San Diego, CA) was used to plot graphs. All tests were two-sided with p-values < 0.05 denominating statistical significance.
Results
One hundred forty consecutive patients with mCRPC (median age 72 [IQR 67–78] years) met the eligibility criteria for RLT and underwent treatment at our institution. Patient characteristics at baseline are summarized in Table 1. Upon treatment initiation, 109 (78%) patients had low-grade anemia (85 grade 1, 24 grade 2), 13 (9%) leukopenia (10 grade 1, 3 grade 2) and 15 (11%) thrombocytopenia (13 grade 1, 2 grade 2). Two patients with hemoglobin levels slightly below the inclusion threshold (both 7.7 g/dL) were treated after individual consent and lack of therapeutic alternatives. Patients received a total of 497 cycles of 177Lu-PSMA-617 with a mean treatment activity of 6.9 1.3 GBq given in a median of 3 (IQR 2–5) treatment cycles. RLT cycles were administered at intervals of 4–8 weeks, reaching a mean cumulative activity of 24.6 15.9 GBq. The median follow-up period was 8 (IQR 4–13) months from the start of treatment.
Table 1.
Baseline characteristics for 140 patients
| All patients (n = 140) | |
|---|---|
| Age | 72 (67–78) |
| PSA (µg/L) | 86 (12–258) |
| Hemoglobin (g/dL) | 11.9 (10.4–13.2) |
| White blood cells (109/L) | 6.4 (5.0–7.8) |
| Platelets (109/L) | 238 (188–286) |
| eGFR (mL/min/1.73 m2) | 81.8 (68.0–93.7) |
| Alkaline phosphatase (U/L) | 93 (67–200) |
| LDH (U/L) | 240 (205–303) |
| Gleason score* | |
| < 8 | 42 (34) |
| ≥ 8 | 82 (66) |
| ECOG performance status | |
| 0 | 42 (30) |
| 1 | 81 (58) |
| 2 | 17 (12) |
| Sites of metastases | |
| Bone | 125 (89) |
| Uni-/oligo-/multifocal | 48 (34) |
| Disseminated/diffuse | 77 (55) |
| Lymph nodes | 125 (89) |
| Visceral | 33 (24) |
| Previous mCRPC therapies | |
| Abiraterone | 87 (62) |
| Enzalutamide | 75 (54) |
| 223Radium-dichloride | 45 (32) |
| Docetaxel | 70 (50) |
| Cabazitaxel | 27 (19) |
| Other chemotherapies† | 7 (5) |
| EBRT (bone metastases) | 49 (35) |
Data presented as median with interquartile range (IQR) or n (%)
ECOG Eastern Cooperative Oncology Group, PSA prostate-specific antigen, eGFR estimated glomerular filtration rate, LDH lactate dehydrogenase, EBRT external beam radiotherapy
*For available patients (n = 124), †: cisplatin, 5-FU, carboplatin, mitoxandrone
Hematologic laboratory values and adverse events
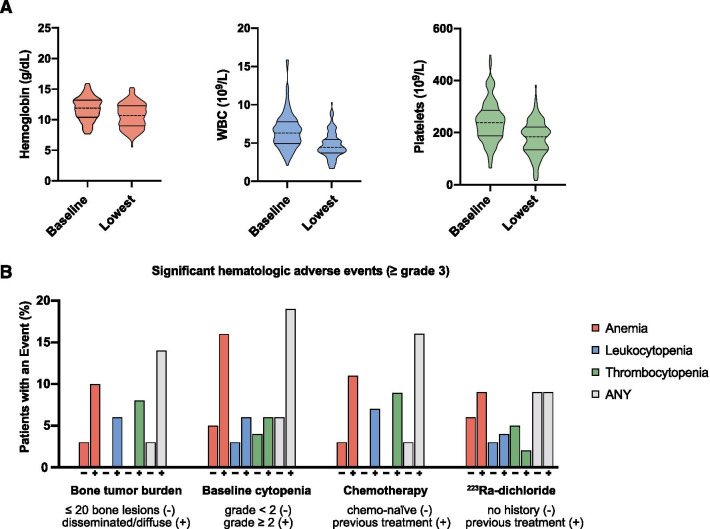
Hematological parameters showed a slight but significant absolute decline through the course of RLT (Fig. 1A). Median hemoglobin decreased from 11.8 (IQR 10.4–13.2) g/dL at baseline to 10.7 (IQR 9.0–12.3) g/dL at the maximum level of deterioration (p < 0.001); median WBC counts shifted from 6.35 (IQR 4.97–7.82) × 109/L to 4.49 (IQR 3.76–5.52) × 109/L (p < 0.001) and thrombocytes from 238 (IQR 188–286) × 109/L to 184 (IQR 134–222) × 109/L (p < 0.001).
Fig. 1.
Violin plots for hemoglobin, white blood cell counts (WBC), and platelets at baseline and upon maximum deterioration (A). Incidence of grade ≥ 3 hematologic adverse events by risk factor: extent of bone tumor burden with 1) none, uni-/oligo-/multifocal (≤ 20) or 2) disseminated and diffuse bone metastases, chemo-naïve or after previous taxane-based chemotherapy, patients previously receiving 223Ra-dichloride or patients with previous hematological decline (CTCAE grade) (B)
Significant hematologic adverse events (grade ≥ 3) during RLT occurred in 13 (9.3%) patients, with anemia in 10 (7.1%), leukopenia in 5 (3.6%) and thrombocytopenia in 6 (4.3%), as shown in Table 2. Median cumulative activity prior to grade ≥ 3 toxicity was 20.7 (IQR 7.4–29.6) GBq. Of 13 patients affected by significant hematologic toxicity, 11 (85%) had initially presented with disseminated or diffuse osseous involvement, 6 (46%) with initial grade 2 cytopenia, 11 (85%) had a history of taxane-based chemotherapy, and 4 (31%) had undergone 223Ra-dichloride prior to RLT (Fig. 1A). The four patients with more than one cell line affected (2 with bicytopenia and 2 with pancytopenia) all had grade ≥ 1 myelosuppression at treatment initiation. Of 497 cycles administered, 17 (3.4%) were subject to subsequent grade ≥ 3 toxicity, which occurred within a median of 6 weeks after administration. Throughout the follow-up period, no case of late onset severe myelosuppression or myelodysplastic syndrome (MDS) was observed.
Table 2.
Baseline and intra-/posttherapeutic hematologic toxicity grades based on CTCAE v5.0
| Baseline (%) | Intra-/posttherapeutic (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Anemia | 85 (61) | 24 (17) | 2 (1) | 0 (0) | 77 (55) | 42 (30) | 10 (7) | 0 (0) |
| Leukopenia | 10 (7) | 3 (2) | 0 (0) | 0 (0) | 27 (19) | 11 (8) | 5 (4) | 0 (0) |
| Thrombocytopenia | 13 (9) | 2 (1) | 0 (0) | 0 (0) | 39 (28) | 3 (2) | 5 (4) | 1 (1) |
Course of patients with significant toxicity
Three out of 13 patients with grade ≥ 3 hematologic toxicity spontaneously recovered to lower levels (grade ≤ 2) within 4 to 6 weeks. Nine (69%) patients with significant myelosuppression received transfusion therapy, eight of which were transfused with packed red blood cells and two received platelet concentrates (Table 3). Four (31%) patients could receive additional cycles of RLT either after spontaneous recovery or blood transfusion. Cytopenia was successfully managed in 10 patients. Two patients who experienced significant disease progression following their last cycle died briefly thereafter; one patient was lost to further follow-up. Of the two aforementioned study patients with grade 3 anemia upon treatment initiation, one spontaneously recovered to grade 2 after responding to RLT and one received packed red blood cells throughout the course of RLT and remained at stable grade 2 hemoglobin levels prior to discontinuing RLT due to disease progression after two cycles.
Table 3.
Previous therapies and course of 13 patients with grade ≥ 3 hematologic adverse events
| Patient | Previous therapies | Toxicity (CTCmax) | Reversibility | Time to toxicity (weeks) | Time to reversibility (weeks) | Course of treatment/disease | ||
|---|---|---|---|---|---|---|---|---|
| Hb | WBC | Platelets | ||||||
| 1 | RP, Rx, ADT, DOCE, ABI, CABA | 3 | 3 | 3 | Yes | 8 | 12 | Recovery after transfusion (2xRBC) |
| 2 | ADT, DOCE, Ra-223 | 3 | 1 | 0 | Yes | 4 | 4 | Recovery after transfusion (2xRBC) |
| 3 | ADT, local Rx | 3 | 2 | 0 | Yes | 4 | 6 | Transfusion therapy, disease progression, death 18 months after PSMA therapy |
| 4 | RP, ADT, DOCE, ENZA | 2 | 1 | 3 | – | 8 | – | Lost to follow-up |
| 5 | ADT, bicalutamide, ABI, DOCE, Rx | 3 | 0 | 0 | Yes | 6 | 4 | Spontaneous recovery, discontinuation of RLT due to PD, continuation of ABI |
| 6 | ADT, ABI, Rx, DOCE, bicalutamide, ENZA | 3 | 3 | 3 | Yes | 8 | 8 | Recovery of all cell lines after 4xRBC, diagnosed with NSCLC after PR under RLT |
| 7 | RARP, Rx, ADT, ABSI, DOCE, ENZA | 3 | 1 | 0 | Yes | 4 | 6 | Continuation of RLT after transfusion (2xRBC) |
| 8 | ADT, bicalutamide, Ra-223 | 3 | 2 | 1 | Yes | 8 | 4 | Carbamazepin intoxication, discontinuation of RLT, spontaneous recovery |
| 9 | ADT, palliative Rx, ABI, ENZA, DOCE, CABA, 5-FU | 1 | 1 | 3 | Yes | 3 | 6 | Continuation of RLT after spontaneous recovery of platelet count, 3 more cycles, PD |
| 10 | RP, ADT, DOCE, ABI, ENZA | 3 | 2 | 1 | Yes | 8 | 8 | Continuation of RLT after transfusion (2xRBC) |
| 11 | RP, salvage Rx, ADT, DOCE, Ra-223, ABI | 3 | 3 | 2 | Yes | 4 | 12 | Transfusion therapy in 4 week intervals (2 × 2 RBC) |
| 12 | RARP, Rx, ADT, DOCE, ABI, CABA, carboplatin, etoposid, mitoxandrone, 5-FU | 1 | 3 | 3 | No | 8 | – | Transfusion of thrombocytes (2xBP), hepatic disease progression |
| 13 | RP, salvage Rx, ADT, Ra-223, ABI | 3 | 0 | 4 | No | 7 | – | Transfusion (RBC, BP) disease progression, death 8 weeks after last cycle |
RP radical prostatectomy, RARP robot-assisted radical prostatectomy, Rx radiotherapy, Ra-223 radium-223-dichloride, ADT androgen deprivation therapy, DOCE docetaxel, CABA cabazitaxel, ABI abiraterone, ENZA enzalutamide, NSCLC non-small cell lung cancer, RBC packed red blood cells, BP blood platelet concentrates, Hb hemoglobin, WBC white blood cells, Plt platelets
Analysis of predisposing factors for hematologic adverse events
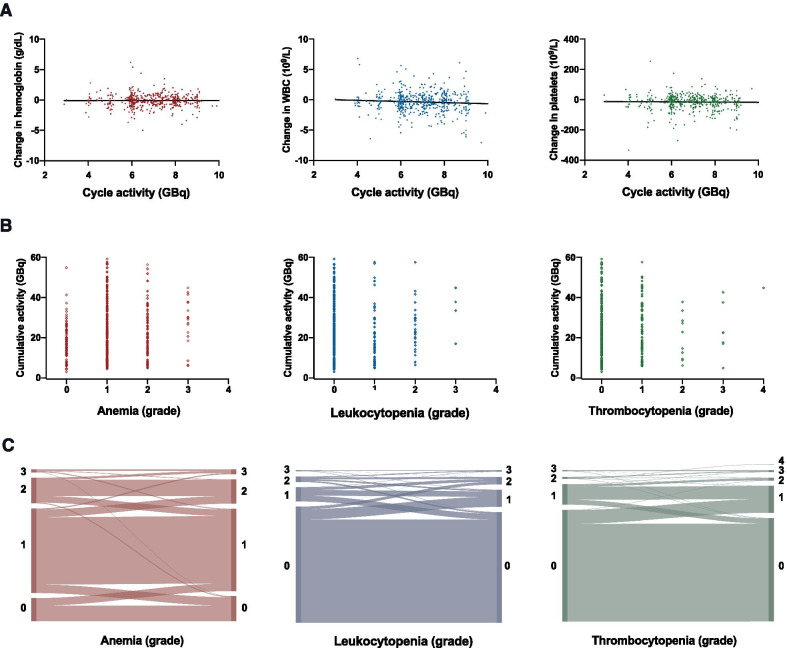
Baseline parameters significantly associated with occurrence of grade ≥ 3 toxicities were: high bone tumor burden (no/one/ ≤ 3 vs. disseminated/diffuse bone metastases, odds ratio [OR]: 5.08, 95% confidence interval [95%CI] 1.08–23.86, p = 0.04), previous treatment with taxane-based chemotherapy (OR: 4.62, 95%CI 1.23–17.28, p = 0.02) and pre-existing grade 2 cytopenia in either cell line (OR: 3.50, 95%CI 1.08–11.32, p = 0.04). Previous treatment with 223Ra-dichloride (OR: 0.93, 95%CI 0.27–3.20, p = 0.58) and presence of bone metastases per se were not significantly associated with occurrence of hematotoxicity (OR: 1.48, 95%CI 0.13–9.22, p = 0.71) (Fig. 1B). Of laboratory values assessed at baseline, alkaline phosphatase (ALP) was correlated with grade ≥ 3 toxicities (rs = 0.23, p = 0.01) and a moderate inverse correlation of eGFR with the occurrence of grade ≥ 3 thrombopenia was observed (rs = -0.19, p = 0.03). Treatment activity per cycle and administered cumulative activity preceding hematotoxic adverse events showed no significant association with incidence of ≥ grade 3 myelosuppression (p = 0.29, 0.32) (Fig. 2B, C, Table 4).
Fig. 2.
Cycle-based analysis (n = 497): (A) association of absolute change in hemoglobin, white blood cells (WBC) and platelets after each treatment cycle with cycle activity, linear trendline, (B) association of toxicity grade after each treatment cycle and cumulative treatment activity, (C) Sankey diagrams for change in CTC grade after each treatment cycle
Table 4.
Association of significant hematologic toxicity and potential risk factors
| Significant toxicity (grade ≥ 3) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | Anemia | Leukopenia | Thrombocytopenia | |||||||||
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |
| A | ||||||||||||
| Bone metastases | ||||||||||||
| Presence | 1.49 | 0.18–12.32 | 0.71 | 1.09 | 0.13–9.22 | 0.94 | – | – | – | – | – | – |
| Disseminated/diffuse | 5.08 | 1.08–23.86 | 0.04 | 3.54 | 0.73–17.29 | 0.12 | – | – | – | – | – | – |
| Baseline hematologic values | ||||||||||||
| Grade ≥ 2 myelosuppression | 3.50 | 1.08–11.32 | 0.04 | 4.00 | 1.08–14.85 | 0.04 | 2.44 | 0.39–15.28 | 0.34 | 1.81 | 0.32–10.38 | 0.51 |
| Previous mCRPC therapies | ||||||||||||
| Taxane-based chemotherapy | 4.62 | 1.23–17.28 | 0.02 | 3.45 | 0.86–13.93 | 0.08 | 10.7 | 0.93–124.74 | 0.06 | 11.37 | 1.11–116.98 | 0.04 |
| 223Ra-dichloride | 0.93 | 0.27–3.20 | 0.91 | 1.45 | 0.39–5.41 | 0.58 | 1.43 | 0.23–8.85 | 0.70 | 0.43 | 0.46–3.61 | 0.42 |
| EBRT (bone metastasis) | 2.36 | 0.75–7.67 | 0.14 | 1.96 | 0.54–7.11 | 0.31 | 2.90 | 0.47–17.99 | 0.25 | 1.91 | 0.37–9.58 | 0.44 |
| Significant toxicity (grade ≥ 3) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | Anemia | Leukopenia | Thrombocytopenia | |||||||||
| rs | p | rs | p | rs | p | rs | p | |||||
| B | ||||||||||||
| Baseline laboratory | ||||||||||||
| ALP | 0.23 | 0.01 | 0.17 | 0.05 | 0.19 | 0.03 | 0.19 | 0.03 | ||||
| LDH | 0.16 | 0.07 | 0.10 | 0.25 | 0.14 | 0.11 | 0.16 | 0.06 | ||||
| eGFR | –0.07 | 0.40 | –0.17 | 0.84 | –0.14 | 0.09 | –0.19 | 0.03 | ||||
| Administered activity | ||||||||||||
| Cumulative | 0.09 | 0.32 | 0.08 | 0.33 | 0.05 | 0.54 | 0.11 | 0.21 | ||||
| Per cycle | –0.05 | 0.29 | –0.07 | 0.12 | 0.01 | 0.85 | –0.03 | 0.58 | ||||
(A) Univariate logistic regression. OR: odds ratio, 95%CI: 95% confidence interval and (B) non-parametric rank test, rs = Spearman’s correlation coefficient
Discussion
In the presented retrospective study, 140 patients receiving RLT for mCRPC were assessed for occurrence of hematologic adverse events and the role of contributing factors. Significant (grade ≥ 3) hematologic toxicity occurred in 3.4% (17/497) of all treatment cycles and in 9.3% (13/140) of all patients undergoing RLT. Hematologic adverse events were manageable and most likely to occur in patients with extensive bone tumor burden (disseminated or diffuse, OR: 5.08, 95%CI 1.08–23.86, p = 0.04). Patients with baseline myelosuppression (grade ≥ 2 cytopenia OR: 3.50, 95%CI 1.08–11.32, p = 0.04) or previous taxane-based chemotherapy (OR: 4.62, 95%CI 1.23–17.28, p = 0.02) also had higher odds of experiencing grade ≥ 3 myelosuppression.
Available taxane-based chemotherapeutic agents for progressive mCRPC bear a risk hematotoxicity, especially to white blood cells. The phase 3 TAX 327 trial for docetaxel yielded grade ≥ 3 neutropenia in 32%; the TROPIC trial reported grades ≥ 3 leukopenia in 68% of patients receiving cabazitaxel [3, 4, 25]. Myelosuppression is also a known side effect in radionuclide therapy [26]. Toxic effects to hematopoietic cells are mediated by both blood-driven recirculating ß-irradiation and scatter radiation from bone metastases. Long-standing experience from peptide receptor radionuclide therapy (PRRT) in neuroendocrine neoplasias (NEN) with 177Lu-labeled DOTA0-Tyr3-octreotate (177Lu-DOTATATE) yielded moderate grade ≥ 3 hematotoxicity rates in the range of 8 to 11.3% [26–29]. Beyond the emergence of subacute toxicity, myelodysplastic syndrome (MDS) may develop as a rare, but severe long-term sequel after PRRT in 1–2% of all patients treated [26, 30]. Apart from effects attributable to diverging biokinetics, it may be hypothesized that MDS is less likely observed after 177Lu-PSMA-617 due to shorter survival of patients with mCRPC as compared to NEN.
Initial radioimmunological approaches targeting an extracellular PSMA epitope were limited by high rates of myelotoxicity related to the longer plasma half-life inherent to circulating antibodies [31]. In a phase 2 study with the 177Lu-labeled monoclonal antibody J591 conducted by Tagawa et al., 47% of all patients developed grade 4 thrombocytopenia necessitating aggressive management and transfusion therapy in 30% of all patients enrolled [32, 33]. Following the advent of the small-molecule ligands 177Lu-PSMA-617 and 177Lu-PSMA-I&T multiple studies, predominantly within compassionate use programs have included assessment of hematologic adverse events in heterogeneous mCRPC cohorts. Reported overall incidence rates are summarized in Table 5 [9–18, 34–43]. The landmark phase 2 trial conducted by Hofman et al. in 30 patients receiving 177Lu-PSMA-617 reported grade ≥ 3 anemia, neutropenia and thrombocytopenia in 13%, 37% and 4% [8]. Results from a large retrospective study by Heck et al. in 100 patients receiving 317 cycles of 177Lu-PSMA I&T indicated lower rates, with grade ≥ 3 anemia, neutropenia and thrombocytopenia in 9%, 6% and 6%, respectively [9]. It has to be acknowledged that comparison of white blood cell toxicity in our study was impeded by the fact that differential blood counts for neutrophils and lymphocytes were not available for analysis in all our patients. Recently, Barber et al. contributed a comparative retrospective study using both 177Lu-PSMA-617 and 177Lu-PSMA I&T in 83 patients previously treated with taxane-based chemotherapy and 84 taxane-naïve controls. Grade ≥ 3 anemia, leukopenia and thrombocytopenia occurred in 8% vs. 1%, 2 vs. 0% and 4% vs. 1% of all study patients [35]. The latter findings indicate an adverse impact of previous taxane-based chemotherapy on subsequent hematotoxcity during RLT, as described in our cohort. In the most recent randomized, multicentric phase 2 trial by Hofman et al. (TheraP, ANZUP 1603) previous treatment with docetaxel was an inclusion criterion. Here, grade ≥ 3 anemia, leukopenia and thrombocytopenia occurred in 8%, 1% and 11% of 98 patients receiving 177Lu-PSMA-617, as compared to 8%, 1% and 0% in the standard-of-care arm (n = 85) treated with cabazitaxel [44].
Table 5.
177Lu-PSMA studies reporting hematologic adverse events
| Study | Ref | n | Cycles | Ligand | Activity (GBq/cycle, range) | Anemia grade 1/2 | Leukopenia/*neutropenia grade 1/2 | Thrombocytopenia grade 1/2 | Anemia grade 3/4 | Leukopenia/*neutropenia grade 3/4 | Thrombocytopenia grade 3/4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmadzadehfar et al. 2015 | [10] | 10 | 10 | PSMA-617 | 5.6 | 1 (10%) | 3 (30%) | 2 (20%) | 1 (10%) | 0 | 0 |
| Ahmadzadehfar et al. 2016 | [11] | 24 | 46 | PSMA-617 | 6 (4.1–7.1) | 7 (29%) | 5 (21%) | 4 (17%) | 2 (8%) | 0 | 0 |
| Barber et al. 2019 | [35] | 84/83 | n/a | PSMA-617,-I&T | 6.3 (5.7–6.8) | 81(96%)/73(88%) | 22(26%)/23(28%) | 21(25%)/27(33%) | 1(1%)/7(8%) | 0/2(2%) | 1(1%)/3(4%) |
| Baum et al. 2016 | [12] | 56 | 125 | PSMA-I&T | 5.8 | n/a | n/a | n/a | 0 | 0 | 0 |
| Bräuer et al. 2017 | [13] | 59 | 159 | PSMA-617 | 6.1 (5.9–6.3) | n/a | n/a | n/a | 11 (19%) | 2 (4%) | 2 (4%) |
| Derlin et al. 2020 | [34] | 31/40 | n/a | PSMA-617 | 6.0–7.4 | 9 (29%)/6 (15%) | 5 (16%)/4(10%) | 3(10%)/4(10%) | 0 | 0 | 0 |
| Emmett et al. 2018 | [36] | 14 | n/a | PSMA-617 | 6.0–8.0 | 2 (14%) | n/a | 1/14 (7%) | 0 | 0 | 0 |
| Fendler et al. 2016 | [14] | 30 | 30 | PSMA-617 | 3.7 or 6 | n/a | n/a | n/a | 1 (3%) | 3 (3%) | 0 |
| Heck et al. 2016 | [15] | 22 | 43 | PSMA-I&T | 3.7–7.4 | 3 (14%) | 1*(5%) | 5 (23%) | 0 | 0 | 0 |
| Heck et al. 2018 | [9] | 100 | 317 | PSMA-I&T | 7.4 | n/a | n/a | n/a | 9 (9%) | 6* (6%) | 4 (4%) |
| Hofman et al. 2018 | [8] | 30 | 86 | PSMA-617 | 7.5 | 4 (13%) | 11* (37%) | 8 (27%) | 4 (13%) | 11* (37%) | 4 (13%) |
| Hofman et al. 2021 | [44] | 98 | n/a | PSMA-617 | 6.0–8.5 | 19 (19%) | 10 (10%) | 18 (18%) | 8 (8%) | 1 (1%) | 11 (11%) |
| Kratochwil et al. 2016 | [16] | 30 | 70 | PSMA-617 | 3.7–6.0 | 9 (30%) | 8 (27%) | 4 (18%) | 1 (5%) | 0 | 1 (5%) |
| Paganelli et al. 2020 | [37] | 43 | n/a | PSMA-617 | 3.7–5.5 | 28 (65%) | 3*(6.9%) | 3(6.9%) | 2 (4.8%) | 0 | 0 |
| Rahbar et al. 2016 | [18] | 74 | 74 | PSMA-617 | 6.0 | 26 (35%) | 9 (12%) | 16 (22%) | 1(1%) | 0 | 1 (1%) |
| Rahbar et al. 2016 | [38] | 28 | 50 | PSMA-617 | 5.9 | 3 (14%) | 3 (14%) | 5 (23%) | 0 | 0 | 0 |
| Rathke et al. 2018 | [39] | 40 | 120 | PSMA-617 | 4–9.3 | 0 | 8 (20%) | 2 (5%) | 0 | 1 (3%) | 2 (5%) |
| Scarpa et al. 2017 | [40] | 10 | 29 | PSMA-617 | 6 (5.4–6.5) | n/a | n/a | n/a | 0 | 0 | 0 |
| Seifert et al. 2020 | [41] | 37/41 | n/a | PSMA-617 | 6.0/7.5 | (35%)/(27%) | (49%)/(49%) | (41%)/(34%) | (22%)/(24%) | (8%)/(2%) | (8%)/(2%) |
| Violet et al. 2019 | [42] | 50 | n/a | PSMA-617 | 7.5 | 9 (18%) | 12* (24%) | 14 (28%) | 5 (10%) | 3* (6%) | 5 (10%) |
| Yadav et al. 2016 | [43] | 31 | 65 | PSMA-617 | 1.1–7.4 | 9 (31%) | n/a | 1 (3%) | 1 (3%) | n/a | 0 |
| Yadav et al. 2020 | [48] | 90 | 281 | PSMA-617 | 1.1–7.8 | 72 (78%) | 10 (11%) | 14 (16%) | 2 (2%) | 1 (1%) | 1 (1%) |
n/a Not available
We report new onset grade ≥ 3 anemia, leukopenia and thrombopenia in 7% (10/140), 4% (5/140) and 4% (6/140) of patients, respectively. Despite delimiting pre-existing cytopenia from therapy-emergent toxicity, differentiation of hematologic decline due to disease progression from true therapy-emergent toxicity remains challenging due to frequently overlapping phenomena. For a conservative estimate, we considered all new onset grade ≥ 3 toxicities in our analysis, regardless of disease progression being a likely contributing factor in a number of cases. Overall, our results appear well in line with data from the foregoing retrospective and prospective studies, taking into account the significant portion of patients with extensive tumor burden and baseline low-grade myelosuppression in the examined cohort.
Our study points toward an influence of predisposing factors on emergence of grade ≥ 3 hematologic adverse events, including taxane-based chemotherapy and initial grade 2 cytopenia. This may be explained by DNA damage conferred by cytotoxic agents [45]. In addition, sequential failure on multiple systemic treatments preceding RLT puts patients at higher odds of developing hematologic decline through disease progression over time. In their post hoc hematologic safety analysis of the ALSYMPCA trial, Vogelzang et al. report both previous taxane-based chemotherapy with docetaxel and baseline cytopenia (anemia and thrombocytopenia) to be associated with grade ≥ 2 thrombocytopenia in mCRPC patients undergoing 223Ra-dichloride [19]. Interestingly, the placebo arm also contained relevant rates of new onset toxicity with grade ≥ 3 anemia, neutropenia and thrombocytopenia in 14%, 1% and 3%, underlining the notion that the natural course of mCRPC itself is linked to significant deterioration of bone marrow reserve. In further accordance with our observations in RLT, increased tumor burden (defined as ≥ 6 metastases) was also a predictive factor for hematotoxicity in mCRPC patients receiving 223Ra-dichloride.
In our cohort, a slight trend toward grade ≥ 3 hematologic toxicities, especially thrombocytopenia, was observed with decreasing eGFR values. This effect has been described also in PRRT and attributed to decreased plasma clearance of recirculating radionuclides in chronic kidney disease [28, 46].
Cumulative activity and individual treatment activity play a distinct role in defining appropriate regimens for RLT, and various RLT-schemes have been put forth. Rathke et al. clustered 40 patients into treatment groups receiving 4, 6, 7.4 or 9.3 GBq of 177Lu-PSMA-617 reporting comparable safety and efficacy, while pointing out a lower mean platelet count in the 10 patients having received 9.3 GBq [39]. Our treatment routine allowed for individual dose adaptation and yielded no correlation between higher treatment activities or high cumulative activities with increased rates of hematologic adverse events. Potential bias must be considered when interpreting the bivariate association of treatment activity and hematotoxic events since myelosuppression was one reason for individual dose de-escalation.
A major limitation to the conducted analysis is undoubtably its retrospective design. The presented patient population is highly heterogenous and may differ from previously reported series, taking into account that both a fraction of patients omitting prior chemotherapy after interdisciplinary counseling and a considerable number of patients with wide-spread bone tumor burden were included in our analysis. Prospective phase 3 data are much anticipated, with results from the VISION trial expected in near future [47].
Conclusions
Our findings suggest that repeated cycles of RLT with 177Lu-PSMA-617 can be carried out at acceptable rates of myelosuppression with cytopenia being most frequently reversible, especially in earlier phases of disease progression. High bone tumor burden, previous taxane-based chemotherapy and initial hematologic decline are possible risk factors for developing significant new onset hematologic adverse events. Administered activity per cycle and cumulative activity had in turn no significant impact. These results call for further refining individualized treatment based on given risk factors for hematologic toxicity.
Supplementary Information
Additional file 1: Fig. S1 Maximum intensity projections of 68 Ga-PMSA imaging at baseline: (a) 81-year-old patient (P8 in Table 3) with limited extent of bone metastases (category 1), the patient developed reversible grade 3 anemia after RLT. (b) 75-year-old patient (P 13 in Table 3) with diffuse bone marrow involvement (category 2), developing progressive disease and irreversible hematological decline with grade 3 anemia and grade 4 thrombocytopenia after 6 cycles of RLT.
Acknowledgements
Not applicable.
Abbreviations
- ALP
Alkaline phosphatase
- CI
Confidence interval
- CTCAE
Common Terminology Criteria for Adverse Events
- EBRT
External beam radiotherapy
- ECOG
Eastern Cooperative Oncology Group
- eGFR
Estimated glomerular filtration rate
- GBq
Gigabecquerel
- IQR
Interquartile range
- LDH
Lactate dehydrogenase
- mCRPC
Metastatic castration-resistant prostate cancer
- MDS
Myelodysplastic syndrome
- NEN
Neuroendocrine neoplasia
- OR
Odds ratio
- PROMISE
Prostate Cancer Molecular Imaging Standardized Evaluation
- PRRT
Peptide receptor radionuclide therapy
- PSA
Prostate-specific antigen
- PSMA
Prostate-specific membrane antigen
- RLT
Radioligand therapy
- WBC
White blood cells
Authors' contributions
DG and AS designed study, analyzed and interpreted data, drafted manuscript. CTN, JB, KD, NM, CNN, JW acquired data and revised manuscript. FG, CH, BB interpreted data and substantively revised manuscript. NT, SB, PM, FKHC substantively revised manuscript and approved of its final content. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All patients gave written informed consent prior to each therapy cycle, and retrospective data analysis was approved by the ethics committee of Goethe University Frankfurt (approval number: 310/18).
Consent for publication
All patients gave written informed consent to participation and publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. Ca Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. New Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels J-P, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet Lond Engl. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. New Engl J Medicine. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabot RC, Harris NL, Rosenberg ES, Shepard JAO, Cort AM, Ebeling SH, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 7.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 8.Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. doi: 10.1016/S1470-2045(18)30198-0. [DOI] [PubMed] [Google Scholar]
- 9.Heck MM, Tauber R, Schwaiger S, Retz M, D’Alessandria C, Maurer T, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urol. 2018;75:920–926. doi: 10.1016/j.eururo.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadzadehfar H, Rahbar K, Kürpig S, Bögemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. Ejnmmi Res. 2015;5:36. doi: 10.1186/s13550-015-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmadzadehfar H, Eppard E, Kürpig S, Fimmers R, Yordanova A, Schlenkhoff CD, et al. Therapeutic response and side effects of repeated radioligand therapy with 177 Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2015;7:12477–12488. doi: 10.18632/oncotarget.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–1013. doi: 10.2967/jnumed.115.168443. [DOI] [PubMed] [Google Scholar]
- 13.Bräuer A, Grubert LS, Roll W, Schrader AJ, Schäfers M, Bögemann M, et al. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol. 2017;I(44):1663–1670. doi: 10.1007/s00259-017-3751-z. [DOI] [PubMed] [Google Scholar]
- 14.Fendler WP, Reinhardt S, Ilhan H, Delker A, Böning G, Gildehaus FJ, et al. Preliminary experience with dosimetry, response and patient reported outcome after 177 Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget. 2015;5. [DOI] [PMC free article] [PubMed]
- 15.Heck MM, Retz M, D’Alessandria C, Rauscher I, Scheidhauer K, Maurer T, et al. Systemic radioligand therapy with 177 lu labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate cancer. J Urology. 2016;196:382–391. doi: 10.1016/j.juro.2016.02.2969. [DOI] [PubMed] [Google Scholar]
- 16.Kratochwil C, Giesel FL, Stefanova M, Beneova M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57:1170–6. doi: 10.2967/jnumed.115.171397. [DOI] [PubMed] [Google Scholar]
- 17.Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med Offic Publ Soc Nucl Med. 2016;57:1334–1338. doi: 10.2967/jnumed.116.173757. [DOI] [PubMed] [Google Scholar]
- 18.Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schäfers M, Essler M, et al. PSMA targeted radioligand therapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol. 2017;I(45):12–9. doi: 10.1007/s00259-017-3848-4. [DOI] [PubMed] [Google Scholar]
- 19.Vogelzang NJ, Coleman RE, Michalski JM, Nilsson S, O’Sullivan JM, Parker C, et al. Hematologic safety of radium-223 dichloride: baseline prognostic factors associated with myelosuppression in the ALSYMPCA trial. Clin Genitourin Canc. 2017;15:42–52.e8. doi: 10.1016/j.clgc.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Schmeiser HH, Muehlbauer K-R, Mier W, Baranski A-C, Neels O, Dimitrakopoulou-Strauss A, et al. DNA damage in human whole blood caused by radiopharmaceuticals evaluated by the comet assay. Mutagenesis. 2019;34:239–244. doi: 10.1093/mutage/gez007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceci F, Oprea-Lager DE, Emmett L, Adam JA, Bomanji J, Czernin J, et al. E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol. 2021;I(48):1626–38. doi: 10.1007/s00259-021-05245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-Ligand PET/CT. J Nucl Med Offic Publ Soc Nucl Med. 2017;59:469–478. doi: 10.2967/jnumed.117.198119. [DOI] [PubMed] [Google Scholar]
- 23.Yordanova A, Linden P, Hauser S, Meisenheimer M, Kürpig S, Feldmann G, et al. Outcome and safety of rechallenge [177Lu]Lu-PSMA-617 in patients with metastatic prostate cancer. Eur J Nucl Med Mol. 2019;I(46):1073–1080. doi: 10.1007/s00259-018-4222-x. [DOI] [PubMed] [Google Scholar]
- 24.van Kalmthout LWM, Lam MGEH, de Keizer B, Krijger GC, Ververs TFT, de Roos R, et al. Impact of external cooling with icepacks on 68Ga-PSMA uptake in salivary glands. Ejnmmi Res. 2018;8:56. doi: 10.1186/s13550-018-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesavan M, Turner JH. Myelotoxicity of peptide receptor radionuclide therapy of neuroendocrine tumors: a decade of experience. Cancer Biother Radio. 2016;31:189–198. doi: 10.1089/cbr.2016.2035. [DOI] [PubMed] [Google Scholar]
- 27.Sabet A, Khalaf F, Haslerud T, Al-Zreiqat A, Sabet A, Simon B, et al. Bone metastases in GEP-NET: response and long-term outcome after PRRT from a follow-up analysis. Am J Nucl Med Mol Imaging. 2013;3:437–445. [PMC free article] [PubMed] [Google Scholar]
- 28.Bergsma H, Konijnenberg MW, Kam BLR, Teunissen JJM, Kooij PP, de Herder WW, et al. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol. 2015;I(43):453–463. doi: 10.1007/s00259-015-3193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabet A, Haug AR, Eiden C, Auernhammer CJ, Simon B, Bartenstein P, et al. Efficacy of peptide receptor radionuclide therapy with 177Lu-octreotate in metastatic pulmonary neuroendocrine tumors: a dual-centre analysis. Am J Nucl Med Mol Imaging. 2017;7:74–83. [PMC free article] [PubMed] [Google Scholar]
- 30.Sabet A, Ezziddin K, Pape U-F, Ahmadzadehfar H, Mayer K, Pöppel T, et al. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2013;54:1857–1861. doi: 10.2967/jnumed.112.119347. [DOI] [PubMed] [Google Scholar]
- 31.Vallabhajosula S, Goldsmith SJ, Hamacher KA, Kostakoglu L, Konishi S, Milowski MI, et al. Prediction of myelotoxicity based on bone marrow radiation-absorbed dose: radioimmunotherapy studies using y- and lu-labeled j591 antibodies specific for prostate-specific membrane antigen. J Nucl Med Offic Publ Soc Nucl Med. 2005;46:850–858. [PubMed] [Google Scholar]
- 32.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177 lutetium-labeled j591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–4601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 33.Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of lutetium-177–labeled anti-prostate-specific membrane antigen monoclonal antibody j591 for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2013;19:5182–5191. doi: 10.1158/1078-0432.CCR-13-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derlin T, Sohns JMS, Schmuck S, Henkenberens C, Klot CAJ, Ross TL, et al. Influence of short-term dexamethasone on the efficacy of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Prostate. 2020;80:619–631. doi: 10.1002/pros.23974. [DOI] [PubMed] [Google Scholar]
- 35.Barber TW, Singh A, Kulkarni HR, Niepsch K, Billah B, Baum RP. Clinical outcomes of 177 lu-psma radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J Nucl Med. 2019;60:955–962. doi: 10.2967/jnumed.118.216820. [DOI] [PubMed] [Google Scholar]
- 36.Emmett L, Crumbaker M, Ho B, Willowson K, Eu P, Ratnayake L, et al. Results of a prospective phase 2 pilot trial of Lu177 PSMA 617 therapy for metastatic castrate resistant prostate cancer including Imaging predictors of treatment response and patterns of progression. Clin Genitourin Canc. 2018;17:15–22. doi: 10.1016/j.clgc.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Paganelli G, Sarnelli A, Severi S, Sansovini M, Belli ML, Monti M, et al. Dosimetry and safety of 177Lu PSMA-617 along with polyglutamate parotid gland protector: preliminary results in metastatic castration-resistant prostate cancer patients. Eur J Nucl Med Mol. 2020;I(47):3008–3017. doi: 10.1007/s00259-020-04856-1. [DOI] [PubMed] [Google Scholar]
- 38.Rahbar K, Bode A, Weckesser M, Avramovic N, Claesener M, Stegger L, et al. Radioligand therapy with 177lu-psma-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin Nucl Med. 2016;41:522–528. doi: 10.1097/RLU.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 39.Rathke H, Giesel FL, Flechsig P, Kopka K, Mier W, Hohenfellner M, et al. Repeated177Lu-labeled psma-617 radioligand therapy using treatment activities of up to 93 GBq. J Nucl Med Offic Publ Soc Nucl Med. 2017;59:459–65. doi: 10.2967/jnumed.117.194209. [DOI] [PubMed] [Google Scholar]
- 40.Scarpa L, Buxbaum S, Kendler D, Fink K, Bektic J, Gruber L, et al. The (68)Ga/(177)Lu theragnostic concept in PSMA targeting of castration-resistant prostate cancer: correlation of SUVmax values and absorbed dose estimates. Eur J Nucl Med Mol. 2017;I(44):788–800. doi: 10.1007/s00259-016-3609-9. [DOI] [PubMed] [Google Scholar]
- 41.Seifert R, Kessel K, Schlack K, Weckesser M, Bögemann M, Rahbar K. Radioligand therapy using [177Lu]Lu-PSMA-617 in mCRPC: a pre-VISION single-center analysis. Eur J Nucl Med Mol. 2020;I:1–7. doi: 10.1007/s00259-020-04703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Violet J, Sandhu S, Iravani A, Ferdinandus J, Thang S-P, Kong G, et al. Long-term follow-up and outcomes of retreatment in an expanded 50-patient single-center phase ii prospective trial of 177 lu-psma-617 theranostics in metastatic castration-resistant prostate cancer. J Nucl Med. 2019;61:857–865. doi: 10.2967/jnumed.119.236414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, et al. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol. 2016;I(44):81–91. doi: 10.1007/s00259-016-3481-7. [DOI] [PubMed] [Google Scholar]
- 44.Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021; [DOI] [PubMed]
- 45.Bolton KL, Ptashkin RN, Gao T, Braunstein L, Devlin SM, Kelly D, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet. 2020;52:1219–1226. doi: 10.1038/s41588-020-00710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol. 2015;I(42):5–19. doi: 10.1007/s00259-014-2893-5. [DOI] [PubMed] [Google Scholar]
- 47.Rahbar K, Bodei L, Morris MJ. Is the vision of radioligand therapy for prostate cancer becoming a reality? An overview of the phase III VISION trial and its importance for the future of theranostics. J Nucl Med. 2019;60:1504–1506. doi: 10.2967/jnumed.119.234054. [DOI] [PubMed] [Google Scholar]
- 48.Yadav MP, Ballal S, Bal C, Sahoo RK, Damle NA, Tripathi M, et al. Efficacy and safety of 177Lu-PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer patients. Clin Nucl Med. 2020;45:19–31. doi: 10.1097/RLU.0000000000002833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1 Maximum intensity projections of 68 Ga-PMSA imaging at baseline: (a) 81-year-old patient (P8 in Table 3) with limited extent of bone metastases (category 1), the patient developed reversible grade 3 anemia after RLT. (b) 75-year-old patient (P 13 in Table 3) with diffuse bone marrow involvement (category 2), developing progressive disease and irreversible hematological decline with grade 3 anemia and grade 4 thrombocytopenia after 6 cycles of RLT.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.