Abstract
Background
More patients with ovarian cancer are being treated with poly (ADP-ribose) polymerase (PARP) inhibitors as regulatory agencies have granted these drugs new approvals for a variety of treatment indications. However, PARP inhibitors are expensive. When given as a maintenance therapy, these drugs may be administered for months or years. How much of this cost patients experience as out-of-pocket spending is unknown.
Objectives
To estimate the out-of-pocket spending patients experience during PARP inhibitor treatment, and to characterize which health care services account for that spending.
Study Design
A retrospective cohort study was performed with a sample of ovarian cancer patients treated in 2014–2017 with olaparib, niraparib, or rucaparib. Patients were identified from MarketScan, a health insurance claims database. All insurance claims during PARP inhibitor treatment were collected. The primary outcome was patients’ out-of-pocket spending (copayment, coinsurance, and deductibles) during PARP inhibitor treatment for the medication itself. Other outcomes of interest included out-of-pocket spending for other health care services, the types and frequency of other health care services used, health plan spending, the estimated proportion of patients’ household income used each month for health care, and patients’ out-of-pocket spending immediately prior to PARP inhibitor treatment.
Results
We identified 503 ovarian cancer patients with a median age of 55 years (IQR 50–62); 83% had out-of-pocket spending during PARP inhibitor treatment. Median treatment duration was 124 days (IQR 66–240). Mean out-of-pocket spending for PARP inhibitors was $305 (SD $2,275) per month. On average, this accounted for 44.8% (SD 34.8%) of patients’ overall monthly out-of-pocket spending. Mean out-of-pocket spending for other health care services was $165 (SD $769) per month. Health plans spent, on average, $12,661 (SD $15,668) per month for PARP inhibitors and $7,108 (SD $15,254) per month for all other health care services. Cost-sharing for office visits, laboratory tests, and imaging studies represented the majority of non-PARP out-of-pocket spending. The average amount patients paid for all health care services per month during PARP inhibitor treatment was $470 (SD $2,407), which was estimated to be 8.7% of patients’ monthly household income. Mean out-of-pocket spending in the 12 months prior to PARP inhibitor treatment was $3,110 (SD $6,987).
Conclusions
Patients can face high out-of-pocket costs for PARP inhibitors, although the sum of cost-sharing for other healthcare services used during PARP inhibitor treatment is often higher. Spending on health care consumes a large proportion of these patients’ household income. Ovarian cancer patients experience high out-of-pocket costs for health care, both before and during PARP inhibitor treatment.
Keywords: Ovarian cancer, PARP inhibitor, Financial toxicity, Gynecologic cancer, Out-of-pocket costs, Financial burden, Health care costs, Financial hardship, Costs of care, Health services research
Condensation
Patients with ovarian cancer may experience burdensome out-of-pocket costs during PARP inhibitor treatment due to medication-related cost-sharing as copayments for other health care services.
INTRODUCTION
Since 2014, the poly (ADP-ribose) polymerase (PARP) inhibitors olaparib, rucaparib, and niraparib together have been granted nine indications for use in patients with ovarian cancer. The clinical trials supporting these regulatory approvals show the drugs’ substantial clinical benefit, particularly among patients with a BRCA-mutated or homologous recombination (HR)-deficient tumor.1–10 However, these new therapies are expensive. A PARP inhibitor’s “sticker price” can be as high as $16,999 for a month of treatment.11 Past analyses found the drugs’ cost to be an obstacle to PARP inhibitors being considered as a cost-effective treatment in ovarian cancer.12–15
The cost-sharing experienced by patients for PARP inhibitors is unknown. Whatever this amount is, it is in addition to other out-of-pocket spending for health care that is incurred during cancer treatment. With more patients receiving PARP inhibitor treatment and maintenance strategies potentially lasting for years,1,9,10 patients’ risk of experiencing financial hardship from the cumulative cost of treatment is high. In other cancer types, treatment-related financial burdens have been associated with diminished quality of life and coping strategies including nonadherence to medication, delayed or missed clinic visits, refusal of recommended testing, and reduced spending on non–health care necessities.16,17 In patients with gynecologic cancers, psychologic distress related to the costs of cancer care is common, as is the use of coping strategies.18,19 Our goal with this study was to evaluate the spending that patients and their insurers incurred during treatment with PARP inhibitors.
MATERIALS AND METHODS
We performed an observational retrospective cohort study using the Truven Health MarketScan Commercial Claims and Encounters Database. MarketScan is created from paid and adjudicated health insurance claims and includes deidentified information from a nationwide sample of commercially insured individuals (See Supplemental Appendix for more information about MarketScan). We included all individuals with ovarian cancer who had at least one prescription drug claim for olaparib, niraparib, or rucaparib between January 1, 2014 (the year in which the first PARP inhibitor was approved for use in ovarian cancer) and December 31, 2017 (the most recent year of data available in MarketScan to the investigators). Using a validated approach,20 we identified individuals with ovarian cancer who had at least one inpatient or at least two outpatient insurance claims associated with an ovarian cancer diagnosis code (ICD-9: 183.0, 183.2, 183.8; ICD-10: C56) (Supplemental Figure 1 for cohort selection). We used the National Drug Codes for olaparib, rucaparib, and niraparib to identify individuals treated with PARP inhibitors (Supplemental Table 1). To determine their out-of-pocket costs and health care use, we included all insurance claims that occurred during PARP inhibitor treatment, which we defined as starting the first day a PARP inhibitor prescription was filled until the final prescription fill plus the number of days supplied with the final prescription.
Our primary outcome of interest was patients’ out-of-pocket spending per month for the PARP inhibitor itself. Secondary outcomes of interest included the proportion of patients who had no out-of-pocket spending during PARP inhibitor treatment, patients’ out-of-pocket spending for all other health care services, the proportion of overall out-of-pocket spending accounted for by cost-sharing for PARP inhibitors, total spending (i.e., by the patient and insurer), the types and frequency of other health care services that were used during PARP inhibitor treatment, out-of-pocket spending in the year before starting PARP inhibitor treatment, and the estimated proportion of household income used each month for health care. We adjusted dollar amounts to 2018 values using the Consumer Price Index.
We calculated out-of-pocket spending as the sum of all cost-sharing amounts by the patient including copayments, deductibles, and coinsurance. Patients’ out-of-pocket spending was divided into two categories: cost-sharing for filling the PARP inhibitor prescription itself and cost-sharing for any other health care service. To determine whether the proportion of patients’ out-of-pocket spending that was accounted for by prescription cost-sharing varied by the total amount patients spent each month, we categorized patients by total out-of-pocket spending ($0-$25, $25-$50, $50-$75, $75-$100, $100-$500, and >$500) and compared the composition of spending among these groups. If a patient had spending equal to the cutoff between two categories, that individual was grouped into the lower of the two cost categories. Total spending, for the PARP inhibitor or for other health care services, was the sum of the patient’s out-of-pocket spending added to the reimbursement paid by the patient’s health insurance plan. We estimated the financial burden of treatment as a percentage of monthly household income that was spent on health care by dividing a patient’s overall monthly out-of-pocket spending by monthly household income. This estimate was averaged for each patient’s duration of PARP inhibitor treatment. Because MarketScan does not capture income data, we used median estimates of households income from the United States Census Bureau21 for each patients’ metropolitan statistical area – a local area geographic variable included in our data source.
After observing that some patients had no out-of-pocket spending during PARP inhibitor treatment, we suspected that this may be explained by patients reaching their health plan’s out-of-pocket maximum before treatment. We evaluated this hypothesis by examining patients’ out-of-pocket spending in the year preceding treatment and collected all insurance claims available for the year leading up to the index date. Patients were categorized by whether they had out-of-pocket spending during PARP inhibitor treatment (i.e., any amount of spending vs. none). We compared these groups’ out-of-pocket spending before starting PARP inhibitor treatment. Using the MarketScan Benefit Plan Design supplementary dataset, we also determined the proportion of the cohort that had reached their out-of-pocket maximum in the insurance plan period preceding the one during which they started PARP inhibitor treatment. Finally, we recalculated the outcome measures described above after excluding patients who had no out-of-pocket spending during treatment.
Cost data were analyzed descriptively. We performed group comparisons by using either a Wilcoxon rank-sum test or Kruskal-Wallis test depending on the number of groups being compared and the distribution of the data. This investigation was approved by our Institutional Review Board.
RESULTS
In MarketScan, we identified 503 patients with ovarian cancer with a median age of 55 years (IQR 50–62) who had started treatment with a PARP inhibitor during the study period (Table 1). Patients’ median duration of treatment with a PARP inhibitor was 124 days (IQR 66–240 days). A total of 3,326 patient-months of PARP inhibitor treatment was observed. Consistent with its date of approval, the majority of patients (292/503; 58.1%) were treated with olaparib, and these patients contributed 75.2% of the observed patient-months of treatment. Most patients had health insurance with a preferred provider organization-type plan design (268/503; 53.3%); 91 patients (18.1%) were covered by high-deductible health plans; 106 patients (21.1%) had hospital admissions during PARP inhibitor treatment; and 86 patients (17.1%) had no observed out-of-pocket spending during PARP inhibitor treatment.
TABLE 1.
Patient characteristics (n=503)
| Characteristic | No. (%) of Patients |
|---|---|
| Age, median (IQR), y | 55 (50–62) |
| Insurance type | |
| Preferred provider organization | 268 (53.2%) |
| Health maintenance organization | 51 (10.1%) |
| Comprehensive plan | 46 (9.1%) |
| Other1 | 47 (9.3%) |
| High-deductible health plan | 91 (18.1%) |
| Year of first PARP prescription | |
| 2015 | 122 (24.2%) |
| 2016 | 91 (18.1%) |
| 2017 | 289 (57.5%) |
| Region | |
| Northeast | 113 (22.5%) |
| North central | 109 (21.7%) |
| South | 188 (37.4%) |
| West | 92 (18.3%) |
| PARP inhibitor2 | |
| Olaparib | 292 (58.1%) |
| Niraparib | 158 (31.4%) |
| Rucaparib | 80 (15.9%) |
| Out-of-pocket spending during PARP inhibitor treatment | 417 (82.9%) |
Other includes: basic/major medical, exclusive provider organization, non-capitated/capitated/partially-capitated point-of-service, and consumer-directed type health plans.
Sum of percentages exceeds 100% because some patients were treated with >1 PARP inhibitor.
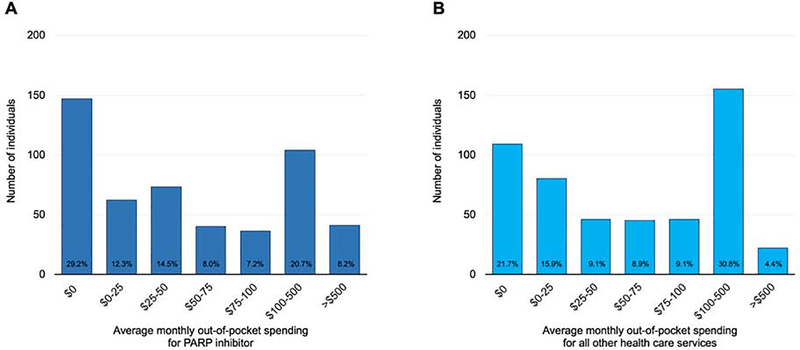
Mean and median out-of-pocket spending for PARP inhibitors was $305 (SD $2,275) and $39 (IQR $0–118) per month, respectively (Table 2) (Figure 1A). Excluding patients who had no out-of-pocket spending during PARP inhibitor treatment, patients’ monthly mean and median spending for their PARP inhibitor was $368 (SD $2,494) and $55 (IQR $17-$141), respectively. 8.2% (41/503) of patients paid, on average, more than $500 per month for their PARP inhibitor. The average amount that patients paid out-of-pocket for all health care services was $470 (SD $2,407). Compared with patients covered by a conventional health plan, monthly out-of-pocket spending for PARP inhibitors by patients covered by high-deductible health plans was not significantly different (median out-of-pocket spending, $39 and $41, respectively; p=.68).
TABLE 2.
Estimates of spending by patients and insurers for PARP inhibitors and other health care service (n=503)
| Spending for PARP Inhibitor per month | Monthly spending for other health care services (n=503) | Monthly overall spending (n=503) | ||||
|---|---|---|---|---|---|---|
| Any PARP Inhibitor (n=503) | Olaparib (n=292) | Rucaparib (n=80) | Niraparib (n=158) | |||
| Out-of-pocket, mean (SD) | ||||||
| All patients | $305 ($2,275) |
$354 ($2,889) |
$157 ($468) |
$237 ($979) |
$165 ($769) |
$470 (2407) |
| Patients with >$0 out-of-pocket spending1 | $368 ($2,494) |
$396 ($3,054) |
$194 ($513) |
$322 ($1,132) |
$199 ($840) |
$567 ($2,634) |
| Out-of-pocket, median (IQR) | ||||||
| All patients | $39 ($0–118) |
$45 ($5–136) |
$24 ($0–76) |
$16 ($0–79) |
$59 ($3–140) |
$125 ($46–283) |
| Patients with >$0 out-of-pocket spending1 | $55 ($17–141) |
$59 ($18–150) |
$38 ($4–88) |
$44 ($13–111) |
$80 ($26–165) |
$162 ($90–328) |
| Third-party, mean (SD) | $12,661 ($15,668) |
$12,350 ($20,151) |
$11,593 ($4,850) |
$11,611 ($6,184) |
$7,108 ($15,254) |
$19,769 ($21,767) |
| Third-party, median (IQR) | $12,449 (9,730–14,047) |
$11,941 ($9,195–13,323) |
$13,498 ($8,645–14,659) |
$11,561 ($8,651–14,508) |
$2,321 ($848–7,140) |
$15,040 ($12,480–19,880) |
| Total, mean (SD) | $12,966 ($17,734) |
$12,705 ($22,934) |
$11,751 ($4,873) |
$11,848 ($6,129) |
$7,274 ($15,406) |
$20,239 ($23,354) |
| Total, median (IQR) | $12,718 ($9,935–14,223) |
$12,144 ($9,368–13,381) |
$13,670 ($8,645–14,778) |
$12,165 ($8,814–14,603) |
$2,583 ($926–7,318) |
$15,307 ($12,944–20,085) |
Values in table are in 2018 dollar amounts.
417 patients (82.9%) had >$0 out-of-pocket spending.
FIGURE 1. Average monthly out-of-pocket spending for PARP inhibitors and all other health care services.
Average monthly out-of-pocket spending for PARP inhibitor (Part A) and all other health care services (Part B) (n=503). The height of column indicates the number of individuals within a given amount of spending as categorized on the x-axis. The percentage at the base of the column indicates the proportion of the total cohort within the spending category. If a patient had spending equal to the cutoff between two categories, that individual was grouped into the lower of the two categories.
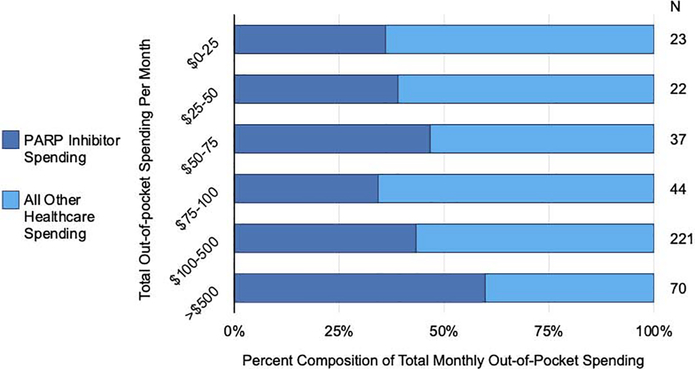
Cost-sharing for PARP inhibitors accounted for an average of 44.8% (SD 34.8%) of patients’ overall out-of-pocket spending (Figure 2). Categorized by their amount of total monthly out-of-pocket spending, cost-sharing for the PARP inhibitor varied as a proportion of patients’ total out-of-pocket spending (p<.001) (Figure 2). For patients with total expenditures exceeding $500 per month, PARP inhibitor spending accounted for more than half of their monthly spending on average.
FIGURE 2. Percent composition of total monthly out-of-pocket spending.
Composition of out-of-pocket spending categorized as cost-sharing for PARP inhibitors and for all other health care services (n=417), with patients categorized by total amount of spending per month (left vertical axis). The horizontal axis indicates the percentage of spending made up of cost-sharing for PARP inhibitors (blue) and for all other health care services (yellow). The number of patients in a given category of total spending per month is indicated on the right. If a patient had spending equal to the cutoff between two categories, that individual was grouped into the lower of the two categories. Patients who did not have out-of-pocket spending during PARP inhibitor treatment are excluded.
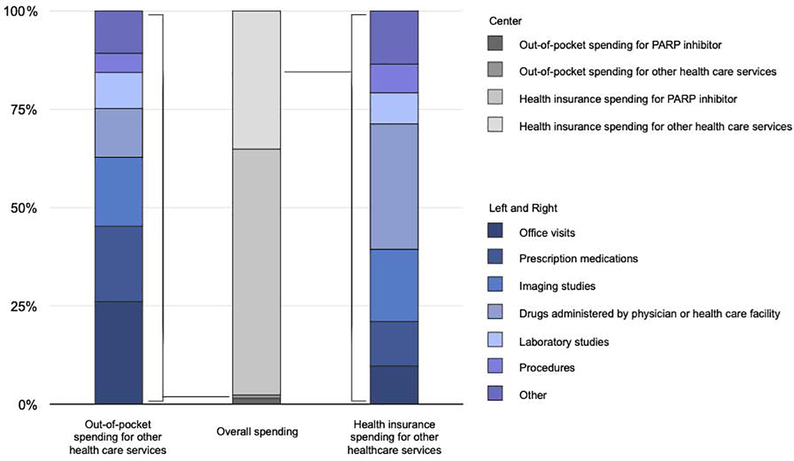
The average monthly out-of-pocket spending for health care services other than the PARP inhibitor was $165 (SD $769) (Table 2) (Figure 1B). Excluding patients who had no out-of-pocket spending during PARP inhibitor treatment, average out-of-pocket spending for other health care services was $199 ($840). Among non-pharmacy drug claims, laboratory studies (percentage of all claims, 34.6%), office visits (15.2%), drugs administered by a physician or health care facility (14.5%), and imaging (7.8%) were the most common types of insurance claims seen. In terms of amount of spending, cost-sharing for office visits (percentage of non-PARP out-of-pocket spending, 26.1%) prescription medications other than PARP inhibitors (19.2%), and imaging studies (17.5%) together accounted for the majority of patients’ non-PARP spending (Figure 3).
FIGURE 3. Composition of spending on other health care services by patients and insurers.
The center column shows spending by the insurer (lighter grey shades) and the patient (darker grey shades). The columns on the sides show the categorized spending of the patient (left) and insurer (right). “Prescription medications” includes all drugs covered by the patient’s prescription benefits other than the PARP inhibitor. “Other” includes home health services, physical and occupational therapy, phlebotomy charges, radiation oncology treatment, transfusion of blood products, emergency department care, non-radiologic diagnostic testing, and material supplies.
Mean and median monthly total spending (i.e., by patient and insurer) for PARP inhibitors was $12,966 (SD $17,734) and $12,718 (IQR $9,935–14,223), respectively (Table 2). The average total amount spent for all health care services by the patient and insurer was $20,239 (SD 23,354) per month of PARP inhibitor treatment. For the 106 patients who were hospitalized during PARP inhibitor treatment, the mean and median total spending for these hospitalizations was $26,894 (SD $25,835) and $17,152 (IQR $11,335-$34,150), respectively.
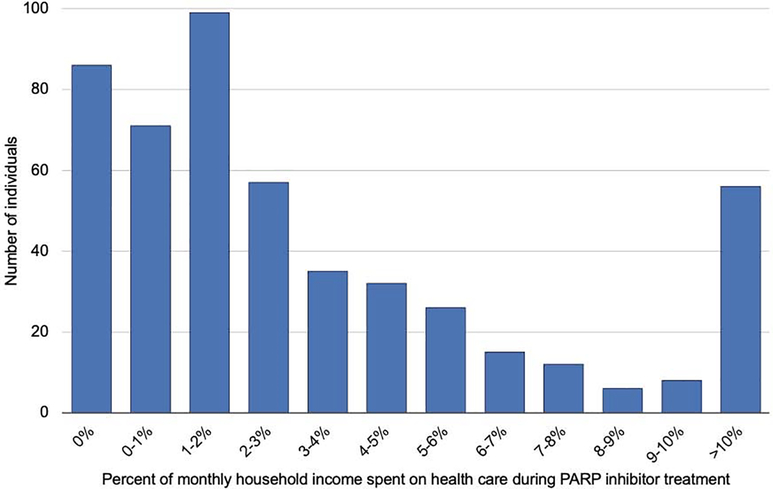
On average, patients spent 8.7% of their total household income per month on health care during PARP inhibitor treatment (Figure 4). Of the 503 study patients, 56 (11.1%) patients had health care spending that exceeded 10% of their estimated monthly household income. Excluding patients who had no out-of-pocket spending during PARP inhibitor treatment, the average percentage of household income spent on health care per month was 10.4%.
FIGURE 4. Distribution of household income spent per month on health care during PARP inhibitor treatment.
Distribution of average household income spent per month on health care during PARP inhibitor treatment (n=503). Along the x-axis, patients are grouped from 0% to 10% in 1% increments. The y-axis indicates the number of patients in that group. The right-most bar includes all patients who spent on average >10% of household income per month on health care. If a patient had spending equal to the cutoff between two categories, that individual was grouped into the lower of the two categories.
Although 17.1% of patients (86/503) did not have out-of-pocket spending during PARP inhibitor treatment, all 86 patients had out-of-pocket costs during the 12 months before their first PARP prescription. Those patients who had no out-of-pocket spending during treatment had higher average out-of-pocket spending in the 12 months preceding their first PARP inhibitor prescription than did patients who did have cost-sharing during treatment ($5,212 vs. $2,676, p<.001); 33.2% of patients had met their out-of-pocket maximum in the insurance plan period preceding the period during which they initiated PARP inhibitor treatment.
COMMENT
Principal Findings
Among a sample of patients with ovarian cancer who were commercially insured and receiving PARP inhibitor treatment, we found patients’ mean out-of-pocket spending each month for these agents was $305. On average, the cost-sharing for these drugs accounted for just less than half of patients’ total monthly health care spending. Cost-sharing for office visits, other prescription medications, and imaging studies accounted for the majority of non-PARP out-of-pocket costs. We estimated that 8.7% of household income, on average, was spent per month on health care during PARP inhibitor treatment.
Results in Context
The FDA has granted PARP inhibitors nine regulatory approvals for use in ovarian cancer’s disease course.22–24 Additional uses have been endorsed by NCCN guidelines.25 These varying indications and recommendations have implications for the expected duration of therapy and patients’ cumulative out-of-pocket spending. A limitation of our data source is that we did not know the precise indication for which a patient was prescribed a PARP inhibitor. Assuming patients were not treated off-label, most of the patients in the first two years of the study received olaparib, likely as monotherapy for recurrent disease. Beginning in 2017, patients may have also been prescribed PARP inhibitors as maintenance therapy after treatment of recurrent disease. For patients in this study, the average treatment length was six months, a duration similar to that seen in early trials evaluating PARP efficacy in patients with recurrent ovarian cancer.8 Therefore, our observations may be most representative of ovarian cancer patients who have experienced one or more recurrences and may be relatively advanced in their disease course.
Although the median estimate for patients’ monthly PARP inhibitor spending was $39, monthly PARP prescription cost-sharing that exceeded $100 was observed in 3 of every 10 patients. As is often the case in health care cost studies, patients’ costs were highly variable and right-skewed, as the differences in mean and median estimates show. Almost 10% of patients had average drug-associated cost-sharing that exceeded $500 per month. For other targeted oral anticancer medications, this amount of out-of-pocket spending has been associated with a more than four-fold increased odds of prescription abandonment.17 Given the PARP inhibitors’ efficacy in treating BRCA-mutated or HR-deficient ovarian cancer, the drug’s cost may be an obstacle to patients receiving these effective and recommended therapies.25 These findings may generalize to frontline ovarian cancer or other cancers types, as the treatment indication (e.g., maintenance vs. monotherapy) may be not affect patients’ cost-sharing amount when filling a PARP inhibitor prescription.
Despite the high cost-sharing observed for PARP inhibitors, this spending accounted for slightly less than half of patients’ monthly out-of-pocket spending overall. The mean non-PARP-related out-of-pocket cost was $165 per month. For many patients, the cost-sharing from their PARP inhibitor prescription fill was likely their single highest cost item, but the spending on other health care services combined was often more. Offices visits, imaging studies, other prescription medications, and laboratory tests together accounted for nearly three-quarters of patients’ non-PARP-related spending. Treatment monitoring and management of toxicities associated with PARP inhibitors contributed to some portion of these services. We are unable to measure patients’ treatment-related costs that are not captured in health insurance claims, such as transportation or housing, so the actual total that patients experience may be higher. Although drug-related cost-sharing offers an attractive single target for policy interventions,26 the reality patients face is that out-of-pocket costs accrue from many sources. These findings suggest that changes in care delivery, such as telehealth visits, remote monitoring of laboratory tests, and avoiding low-value imaging studies may be strategies that could reduce financial burdens for patients receiving PARP inhibitor treatment. Many patients are interested in discussing the costs of care with their oncologist.27 As an initial step that does not require practice patterns to change, clinicians can simply ask if their patients are experiencing any treatment-related financial concerns.
Although the main focus of our investigation was patients’ spending during treatment, we also explored out-of-pocket spending occurring before PARP inhibitor initiation. In the year before patients started treatment, we observed average annual spending of $3,100, an amount that exceeds estimates for out-of-pocket spending for frontline ovarian cancer treatment28,29 and places these patients above the top 5th percentile for out-of-pocket spending on medical care.30 We also found that one in three ovarian cancer patients had reached their out-of-pocket limit in the plan year immediately before the one in which they started a PARP inhibitor. Additionally, those patients who had no cost-sharing during PARP inhibitor treatment often had high out-of-pocket spending in the months prior. While it is possible some of these patients had generous health insurance plans, this finding more likely suggests they had already met their annual out-of-pocket cap before starting a PARP inhibitor. Consistent with other investigations in gynecologic cancer, these findings suggest that patients with ovarian cancer already have persistently high treatment-related financial burdens that continue after starting PARP inhibitor treatment.18,19
The average total amount spent out-of-pocket per month of treatment was $470. Four in 10 American households would have difficulty in covering such an expense if it occurred once,31 and many fewer if this expense occurred month after month. We estimated that an average of 8.7% of household income was spent on these patients’ health care during PARP inhibitor treatment. Similar to other oncology cost studies using MarketScan, we used local area estimates of median household income, since this database does not collect or provide income data 32. It is unclear whether or how this estimate would change if using these patients’ real income. Despite this limitation, the use of median income estimates may be conservative, given the negative effect of cancer on household income.33 In addition, because we do not include out-of-pocket spending for other household members in our calculation, we likely underestimate the true percentage of household income used each month for medical care. The real estimate may be much higher.
In this analysis, we observed numerical differences among the three drugs in the PARP-related out-of-pocket costs. We would interpret this finding with caution, and we deliberately performed no hypothesis testing to compare the out-of-pocket cost estimates for the various PARP inhibitors.
Strengths and Limitations
Constraints from the study data source created limitations in addition to those already discussed. First, our data source can at best be considered a convenience sample of commercially insured patients in the United States and not representative of the entire population. The findings may not generalize to persons covered under a publicly funded health insurance program, to those who are uninsured, to those who are covered by a Marketplace health plan, or to those who have employer-sponsored insurance from a smaller business. Second, we did not attempt to separate cancer-related out-of-pocket spending from that which may be related to other health problems. Third, we do not know what cancer or non–cancer-related care these patients may not have received because of cost. Fourth, we do not attempt to compare these patients with a similar group of ovarian cancer patients who did not receive PARP inhibitors. Fifth, we were unable to assess the extent to which patient assistance programs may have defrayed out-of-pocket costs for some patients. These programs can make expensive medications more accessible for more patients, but can also increase demand by making patients less price-sensitive to a drug’s cost, driving up spending by insurers.34 Sixth, while we present costs to patients and the financial burdens to households, these observations are not linked to any clinical or patient-reported endpoints. We do not know how these costs affected patients’ quality of life or adherence to recommended cancer care. We cannot make conclusions about the financial toxicity of PARP inhibitor treatment without linking the costs observed to a patient-centered outcome. Finally, as stated previously, we are uncertain how these findings apply to patients receiving PARP inhibitor maintenance after primary ovarian cancer treatment or to patients treated with a PARP inhibitor for other types of cancer.
Conclusions
This study finds cost-sharing to be high among ovarian cancer patients treated with PARP inhibitors. Although spending for PARP inhibitors may be the largest contributor to out-of-pocket costs for many patients, on average it makes up less than half of their total health care spending per month. As PARP inhibitors become more widely used in ovarian cancer treatment and are introduced in other diseases, clinicians must be aware that some patients experience considerable financial burdens during treatment. How costs experienced by patients during PARP inhibitor treatment affect adherence and oncologic outcomes is unknown.
Supplementary Material
Highlights.
Ovarian cancer patients can experience high out-of-pocket costs during PARP inhibitor treatment.
Cost-sharing for health care services can consume a large amount of monthly household income.
Mean and median out-of-pocket spending for PARP inhibitors was $305 and $39 per month, respectively.
Cost-sharing for office visits, other prescription medications, and imaging studies account for most non-PARP out-of-pocket spending.
AJOG at a Glance.
A. Why was the study conducted?
The clinical use of poly(ADP-ribose) polymerase (PARP) inhibitor therapy in the management of ovarian cancer is expanding. Like other new anticancer drugs, PARP inhibitors are costly, but how much of that cost patients experience as out-of-pocket spending is unclear.
B. What are the key findings?
In this observational cohort study using insurance claims, copayments for PARP inhibitors were, on average, $305 per month. Cost-sharing for these medications accounted for 45% of an average patient’s total out-of-pocket spending during each month of PARP inhibitor treatment.
C. What does this study add to what is already known?
Prior research found the median amount that patients spend out-of-pocket is approximately $3000 during frontline treatment of ovarian cancer. This investigation finds that high levels of cost-sharing often persist during subsequent lines of therapy.
Acknowledgements
For editing during the preparation of this manuscript, the authors acknowledge and are grateful to Tamara K. Locke from Scientific Publications, Research Medical Library, at the University of Texas MD Anderson Cancer Center, Houston, TX. Ms. Locke has agreed to this acknowledgement.
Funding/Support
This research was supported in part by grant 5T32CA101642 from the National Cancer Institute (supports the training of Dr. Harrison; PI, Dr. Karen H. Lu; National Cancer Institute grant K07CA201013 (Dr. Meyer); CPRIT Grant RP160674 and Komen SAC150061 (Dr Giordano); and National Cancer Institute Cancer Center Support grant P30CA016672.
Role of the Funder/Sponsor
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Disclosures
Larissa Meyer and Charlotte Sun report research funding from AstraZeneca for unrelated research. The remaining authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284. [DOI] [PubMed] [Google Scholar]
- 3.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med. 2016;375(22):2154–2164. [DOI] [PubMed] [Google Scholar]
- 4.Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18(1):75–87. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore KN, Secord AA, Geller MA, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. The Lancet Oncology. 2019. [DOI] [PubMed] [Google Scholar]
- 7.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–1392. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. 2019;381(25):2416–2428. [DOI] [PubMed] [Google Scholar]
- 10.González-Martín A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. New England Journal of Medicine. 2019. [DOI] [PubMed] [Google Scholar]
- 11.“Olaparib”. IBM Watson Health; 2020. https://www.micromedexsolutions.com/micromedex2/librarian/CS/C6248B/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/6F963F/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/redbook.ShowProductSearchResults?SearchTerm=olaparib&searchType=redbookGenericName&searchTermId=931320&searchContent=REDBOOK&searchFilterAD=filterADActive&searchFilterRepackager=filterExcludeRepackager&searchPattern=%5Eolaparib. Accessed 2020.
- 12.Dottino JA, Moss HA, Lu KH, Secord AA, Havrilesky LJ. U.S. Food and Drug Administration-Approved Poly (ADP-Ribose) Polymerase Inhibitor Maintenance Therapy for Recurrent Ovarian Cancer: A Cost-Effectiveness Analysis. Obstet Gynecol. 2019;133(4):795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong L, Tran AT, Tomasino T, Nugent E, Smith JA. Cost-Effectiveness of Niraparib and Olaparib as Maintenance Therapy for Patients with Platinum-Sensitive Recurrent Ovarian Cancer. J Manag Care Spec Pharm. 2018;24(12):1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith HJ, Walters Haygood CL, Arend RC, Leath CA 3rd, Straughn JM Jr. PARP inhibitor maintenance therapy for patients with platinum-sensitive recurrent ovarian cancer: a cost-effectiveness analysis. Gynecol Oncol. 2015;139(1):59–62. [DOI] [PubMed] [Google Scholar]
- 15.Liu AY, Cohen JG, Walsh C, Holschneider CH, Sinno AK. A cost-effectiveness analysis of three PARP inhibitors for maintenance therapy in platinum-sensitive recurrent ovarian cancer. Gynecologic Oncology. 2018;149:9. [Google Scholar]
- 16.Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial Hardships Experienced by Cancer Survivors: A Systematic Review. J Natl Cancer Inst. 2017;109(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Streeter SB, Schwartzberg L, Husain N, Johnsrud M. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. J Oncol Pract. 2011;7(3 Suppl):46s–51s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang MI, Pisu M, Summerlin SS, et al. Extensive financial hardship among gynecologic cancer patients starting a new line of therapy. Gynecol Oncol. 2020;156(2):271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouberhan S, Shea M, Kennedy A, et al. Financial toxicity in gynecologic oncology. Gynecol Oncol. 2019;154(1):8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper GS, Yuan Z, Stange KC, Dennis LK, Amini SB, Rimm AA. The sensitivity of Medicare claims data for case ascertainment of six common cancers. Med Care. 1999;37(5):436–444. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Census Bureau. Explore Census Data. U.S. Census Bureau. https://data.census.gov/cedsci/. Accessed 3/1/2020. [Google Scholar]
- 22.U.S. Food & Drug Administration. Lynparza Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208558Orig1s010lblrpl.pdf. Updated 12/2019. Accessed 5/2020.
- 23.U.S. Food & Drug Administration. Rubraca Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209115s003lbl.pdf. Updated 04/2018. Accessed 5/2020.
- 24.U.S. Food & Drug Administration. Zejula Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208447s015s017lbledt.pdf. Updated 4/2020. Accessed 5/2020.
- 25.National Comprehensive Cancer Network. Ovarian Cancer, Version 1.2020. https://www.nccn.org/professionals/physician_gls/pdf/ovarian_blocks.pdf. Published 2020. Updated 3/11/2020. Accessed 5/2020.
- 26.Green AK, Ohn JA, Bach PB. Review of Current Policy Strategies to Reduce US Cancer Drug Costs. J Clin Oncol. 2020;38(4):372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zafar SY, Chino F, Ubel PA, et al. The utility of cost discussions between patients with cancer and oncologists. Am J Manag Care. 2015;21(9):607–615. [PubMed] [Google Scholar]
- 28.Bercow AS, Chen L, Chatterjee S, et al. Cost of Care for the Initial Management of Ovarian Cancer. Obstet Gynecol. 2017;130(6):1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suidan RS, He W, Sun CC, et al. Total and out-of-pocket costs of different primary management strategies in ovarian cancer. Am J Obstet Gynecol. 2019;221(2):136 e131–136 e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamin Glied SAZ. Catastrophic Out-of-Pocket Health Care Costs: A Problem Mainly for Middle-Income Americans with Employer Coverage. Commonwealth Fund. https://www.commonwealthfund.org/publications/issue-briefs/2020/apr/catastrophicout-of-pocket-costs-problem-middle-income. Updated 4/17/2020. Accessed 5/2020.
- 31.Board of Governors of the Federal Reserve System. Report on the Economic Well-Being of U.S. Households in 2018. Federal Reserve Board;2019. [Google Scholar]
- 32.Pan HY, Jiang J, Hoffman KE, et al. Comparative Toxicities and Cost of Intensity-Modulated Radiotherapy, Proton Radiation, and Stereotactic Body Radiotherapy Among Younger Men With Prostate Cancer. J Clin Oncol. 2018;36(18):1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon SH. The Long-Term Effects of Cancer on Employment and Earnings. Health Econ. 2017;26(5):671–684. [DOI] [PubMed] [Google Scholar]
- 34.Howard DH. Drug companies’ patient-assistance programs--helping patients or profits? N Engl J Med. 2014;371(2):97–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.