Abstract
Protozoan parasites, such as Leishmania major (L. major), remained as a global health problem of the current century. Leishmania major is a major cause of cutaneous leishmaniasis (CL) in developed and developing countries. Traditionally, amphotericin B is prescribed as an alternative drug, while first-line drugs failed. Some active proteins of the innate immune system such as cathelicidins try to inhibit infection Via several proposed mechanisms. Here this research aimed to not only determine the anti-leishmanial activity of nano amphotericin B but also to evaluate which anti-leishmanial drug can induce the cathelicidin gene expression. Both promastigote and amastigote stages of L. major were exposed to various concentrations of nano amphotericin B, amphotericin B and finally compared to glucan time as standard drug for CL treatment. For the gene expression of cathelicidin, macrophages were exposed to the same concentration of anti-leishmanial drugs. The findings demonstrated that nano amphotericin B was more effective at all concentrations than amphotericin B. Additionally, among tested anti-leishmanial drugs, nano amphotericin B has more potency to induce the cathelicidin gene expression in macrophages cells. The findings revealed that nano amphotericin B has potential as an effective anti-leishmanial drug against CL caused by L. major parasites.
Keywords: Nano-amphotericin B, Antimicrobial peptides, Leishmaniasis, Anti-leishmanial drugs, Cathelin-related antimicrobial peptide (CRAMP)
Introduction
Leishmaniasis, a severe zoonotic infectious disease with a remarkable annual incidence rate, arises in the different parts of the world (690/900 to 1.213/300) (Alvar et al. 2012). This infection disease demonstrated a diverse clinical manifestation including visceral leishmaniasis (VL), mucocutaneous leishmaniasis (MCL) and cutaneous leishmaniasis (CL) (McGwire and Satoskar 2013). It creates huge numbers of social and economic problems in human populations (Okwor and Uzonna 2016; Asadi et al 2020). Different species of sand fly play a crucial role as a carrier in the life cycle of leishmaniasis (Desjeux 1996). CL caused by L. major is a zoonotic cosmopolitan protozoan infection in manycountries such as Iran (Akhoundi et al. 2013, 2016; Le Blancq 1986). However, nowadays there are a lot number of anti-leishmanial drugs with different treatment procedures that are routinely administrated for CL but a well-standardized therapy method yet to be determined. The pentavalent antimonials compounds such as sodium stibogluconate (pentostam) and meglumine antimonate (Glucantime), miltefosine and Amphotericin B (Amp B) are the most prescribed anti-leishmanial drugs (Copeland and Aronson 2015; Soto et al. 2004; Sundar et al. 2004). Which pentavalent antimonials are first-line drugs and are widely prescribed intramuscularly or intravenously (Monzote 2009). Despite their effectiveness, pentavalent antimonials also have some limitations including their toxicity effects and also must be injected under the supervision of a physician (Jolliffe 1985). More importantly, some parasite species are resistant to traditional drugs, and the rate of drug-resistance as one of the important complications was increased remarkably (Croft et al. 2006; Grogl et al. 1992). Amp B widely used in cases of unresponsiveness against the first-line drugs and consider as a second choice for treatment of CL; Whereas this component associated with several dangerous side effects such as nephrotoxicity and negligible solubility (Tripathi et al. 2017) Anti-parasitic drugs especially anti-leishmanial drugs componentswith the new formulation in pharmaceutical filed has been developed during the last decade particularly those agents with natural origins and their properties have been perfectly established against various clinical presentations of leishmaniasis. Nanosciences are fast emerging multidisciplinary in the past decade with high potential to bring novel strategies to different branches of science as nanotechnology, biotechnology, Pharmaceutical Sciences and environmental health (Ghormade et al. 2011; Malakootian et al. 2019, Sahoo et al. 2007).
Nanocomposites and nano-drugs with a safe toxicity profile also are attracting attention as novel components and according to their efficacy proposed alternative that can overcome some of the side effects caused by synthetic and classic drugs. Recently several nano-drugs have been applied for in-vitro or in-vivo assessment against various parasitic infections (Keyhani et al. 2018; Keyhani et al. 2020a, b; Shojaee et al. 2019). Besides, not only an effective and safe drug could remove the above limitations but also should be able to induce and amplify the immunity responses. Among the different proteins that contribute to the innate immunity system, cathelicidins play an exquisite role against pathogens and act as a natural antibiotic in living organisms like other protozoan infection, treatment efficacy of CL depends on series of variables such as geographic location, host’s immune andgenetic diversity of parasite (Tavakoli et al. 2018; Faridi et al. 2020). A similar single gene in mouse and humans are responsible for cathelicidin secretion; consequently, they express one protein. Cathelin related antimicrobial peptide (CRAMP) and Interleukin-37 (IL-37) (mouse and human cathelicidins, respectively) are secreted by some epithelial surfaces or hematopoietic cells (Dorschner et al. 2003; Nizet and Gallo 2003). Cathelicidins affect an extensive board of pathogens such as bacteria, fungi, viruses and parasites directly or indirectly by promoting recruitment of the other immune responses (Cavalcante et al. 2017; Kao et al. 2016; Mello et al. 2017; Vieira-Girao et al. 2017). Here the major aim was to investigate the ant-leishmanial activity of nano amphotericin B, and compare to standard agents and also to determine which anti-leishmanial drug can induce the gene expression of cathelicidin.
Material and methods
Drug preparation
Meglumine antimoniate (MA, Rhone-Poulenc, France) and amphotericin B were purchased from the valid centers. Acetic acid, dimethylsulfoxide, tripolyphosphate were provided from Sigma-Aldrich (MO, USA). The preparation of nano amphotericin was performed based on the standard method previously described. Different concentrations of the mentioned drugs including 12.5, 25, 50 and 100 µM/ml were prepared and their anti-leishmanial activity was measured (Mehrizi et al. 2018).
Preparation of the promastigote stage
To have promastigotes forms in log and stationary phases of L. major standard isolate of previously collected Iranian type (MRHO/IR/75/ER) was selected. At the first step, RPMI 1640 medium that containing 1% pen/strep antibiotics and 10% heated fetal bovine serum (HFBS) from Pastor Institute of Iran prepared; Then standard selected type cultured into the prepared medium and incubate at 22 °C. Finally, after the development of parasite the log and stationary phases are chosen for the next usage.
Preparation of amastigote stage
J744 macrophage cell line (Pasteur institute, Iran) was cultured in DMEM enriched with 10% HFBS and 1% pen/strep antibiotic. Subsequently, the cells incubated at 37 °C in 5% CO2 and humid conditions. Macrophage cells (106/well) were transferred into 24 wells of cell culture plates. The cells were incubated at 37 °C for 6 h and floating cells were removed. The stationary phase of L. major promastigotes (107 parasites/ml) was added to the attached-macrophages and incubated at 37 °C for 3 h. The free promastigotes were removed and the cells were incubated at 37 °C.
Co-incubation of anti-leishmanial drugs with promastigote stage:
The log phase of L. major promastigotes (2 × 102 parasite/ml) was exposed to various concentrations of the studied drugs (Glucantime, Amphotericin B and Nano-amphotericin B) for 72 h. Then inhibitory concentration that inhibited at least 50% of growth (IC50) was calculated using the MTT method as a described study. During this test, control group was promastigotes stage without any treating.
Co-incubation of anti-leishmania drugs with amastigote stage:
Macrophages (2 × 102 per/ml) were transferred into a 24 cell culture plated and incubated at 37° for 6 h. The non-adherent cells were then removed and adherent macrophages were incubated with the stationary phase of L. major promastigotes (2 × 106 parasite/ml) for 3 h to form intracellular amastigotes stage. After incubating, the floating promastigotes were removed and anti-leishmanial drugs were separately added at IC50 concentrations. Finally, smears were prepared from cell sediments after 24 h, stained under the Giemsa staining method. Parasite burden (number of parasites/ macrophage) and infection rate (% infected macrophages) were detected under light microscopy.
Co-incubation of anti_leishmanial drugs with macrophages and Real-time PCR
Macrophages (5 × 103 per/ml) were exposed to various anti-leishmanial drugs at 100 µM/ml concentration for 24 h. The cell sediments were collected from exposed (test) and unexposed cells (control) to anti-leishmanial drugs for the gene expression of cathelicidin gene. Briefly, total RNA was extracted from all harvested cells using RNA Purification kit (Jena Bioscience, Germany) and quantified by a NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE). Three μg was transcribed to complementary DNA (cDNA) using AccuPower®RT PreMix random hexaprimer (Bioneer, Korea). Briefly, 3 µl of RNA was adjusted in 20 µl DEPCI-DW and added to each lyophilized tube. The thermal profile was performed as follows: 12 cycles (20 °C for 30 s, 42 °C for 4 min, 55 °C for 30 s) and 95 °C for 5 min. Quantitative Real-time PCR was carried out in Rotor GENE Q (Qiagen, Germany). The below table demonstrates the specifical primer sequences for two intended genes including B2M and CRAMP. NCBI primer Blast was selected for primer design. B2M-F and B2M-R primers were used to amplify B2M cDNA. In the same way, CRAMP-F and CRAMP-R were applied to amplify CRAMP cDNA. Briefly, The 15 μl of each reaction mixture (1 μl cDNA, 7 μl SYBR Green, 5 μl DW, 1 μl primer forward 2.5 Pmol, 1 μL primer reverse 2.5 Pmol) was prepared using SYBR Premix EX Taq2 Master Mix (Takara, Japan). The thermal profile was performed as follows: 95 °C for 1 min, 40 cycles (95 °C for 15 s, 58 °C for 30 s, 72 °C for 20 s) (Daneshvar et al. 2017, 2018).
| B2M-Forward primer | 5′TTCTGGTGCTTGTCTCACTGA-3' |
| B2M-Reverse reverse | 5′CAGTATGTTCGGCTTCCCATTC-3' |
| CRAMP-Forward primer | 5′GGCTGTGGCGGTCACTAT C-3' |
| CRAMP-Reverse primer | 5′-GTCTAGGGACTGCTGGTTGAA-3' |
Statistical analysis
After data collection, SPSS 17 for Windows (SPSS Inc., Chicago) was used for data analysis. Differences between test and control groups were determined by using t test and P values less than 0.05 were considered to be statistically significant. All experiments and average values were performed in triplicates.
Results
Promastigote assay
The anti-leishmanial activity of used drugs over various concentrations are depicted in Table 1 and Fig. 1. As shown in, all anti-leishmanial drugs potently inhibited the growth of L. major parasites. Glucantime exhibited the most anti-leishmanial activity; Consequently, it had the least IC 50 among the anti-leishmania drugs. The least activity was observed for amphotericin B, which is using now. According to the IC50 level, amphotericin B must be used at a higher concentration due to inhibiting the growth of parasites. As a remarkable response; however, nano amphotericin B irrespectively showed a high level of anti-leishmanial activity compared to that of amphotericin B. The highest mortality rate of amphotericin B and nano-amphotericin B was observed in the concentration of 150 µM/ml.
Table 1.
The anti-leishmanial activity of studied drugs
| Drug | IC50 (µM/ml) |
|---|---|
| Glucantime | 10 |
| Amphotericin B | 40 |
| Nano amphotericin B | 20 |
Fig. 1.
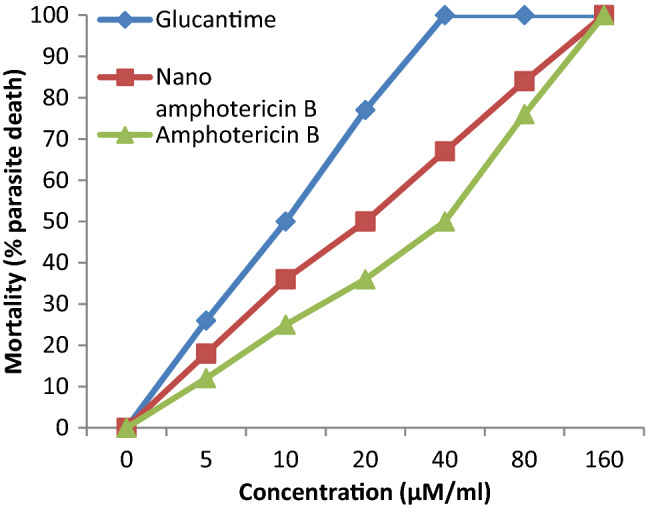
The anti-leishmanial activity of studied drugs
Inhibitory concentration fifty (IC50) was defined as the lowest concentration of anti-leishmanial drugs that inhibited at least 50% of growth of L. major parasites.
Amastigote assay
After the amastigote forms arose into macrophages, they were challenged with anti-leishmanial drugs and incubated for 24 h. At the next step, macrophages were stained for the detection of parasite burden and infection severity was calculated. As shown in Fig. 2a, glucantime had considerably reduced the intracellular amastigotes compared to control groups. The level of parasite burden nano amphotericin B and amphotericin B, respectively. Macrophages treated by glucantime revealed a significant reduction of infection rate (% infected macrophages) among the aforementioned drugs. Nano amphotericin B had a tremendously reduced infection rate compared to amphotericin B and control group. These infection rate reduction nano amphotericin B and amphotericin B, respectively (Fig. 2b). The parasite burden rates of the nano amphotericin B was significant (P < 0.05) for all concentrations of chitosan compared with amphotericin B and also the control group.
Fig. 2.
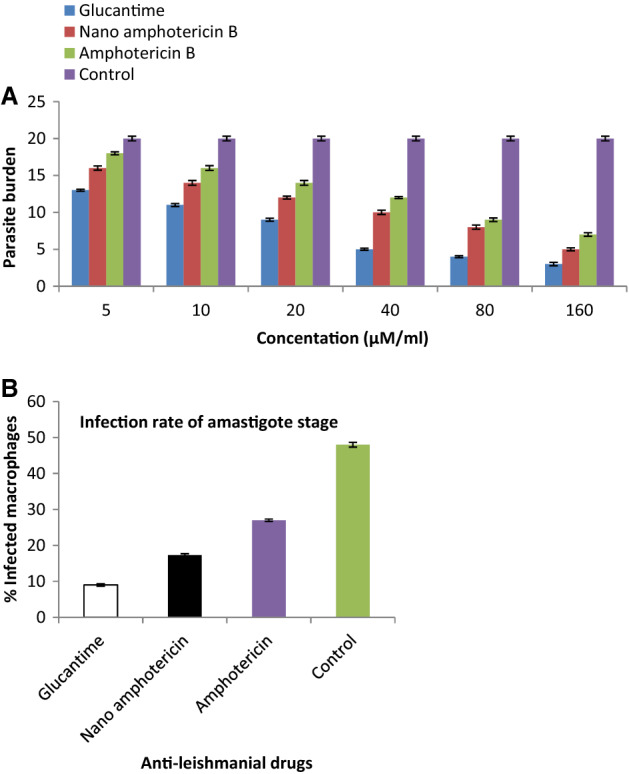
a The effect of different concentration of Nano amphotericin B on parasite burden. b Infection rate of amastigote stage in the test and control groups
Effect of anti-leishmanial drugs on cathelicidin gene expression
Cathelicidins are found in different numbers in living organisms. Although they are secreted first as propeptide, cathelicidins are finally converted to active peptides called cationic peptides. This active form can avoid the establishment of pathogens inside and outside of the body. Due to the essential role of cathelicidins, we aimed to show which anti-leishmanial drugs can induce this system. Among the anti-leishmanial drugs, nano amphotericin B could increase the murine cathelicidin gene expression genes that could be beneficial for immunity response (Fig. 3).
Fig. 3.
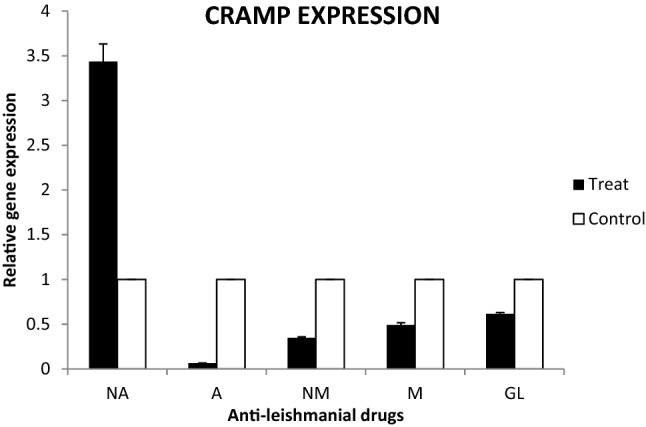
Effect of anti-leishmanial drugs on cathelicidin gene expression
Discussion
Leishmaniasis has been an important parasitic infection during the past decades. After malaria infection, it is a major cause of mortality and morbidity among parasitic infections (Tavakoli et al. 2019). Depending on specious, three clinical forms have been identified. CL talking about is caused by L. major staring with lesions on unprotected area especially face and hands (Alvar et al. 2012). Different kinds of anti-leishmanial drugs are prescribed for treatment. The glucantime and pentostam are two commonly prescribed anti-leishmanial drugs. However, their efficacy has been proved but in most cases associated with toxic and serious side effects. The mechanism of their action is unclear (McGwire and Satoskar 2013). Miltefosine is used to treat some types of cancers but has been exerted for the treatment of some Leishmania species. It inhibits the growth of parasite through the blockage of cytochrome C oxidase enzyme. Amphotericin is a substitute drug wherever first-line drugs failed. It binds to the sterols of plasma membrane of parasites to kill or inhibit them. Although amphotericin B is safer than other drugs, it demonstrated little anti-leishmanial activity. Due to the mentioned complications, recent research has been focused on natural origins components (Tripathi et al. 2017). Even though a different kind of planet extract shave promising results in the in-vitro model, they have not been produced commercially. Moreover, reformulation of old prescribed drugs or novel drugs designing by advanced methods such as nano-drug preparation terminates to promising results in very recent studies (Keyhani et al. 2020b; Jahanbakhsh et al. 2016). Most research has been focused on the production of nano drugs (Mahmoudvand et al. 2019, 2020; Tavakoli Kareshk et al. 2015). Nevertheless, it is progressing right now. Production of different kinds of nano-drugs such as anti-leishmanial drugs has opened a new horizon in future pharmacology. The major aim of the present study was to determine the efficacy of nano-amphotericin B against the amastigote and promastigote forms of L. major parasites. We found that nano-amphotericin B reduces both parasite burden and infection rates tremendously compared to amphotericin B. It has been proven that amphotericin B is more utilized to cure a wide board of fungi organisms, and inhibits them through disrupting membrane integrity and permeability of plasma membrane. It is also effective versus an extensive variety of other organisms such as leishmania parasite. It is a safe drug among the antileishmanial drugs, and its nano-form surprisingly showed a high anti-leishmanial effect than the original form. Additionally, it has been reported that nano form drugs have more potency to induce both innate and adaptive immune responses. Antimicrobial peptides (AMPs), such as cathelicidins and defensins, are small heterogeneous proteins of the innate immune system and sometimes are called natural antibiotics (Kao et al. 2016). Historically, the first AMPs were discovered in 1939. Over 5000 have been determined up to date. According to their structures, AMPs are divided into classes: α-defensins, β-defensins, θ-defensins and cathelicidins. Although few assumptions about the mechanism of antimicrobial activity of AMPs have been proposed up to now, the exact mode of mechanism has yet to be determined (Monzote 2009). Binding and interacting with specific molecules on the cell surface of targeting pathogens could lead to cell lysis through cell rupturing. Some of the AMPs have specific intracellular receptors that this linking could inhibit pathogen development or replication. Due to their important role, the second aim of present study was to show which antimicrobial peptides can synergically induce the cathelicidin pathway. Except for nano amphotericin B, all studied anti-leishmanial drugs down-regulated CRAMP gene expression level. This teamwork is the first report about the effect of nano amphotericin on CRAMPS gene expression. Collectively and according to our findings, it could be concluded that nano amphotericin B has potential to inhibits Leishmania parasites not only through direct effects but also via modulating some effectors such as cathelicidins.
Acknowledgements
We would like to acknowledge all staff from the Leishmania research center in Iran and Department of Medical Parasitology and Mycology, Kerman University of Medical Sciences, Kerman, Iran for their useful assistance.
Author contributions
All authors read and approved the final version of the manuscript.
Funding
No research grants have been considered for this study.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest in this work.
Ethical approval to work on animals
The present study is approved by Ethical Review Board of Kerman University of Medical Sciences (Kerman, Iran) code IR.KMU.REC.1394.208.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akhoundi M, et al. Geographical distribution of Leishmania species of human cutaneous leishmaniasis in Fars province, southern Iran. Iran J Parasitol. 2013;8(1):85. [PMC free article] [PubMed] [Google Scholar]
- Akhoundi M, et al. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoSNegl Trop Dis. 2016;10(3):e0004349. doi: 10.1371/journal.pntd.0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvar J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi A, TavakoliKareshk A, Sharifi I, Firouzeh N. Murine cathelicidin: as a host defensive response against Leishmania major infection. J Parasit Dis. 2020 doi: 10.1007/s12639-020-01238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante CS, et al. Anti-fungal activity of Ctn[15-34], the C-terminal peptide fragment of crotalicidin, a rattlesnake venom gland cathelicidin. J Antibiot. 2017;70(3):231–237. doi: 10.1038/ja.2016.135. [DOI] [PubMed] [Google Scholar]
- Copeland NK, Aronson NE. Leishmaniasis: treatment updates and clinical practice guidelines review. CurrOpin Infect Dis. 2015;28(5):426–437. doi: 10.1097/QCO.0000000000000194. [DOI] [PubMed] [Google Scholar]
- Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. ClinMicrobiol Rev. 2006;19(1):111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshvar H, Sharifi I, Keyhani A, TavakoliKareshk A, Asadi A. Comparative analysis of antimicrobial peptides gene expression in susceptible/resistant mice macrophages to Leishmania major infection. Middle East J Fam Med. 2017;7(10):18. doi: 10.5742/MEWFM.2017.93096. [DOI] [Google Scholar]
- Daneshvar H, TavakoliKareshk A, SharifiI KA, TavakoliOliaee R, Asadi A, et al. Host-parasite responses outcome regulate the expression of antimicrobial peptide genes in the skin of BALB/c and C57BL/6 murine strains following Leishmania major MRHO/ IR/75/ER infection. Iran J Parasitol. 2018;13(4):515–523. [PMC free article] [PubMed] [Google Scholar]
- Desjeux P. Leishmaniasis: public health aspects and control. ClinDermatol. 1996;14(5):417–423. doi: 10.1016/0738-081x(96)00057-0. [DOI] [PubMed] [Google Scholar]
- Dorschner RA, et al. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr Res. 2003;53(4):566–572. doi: 10.1203/01.PDR.0000057205.64451.B7. [DOI] [PubMed] [Google Scholar]
- Faridi A, TavakoliKareshk A, Sadooghian S, et al. Frequency of different genotypes of Giardia duodenalis in slaughtered sheep and goat in east of Iran. J Parasit Dis. 2020;44:618–624. doi: 10.1007/s12639-020-01237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghormade V, Deshpande MV, Paknikar KM. Perspectives for nano-biotechnology enabled protection and nutrition of plants. BiotechnolAdv. 2011;29(6):792–803. doi: 10.1016/j.biotechadv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Grogl M, Thomason TN, Franke ED. Drug resistance in leishmaniasis: its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am J Trop Med Hyg. 1992;47(1):117–126. doi: 10.4269/ajtmh.1992.47.117. [DOI] [PubMed] [Google Scholar]
- Jahanbakhsh S, Azadpour M, TavakoliKareshk A, et al. Zataria multifloraBioss: lethal effects of methanolic extract against protoscoleces of Echinococcus granulosus. J Parasit Dis. 2016;40(4):1289–1292. doi: 10.1007/s12639-015-0670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe D. Nephrotoxicity of pentavalentantimonials. The Lancet. 1985;325(8428):584. doi: 10.1016/S0140-6736(85)91245-0. [DOI] [PubMed] [Google Scholar]
- Kao C, et al. Cathelicidin antimicrobial peptides with reduced activation of toll-like receptor signaling have potent bactericidal activity against Colistin-resistant bacteria. MBio. 2016;7(5):01418–1516. doi: 10.1128/mBio.01418-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyhani A, Mahmoudvand H, Shakibaie M, TavakoliKareshk A, et al. Histopathological and toxicological study of selenium nanoparticles in BALB/C mice. EntomolApplSciLett. 2018;5(1):31–35. [Google Scholar]
- Keyhani A, Ziaal N, Shakibaie M, TavakoliKareshk A, et al. Biogenic selenium nanoparticles target chronic toxoplasmosis with minimal cytotoxicity in a mouse model. J Med Microbiol. 2020;69(1):104–110. doi: 10.1099/jmm.0.001111. [DOI] [PubMed] [Google Scholar]
- Keyhani A, Shakibaie M, Mahmoudvand H, Jahanbakhsh S, TavakoliKareshk A, Shojaee S, Ziaali N. Prophylactic activity of biogenic selenium nanoparticles against chronic Toxoplasma gondii infection. Recent Pat Antiinfect Drug Discov. 2020 doi: 10.2174/1574891X15666200604115001. [DOI] [PubMed] [Google Scholar]
- Le Blancq S, Schnur L, Peters W. Leishmania in the Old World: 1. The geographical and hostal distribution of L. majorzymodemes. Trans R Soc Trop Med Hyg. 1986;80(1):99–112. doi: 10.1016/0035-9203(86)90206-3. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H, TavakoliKareshk A, Moradi M, et al. Efficacy and safety of Zataria multifloraBoiss essential oil against acute toxoplasmosis in mice. Iran J Parasitol. 2020;15(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Mahmoudvand H, Pakravanan M, Aflatoonian M, KhudairKhalaf A, Niazi M, Reza MS, TavakoliKareshk A, Khatami M. Efficacy and safety of Curcuma longa essential oil to inactivate hydatid cyst protoscoleces. BMC Complement Altern Med. 2019;19(1):187. doi: 10.1186/s12906-019-2527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakootian M, Khatami M, Ahmadian M, Asadzadeh SN. Biogenic silver nanoparticles/hydrogen peroxide/ozone: efficient degradation of reactive blue 19. BioNanoScience. 2019;10(5):1–8. [Google Scholar]
- McGwire B, Satoskar A. Leishmaniasis: clinical syndromes and treatment. Qjm Int J Med. 2013;107(1):7–14. doi: 10.1093/qjmed/hct116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrizi T, ShafieeArdestani M, MollaHosein MI, Ramezani A. Novel nano-sized chitosan amphotericin B formulation with considerable improvement against Leishmania major. Nanomedicine. 2018 doi: 10.2217/nnm-2018-0063. [DOI] [PubMed] [Google Scholar]
- Mello CP, et al. Evaluation of the antichagasic activity of batroxicidin, a cathelicidin-related antimicrobial peptide found in Bothrops atrox venom gland. Toxicon. 2017;130:56–62. doi: 10.1016/j.toxicon.2017.02.031. [DOI] [PubMed] [Google Scholar]
- Monzote L. Current treatment of leishmaniasis: a review. Open Antimicrob Agents J. 2009;1:9–19. [Google Scholar]
- Nizet V, Gallo RL. Cathelicidins and innate defense against invasive bacterial infection. Scand J Infect Dis. 2003;35(9):670–676. doi: 10.1080/00365540310015629. [DOI] [PubMed] [Google Scholar]
- Okwor I, Uzonna J. Social and economic burden of human Leishmaniasis. Am J Trop Med Hyg. 2016;94(3):489–493. doi: 10.4269/ajtmh.15-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo SK, Parveen J, Panda J. The present and future of nanotechnology in human health care. Nanomed Nanotech Biol Med. 2007;3(1):20–31. doi: 10.1016/j.nano.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Shojaee S, Firouzeh N, Keshavarz H, et al. Nanosilver colloid inhibits Toxoplasma gondiitachyzoites and bradyzoites in vitro. Iran J Parasitol. 2019;14(3):362–367. [PMC free article] [PubMed] [Google Scholar]
- Soto J, et al. Miltefosine for new world cutaneous leishmaniasis. Clin Infect Dis. 2004;38(9):1266–1272. doi: 10.1086/383321. [DOI] [PubMed] [Google Scholar]
- Sundar S, et al. Amphotericin B treatment for Indian visceral leishmaniasis: conventional versus lipid formulations. Clin Infect Dis. 2004;38(3):377–383. doi: 10.1086/380971. [DOI] [PubMed] [Google Scholar]
- Tavakoli OR, Sharifi I, Afkar A, Jafarzadeh A, TavakoliKareshk A, et al. Differential expression of TLRs 2, 4, 9, iNOS and TNF-a and arginase activity in peripheral blood monocytes from glucantime unresponsive and responsive patients with anthroponotic cutaneous leishmaniasis caused by Leishmania tropica. MicrobPathog. 2019;126:368–378. doi: 10.1016/j.micpath.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Tavakoli OR, Sharifi I, Afkar A, TavakoliKareshk A, Asadi A, et al. Unresponsiveness to meglumineantimoniate in anthroponotic cutaneous leishmaniasis field isolates: analysis of resistance biomarkers by gene expression profiling. Trop Med Int Health. 2018;23(6):622–633. doi: 10.1111/tmi.13062. [DOI] [PubMed] [Google Scholar]
- TavakoliKareshk A, et al. Efficacy of the Bunium persicum (Boiss) essential oil against acute toxoplasmosis in mice model. Iran J Parasitol. 2015;10(4):625–631. [PMC free article] [PubMed] [Google Scholar]
- Tripathi P, Kumar Jaiswal A, Dube A, Ranjan Mishra P. Hexadecylphosphocholine (Miltefosine) stabilized chitosan modified Ampholipospheres as prototype co-delivery vehicle for enhanced killing of L. donovani. Int J BiolMacromol. 2017;105(Pt 1):625–637. doi: 10.1016/j.ijbiomac.2017.07.076. [DOI] [PubMed] [Google Scholar]
- Vieira-Girao PR, et al. Antiviral activity of Ctn[15-34], a cathelicidin-derived Eicosapeptide, against infectious myonecrosis virus in Litopenaeus vannamei primary hemocyte cultures. Food Environ Virol. 2017;16(10):017–9285. doi: 10.1007/s12560-017-9285-5. [DOI] [PubMed] [Google Scholar]


