Abstract
Background:
Targeted temperature management (TTM) improves neurologic outcome after cardiac arrest. However, better neurological prognostication is needed.
Objective:
To test the hypothesis that noninvasive recording of skin sympathetic nerve activity (SKNA) and its association to heart rate (HR) during TTM may serve as a biomarker of neurologic status.
Methods:
SKNA recordings were analyzed from 29 patients undergoing TTM. Patients were grouped based on the Clinical Performance Category (CPC) score into Group 1 (CPC of 1-2) representing a good neurologic outcome and Group 2 (CPC of 3-5) representing a poor neurologic outcome.
Results:
Out of all study participants, 18 of 29 (62%) were deemed to have poor neurologic outcome. At all timepoints, low average SKNA (aSKNA) was associated with poor neurologic outcome (Odds Ratio (OR) = 22.69, p=0.002) and remained significant (p=0.03) even when adjusting for presenting clinical factors. The changes in aSKNA and HR during warming in Group 1 were significantly correlated (ρ=0.49, p<0.001) even when adjusting for corresponding temperature and mean arterial pressure measurements (p=0.017), while this correlation was not observed in Group 2. Lastly, corresponding to high aSKNA, there was increased nerve burst activity during warming in Group 1 compared to Group 2 (0.739±0.451 vs. 0.176±0.231, p=0.013).
Conclusions:
Neurological recovery was retrospectively associated with SKNA. Patients undergoing TTM who did not achieve neurological recovery were associated with low SKNA and lacked a significant correlation between SKNA and HR. These preliminary results indicate that SKNA may potentially be a useful biomarker to predict neurological status in patients undergoing TTM.
Keywords: targeted temperature management, hypothermia, sympathetic nerve activity, neurological function, brain death, cardiac arrest
Introduction
There are more than 350,000 out-of-hospital cardiac arrests taking place every year and they are associated with poor survival rates, with 31% for shockable rhythms and 11% for all rhythms combined.1 Anoxic brain injury accounts for a significant majority of morbidity and mortality in post-cardiac arrest patients. In this context, targeted temperature management (TTM) is one of the only interventions that has been shown to improve neurologic outcomes after cardiac arrest.2, 3 One decade after TTM was incorporated into the guidelines, one-half instead of one-third of patients with return of spontaneous circulation (ROSC) after cardiopulmonary resuscitation were expected to survive hospitalization.4, 5 Despite this, cerebral ischemia may persist for several hours and an accurate short-term assessment of neurological outcome in patients undergoing TTM remains a challenge.6
Once the patient is started on TTM, signs of neurological recovery are often delayed making it difficult to evaluate neurological function7. In addition, it takes multiple modalities of neurological testing to accurately determine poor prognosis.8 However, interpretation of these studies can be technically challenging and is sometimes subject to high reader variability.9,10 Development of robust prognostic tools is critical to aid the multimodal assessment of brain function, suggesting the need to develop an additional biomarker which can predict the neurologic prognosis in patients undergoing TTM.
We have developed a non-invasive method to simultaneously record the electrocardiogram (ECG) and skin sympathetic nerve activity (SKNA) using conventional ECG patch electrodes. This has been validated in dog models, showing that the SKNA recordings can act as a surrogate for stellate ganglion nerve activity11. Recordings have also been performed in multiple clinical applications.12-14 These pre-clinical and clinical studies have shown that SKNA can be used to estimate sympathetic tone. Appropriate autonomic nervous system adjustments are critical for thermoregulatory and cardiovascular responses to thermal stress. Significantly low SKNA or an altered SKNA relationship with heart rate (HR) during TTM could signify autonomic dysregulation. We sought to apply this novel non-invasive method to simultaneously record ECG and SKNA, and leveraging it to estimate sympathetic tone under thermal stress in order to predict neurological outcome in TTM.
The purpose of this study is to simultaneously record SKNA and HR in patients undergoing TTM post-cardiac arrest to determine if there is a correlation between SKNA and neurologic outcome. We hypothesized that low SKNA would be associated with poor neurological outcome. We then hypothesized that in those with poor neurologic outcome undergoing TTM, there would be an altered relationship between SKNA and HR, and low nerve burst activity.
Methods
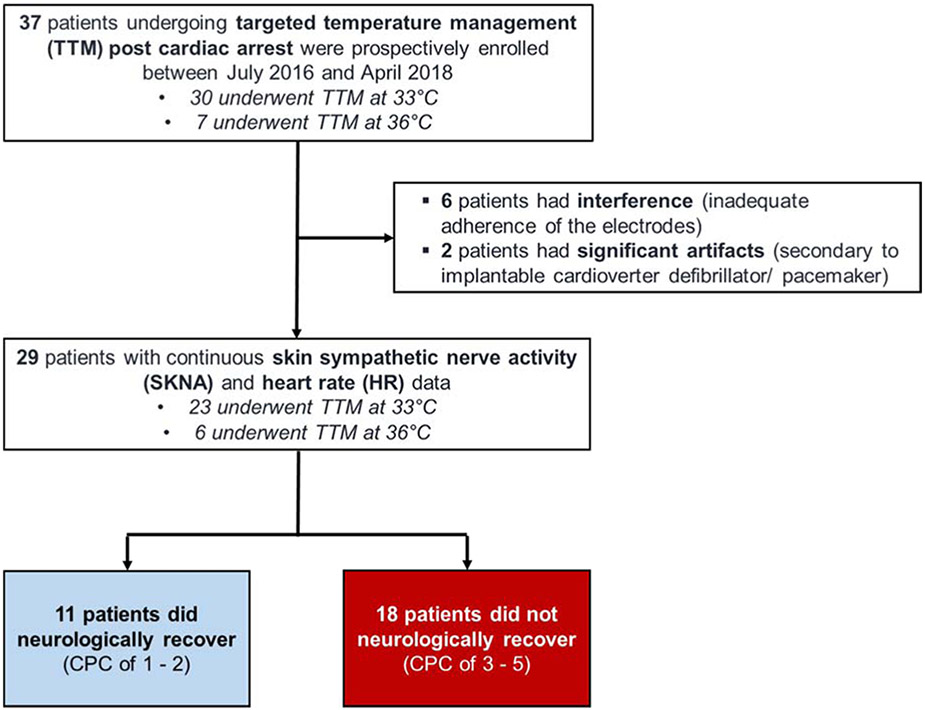
The protocol for this study was approved by the Institutional Review Board of Indiana University School of Medicine. A total of 37 consecutive patients were enrolled between July 2016 and April 2018 in a prospective observational study. All 37 patients underwent TTM, with 30 patients at 33°C and 7 at 36°C (Figure 1).
Figure 1. Recruitment Diagram.
The 33°C protocol consisted of 3 phases: cooling to 33°C for 24 hours, warming to 36°C for 24 hours, then maintenance near 36°C (near-normothermia) for 24 hours. The normothermia protocol spanned 72 hours at 36°C, thus referred to as 3 phases of normothermia, each phase lasting 24 hours. Both protocols were followed by 4 hours of warming at 0.25°C per hour to reach 37°C.
Simultaneous SKNA and HR recordings were started within 12 hours of initiation of TTM. Conventional skin patch electrodes were used to record SKNA from the ECG Lead I configuration12, while temperature values and mean arterial pressure (MAP) were obtained from the values recorded hourly. Quantitative SKNA analyses was performed retrospectively at a later time as previously described, and specific details are described in the Data Supplement (eFigure 1).12, 15
Neurologic outcome was defined in accordance with the Clinical Performance Category (CPC) score, a five-point scale (1 to 5): good recovery, moderate disability, severe disability, persistent vegetative state, and brain death.16 Patients who were terminally weaned or had brain death were scored as a 3-5 on CPC scale (Group 2), whereas those who recovered were defined as a score of 1-2 (Group 1). Multimodal assessment of neurologic outcome was at the discretion of the intensive care unit (ICU) physician and consulting neurologist as needed. Patients were de-identified and the CPC grade was blinded until the SKNA data analysis was completed.
Statistical Analysis
Data were reported as mean with standard deviation for continuous variables and percentages for categorical variables. Continuous variables were compared between the two outcome groups using student t-test and dichotomous variables were analyzed using Fisher’s exact test. In both TTM protocols (TTM at 33°C and 36°C), the median average SKNA (aSKNA) value of 0.77 μV was identified at hour 25 of analysis, corresponding to the beginning of the final phase of TTM and a body temperature of 36°C (near-normothermia) for all patients. Subsequently, aSKNA values across all hours of TTM were dichotomized, based off the median value of 0.77 μV, into low SKNA or high SKNA categories.
The associations of poor neurologic status post-TTM (Group 2) with patient’s low or high aSKNA category at hour 25, the temporal trajectory of aSKNA, and the overall bursts per minute throughout TTM were explored using multivariable logistic regression while adjusting for clinical factors and factors from the initial presentation. Adjustments were made by the inclusion into a multivariate model of the other independent variables to predict a categorical dependent variable, poor neurologic recovery. This allowed for the comparison of the strengths of the predictor of interest within the models. Correlations were tested against 0 using Fisher’s Z-transformation with two-sided p-values. As the analyses were exploratory, nominal p-values were reported without adjustment for multiplicity. All statistical analyses were performed using STATA 15 (Stata Corp., College Station, TX, USA) and R version 3.5.3.
Results
Of the initial 37 patients recruited, 8 patients were excluded from the analysis; 6 were excluded due to nerve activity interference from detachment of the electrodes and loss of data during the rewarming phase, and 2 had an implantable cardioverter defibrillator/ pacemaker where the pacing stimuli produced significant artifacts. Patient characteristics are shown in Table 1. Study participants were predominantly male, obese and had highly prevalent comorbid traditional risk factors at baseline. Patients presented with a history of shockable initial rhythms in 10 of 29 cases (34%), administration of 2.69±1.63 adrenaline dosages and an average ROSC time of 14±12.8 minutes. On arrival to the hospital, point of care blood pH and lactate levels were 7.24±0.22 and 6.1±4.7 mmol/L, respectively. After admission to the ICU, 6 of 29 patients (20%) were placed on TTM at 36°C. There were no significant differences in clinical characteristics or initial presentation compared to those undergoing TTM at 33°C.
Table 1.
Patient characteristics.
| All (n=29) |
TTM at 36°C (n=6) |
TTM at 33°C (n=23) |
p- value |
|
|---|---|---|---|---|
| Demographics | ||||
| Age | 51.6±18.9 | 52.2±18.8 | 51.5±19.3 | 0.94 |
| Gender (Male) | 21 (72) | 5 (83) | 16 (70) | 0.65 |
| Race (African American) | 10 (34) | 1 (17) | 9 (39) | 0.63 |
| Medical History | ||||
| Body Mass Index (kg/m2) | 30.63±8.99 | 28.37±4.91 | 31.22±9.78 | 0.33 |
| Hypertension | 18 (62) | 5 (83) | 13 (57) | 0.36 |
| Diabetes Mellitus | 10 (34) | 2 (33) | 8 (35) | 1.00 |
| Stroke | 4 (14) | 0 (0) | 4 (17) | 0.55 |
| Coronary artery disease | 12 (41) | 2 (33) | 10 (43) | 1.00 |
| Social History | ||||
| Smoking | 13 (45) | 5 (83) | 8 (35) | 0.06 |
| Alcohol use | 11 (38) | 4 (67) | 7 (30) | 0.16 |
| Illicit drug use | 6 (21) | 1 (17) | 6 (26) | 0.30 |
| Initial Presentation | ||||
| Shockable rhythm (VF or VT) | 10 (34) | 2 (33) | 8 (35) | 1.00 |
| Time to ROSC (min) | 14.24±12.78 | 14.67±17.14 | 14.13±11.87 | 0.93 |
| Adrenaline doses | 2.69±1.63 | 3.50±2.43 | 2.48±1.34 | 0.36 |
| Blood pH | 7.20±0.22 | 7.31±0.14 | 7.17±0.23 | 0.08 |
| Blood lactate (mmol/L) | 6.12±4.67 | 5.52±5.77 | 6.32±4.47 | 0.76 |
| Outcome | ||||
| CPC 3-5 (No Recovery) | 18 (62) | 3 (50) | 15 (65) | 0.65 |
Values are reported as mean ± standard deviation for continuous data and N (%) for categorical data. VT – ventricular tachycardia. VF – ventricular fibrillation. ROSC – restoration of spontaneous circulation. CPC – cerebral performance category.
Out of all the study participants, 18 of 29 (62%) were deemed to have poor neurologic status post-cardiac arrest (CPC of 3-5), and their data are summarized in Table 2. Initial head computed tomography showed evidence of cerebral edema in 6 of 18 cases (33%) while electroencephalogram (EEG) showed various degrees of encephalopathy in all. Additional testing (i.e. magnetic resonance imaging, somatosensory evoked potential [SSEP], nuclear perfusion, neuron-specific enolase), when ordered, were often abnormal. On serial neurological exams, 2/3 of patients had absent or abnormal pupillary and corneal reflexes, GCS was 3 (Interquartile Range, IQR 3-5), and more than half of the patients had significant myoclonus present. A CPC score of 4 (coma or vegetative state) was the neurologic outcome in 10 of 18 cases (56%). In more than half of the cases, the determination of neurological status was done with the involvement of a consulting neurologist. The decision to withdraw care was based on poor neurologic prognosis in 13 of 18 cases (73%) and brain death in 4 of 18 cases (22%). The median time for withdrawal of care was approximately 134 (IQR 96–165) hours post-cardiac arrest.
Table 2.
Summary of clinical data for patients in Group 2 with poor neurologic prognosis (n=18 patients).
| Clinical Studies | Findings |
|---|---|
| Initial head CT, with evidence of cerebral edema (early diffuse) | 6 (33%) |
| EEG | |
| Encephalopathy, nonspecific (moderate - severe) | 18 (100%) |
| Periodic EEG patterns | 7 (39%) |
| MRI, with evidence of diffuse hypoxic ischemic injury | 7 of 8 (88%) |
| SSEP, with total absence of cortical response on both median and posterior tibial stimulation | 3 of 4 (75%) |
| NM-CBF, with no intracranial blood flow | 3 of 3 (100%) |
| NSE (n=5), serum level (ng/mL) | 58.0 (30.5-162.0) |
| Key Exam Findings | |
| Pupillary & corneal reflex (absent or abnormal) | 12 (67%) |
| Myoclonus presence | 10 (56%) |
| Glasgow coma scale | 3 (3-5) |
| Prognosis | |
| Cerebral performance category | |
| (3) Severe cerebral disability | 4 (22%) |
| (4) Coma or vegetative State | 10 (56%) |
| (5) Brain death | 4 (22%) |
| Neurological Prognosis Determination | |
| ICU physician | 18 (100%) |
| Neurologist | 10 (56%) |
| Withdrawal of Care | |
| Rational for Family and Physician Decision | |
| Poor neurologic prognosis | 10 (56%) |
| Poor neurologic and medical prognosis | 3 (17%) |
| Brain death | 4 (22%) |
| Early termination, coinciding with patient’s wishes | 1 (5%) |
| Median time of withdrawal post-cardiac arrest (hour:minute) | 133:57 (95:49-165:00) |
Values are reported as median (interquartile range) for non-parametric continuous variables and N (%) for categorical data. CT – computed tomography. EEG – electroencephalogram. MRI – magnetic resonance imaging. SSEP – somatosensory evoked potential. NM-CBF – nuclear medicine cerebral blood flow. NSE – neuron-specific enolase (reference interval 0.0-16.3 ng/mL).
Low SKNA is associated with poor neurologic outcome in TTM
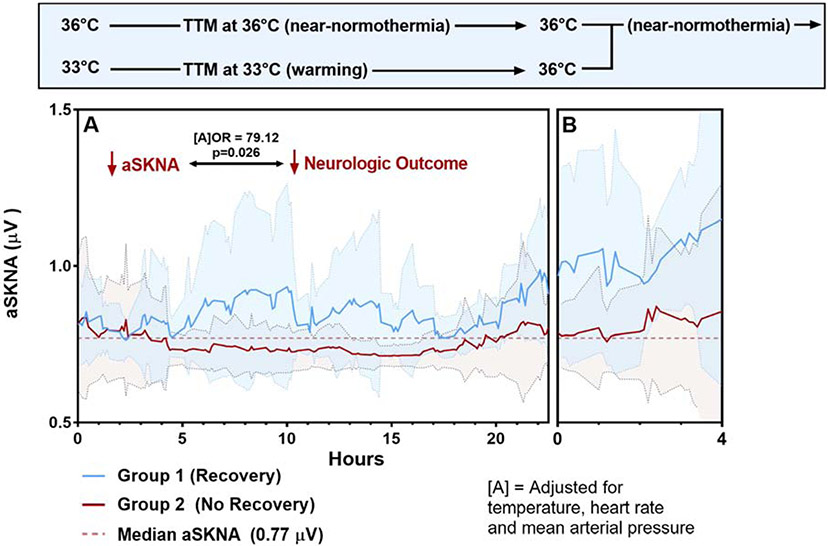
At 36°C (near-normothermia) for both protocols, low aSKNA (values less than median aSKNA value of 0.77 μV) coincided with poor neurologic status post-TTM, compared to those with high aSKNA response (13 of 14 (93%) vs. 5 of 15 (33%), Odds Ratio (OR)=22.69, p=0.002) (eTable 1). Significant confounders of aSKNA included clinical factors; male gender (multiple linear regression coefficient β = 0.16), body-mass index (BMI) (β = −0.23), diabetes (β = −0.20), ongoing illicit drug use (β = −0.18) and factors from the initial presentation; initial shockable rhythm (β = 0.23) and blood lactate level on arrival (β = −0.28). After accounting for these factors using multivariable logistic regression, a value of aSKNA lower than 0.77 μV at 36°C was still significantly associated with poor neurologic status post-TTM (OR=90.24, p=0.03). In addition, throughout TTM, the proportion of low aSKNA under 0.77 was also associated with poor neurologic status post intervention (OR=57.66, p=<0.001) even after adjusting for corresponding mean temperature, HR, and MAP values (OR=79.12, p=0.026) (Figure 2). When comparing aSKNA to neurologic status post-TTM, those in the Group 1 had significantly higher aSKNA values overall compared to those in Group 2, (0.90±0.20 μV vs. 0.76±0.13 μV, p<0.0001).
Figure 2: SKNA during the rewarming TTM protocol.
(A) Represents the warming phase of TTM at 33°C (n=23), while (B) represents the near-normothermia phase of TTM at 33°C and 36°C (n=29). At all hourly timepoints, an aSKNA value under 0.77 was associated with poor neurologic status post intervention even after adjusting for mean temperature, HR, and MAP values (OR=79.12, p=0.026). Background color related to each curve represents standard deviation.
SKNA and Nerve burst activity in TTM at 33°C
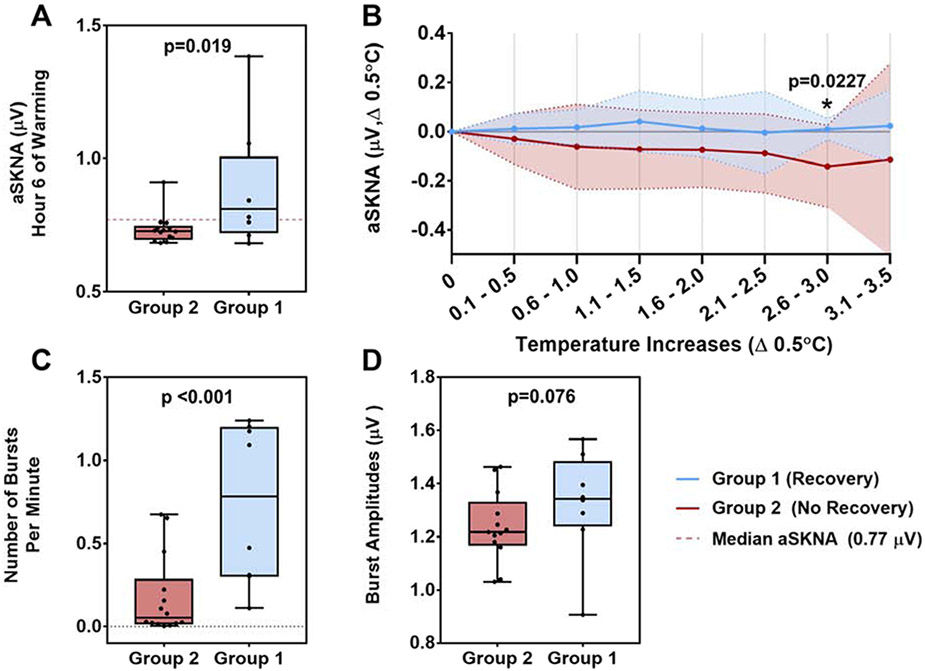
Throughout warming, patients in Group 1 showed a trend of increasing likelihood of high aSKNA (values greater than 0.77 μV) over each hour of TTM, with a significant p for trend of <0.001, that was not seen in Group 2, p=0.06. Group 1 patients also exhibited higher standard deviations per hour in their respective aSKNA measurements during warming relative to Group 2 (Figure 2). Beginning at hour 6, significant differences in SKNA were evident, with increased aSKNA values in Group 1 compared to Group 2 (0.703±0.021 vs. 0.828±0.176, p=0.019) (Figure 3A). Moreover, throughout warming, aSKNA changes in Group 1 tended to be stable over each 0.5°C temperature increment, while changes in Group 2 tended to decrement with the temperature change. There was a significant difference between the two groups coinciding over the 2.6-3°C temperature range (0.0099±0.045 vs. −0.142±0.167, p=0.0227) (Figure 3B).
Figure 3. SKNA Characteristics.
(A) average SKNA (aSKNA) during hour 6 of warming was significantly lower in Group 2 compared to Group 1 (0.703±0.021 vs. 0.828±0.176, p = 0.019). (B) aSKNA changes in Group 1 remained stable per 0.5°C changes compared to Group 2 aSKNA changes, with a significant difference coinciding over the 2.6-3°C temperature range (0.0099±0.045 vs. −0.142±0.167, p=0.0227). (C) Nerve burst activity during warming was found to be significantly higher in Group 1 compared to Group 2 (0.739±0.451 vs. 0.176±0.231, p<0.001). (D) There was a non-significant increase in burst amplitude in Group 1 compared to Group 2 (1.322±0.189 vs. 1.237±0.123, p=0.076).
Nerve burst activity (mean bursts per minute) during warming was significantly higher in Group 1 compared to Group 2 (0.739±0.451 vs. 0.176±0.231, p=0.013) (Figure 3C). Lower bursts per minute remained associated with poor neurologic status post-TTM after adjusting, using multivariable regression, for patient characteristics known to influence sympathetic nerve burst outflow; age, race, BMI, and history of coronary artery disease.17 Although there tended to be a decrease in amplitude or area of these bursts in Group 1 compared to Group 2, there was no significant difference between the groups with regards to burst amplitude (Group 1 vs. Group 2, 1.322±0.189 vs. 1.237±0.123, p=0.30) (Figure 3D) or burst area (Group 1 vs. Group 2, 26.01±11.80 vs 24.56±16.52, p=0.82).
The relationship of aSKNA and HR in TTM at 33°C
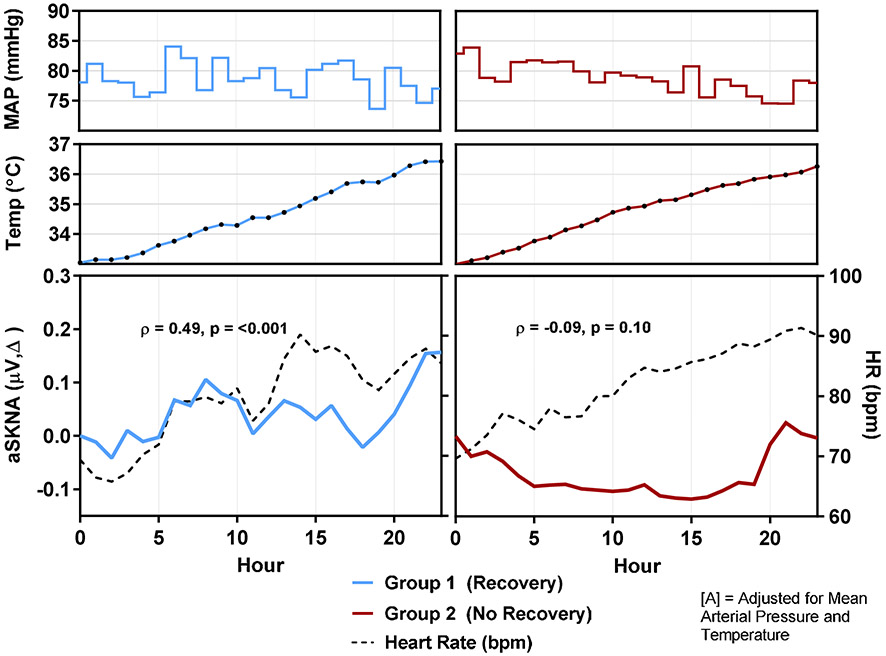
For both groups, MAP remained within 74-84 mmHg and temperature steadily increased throughout warming (Figure 4). Changes in aSKNA and HR increased or decreased simultaneously in Group 1 and were significantly correlated (Spearman's correlation coefficient, ρ = 0.49, p<0.001), even when adjusting for corresponding MAP and temperature values. In Group 2, there was no significant relationship between aSKNA and HR (ρ = −0.09, p=0.10). Compared to Group 1, on a per-patient analysis, patients in Group 2 were more likely to lack a significant positive association between SKNA and HR when adjusting for MAP and temperature (13 of 15 (87%) vs. 1 of 8 (13%), p=0.001) (eTable 2). A lack of a positive association between aSKNA and HR was associated with a poor neurologic status post-intervention (OR=45.5, p=0.004).
Figure 4: SKNA and HR during Warming.
During TTM at 33°C, for both groups, MAP remained within 74-84 mmHg and temperature steadily increased throughout warming. As a whole, changes in aSKNA and HR tended to increase or decrease simultaneously in Group 1 and were significantly correlated (Spearman's correlation coefficient, ρ = 0.49, p<0.001), unchanged even when adjusting for corresponding MAP and temperature values (p=0.017). In Group 2, there was no significant relationship between changes in aSKNA and HR (ρ = −0.09, p=0.10).
Arrhythmias
During SKNA recording, one patient from Group 1 had ventricular fibrillation (VF) at hour 18 of warming when their temperature was 35.4°C (Figure 5A). There was no increase in aSKNA or burst activity prior to arrhythmia onset. The episode of VF and patient intervention lasted approximately 4 minutes and 9 seconds from onset (Figure 5B). No surge of nerve activity was noted at least 20 minutes prior to the onset of arrhythmia. As the arrhythmia continued, nerve activity began to increase, with a large increase in nerve activity prior to termination followed by sudden decrease just as the arrhythmia terminates.
Figure 5. VF Arrhythmia:
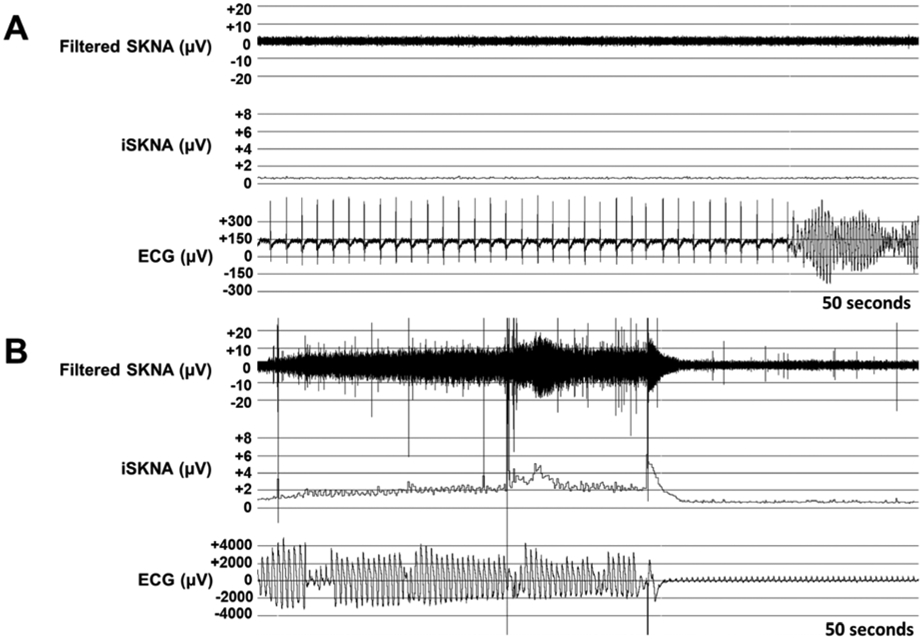
(A) Onset of ventricular fibrillation (VF) at hour 18 of TTM during the warming phase when temperature was 35.4°C. (B) End of VF and patient intervention, approximately 4 minutes and 9 seconds from onset. There was a gradual and large increase in nerve activity prior to termination, lasting 32 seconds, with a sudden decrease just as the arrhythmia terminates. SKNA signal was sampled at 10 KHz and high pass filtered at 500 Hz to analyze the nerve activity; iSKNA – integrated SKNA data over 100 millisecond periods.
Discussion
In this study, we showed several findings: (1) low SKNA activity during TTM was associated with poor neurologic outcome independent of clinical characteristics; (2) during TTM at 33°C, altered SKNA and HR relationship and (3) low nerve burst activity were associated with a poor neurologic outcome. These findings provide insight into how altered sympathetic tone in patients undergoing TTM post-cardiac arrest may be an early marker of poor neurologic outcome.
We have previously shown that SKNA represents sympathetic tone and autonomic activity.12 Humans regulate their core temperature in a narrow range around a “set point” of ~37°C through integrated autonomic reflexes, all of which are efferent responses controlled by higher brain centers. Appropriate autonomic nervous system adjustments are critical for thermoregulatory and cardiovascular responses to thermal stress. Significantly low SKNA or an altered SKNA relationship with HR during TTM could signify autonomic dysregulation or damage to fundamental brain centers that could lead to or be an early warning sign of an adverse neurological outcome. Altered sympathetic regulation during thermal stress occurs in individuals of older age, with cardiovascular comorbidities and in diabetes- factors that were accounted for in our analysis. Moreover, the significantly positive correlation between aSKNA and HR exhibited in Group 1 rather than Group 2 reflects what has been shown in other clinical recordings.12, 14, 15 Thus, low SKNA (and neve burst activity) and altered relationship with HR during TTM are likely poor prognostic signs and may potentially be used as a biomarker that might predict neurological outcome.
Hypothermia and Arrhythmia
Hypothermia has been shown to alter depolarization and repolarization, inducing bradycardia and prolonging atrioventricular nodal conduction.18 However, the cause and triggering mechanism of VF in hypothermia are still not well elucidated. Studies have shown that the QT interval is prolonged with cooling, especially in context of hypokalemia and hypomagnesemia, which are known to occur during hypothermia.9, 19 We have also shown that hypothermia induces J-wave elevation and increases vulnerability to VF.20 However, the risk of developing clinically significant arrhythmias due to hypothermia is extremely low as long as the patient’s core temperature remains higher than 30°C.9 We have previously shown that nerve activity can influence the onset of VF and that there is an increase in nerve activity after initiation of VF, which could be partly related to hypotension.11, 13 In the current study, one patient was noted to have VF during therapeutic hypothermia. However, it was noted that there was no significant change in nerve activity up to 20 minutes prior to the initiation of the arrhythmia and also during the onset of the arrhythmia. It might be that in this case, the mechanism of VF was intrinsic to the myocardium (for example, early afterdepolarization and triggered activity) rather than being influenced by extrinsic sympathetic nerve activity.
Clinical Indication
Neurological recovery is delayed after TTM due to reduced cellular metabolism of the nervous system. This delays the appropriate time for testing until after completion of TTM for a true assessment of neurological recovery.7 Clinical tools used to determine neurological prognosis include spontaneous neurological function, brainstem reflexes, different coma scales, multiple imaging modalities, electrophysiological testing, EEG and SSEP. However, interpretations of these studies can be technically challenging and delayed for hours to 2-3 days after completion of TTM.9, 10 Therefore, delays in recognizing brain death invariably occurs after TTM in patients with uncertain neurological status. Knowing the prognosis of patients will have a significant effect on the course of medical treatment and the resources allotted to them. Hence, the lack of a predictor of poor neurological outcome that can occur earlier in the rewarming process has prompted a number of recent studies including measuring the spectral content of EEG recordings, lymphocyte count, serum biomarkers, IL-6 levels, and the ratio of grey to white brain matter.21-26 Most of these attempts have not improved the predictive outcome of neurological prognosis. Multifactorial prediction models have also been developed that have shown some predictive assessment at 24 hours.27 However, a universally accepted predictor of neurological recovery that can be reliably used early in the rewarming phase is still lacking. SKNA may support the currently used modalities in the ICU and may have the potential to provide an early indication of neurological outcome that can perhaps guide therapy in a cost-effective fashion.
Limitations
First, improper contact between the electrodes and the skin, motion artifacts during routine care, and the presence of a pacemaker/defibrillator may lead to interrupted recordings or electrical interference that renders nerve activity uninterpretable. Second, the neurological status was determined by a non-uniform group of physicians which might have led to inconsistencies in the neurological assessment of patients in the study. Neurological injury was not localized to specific brain areas, hence the impact of particular brain regions on sympathetic outflow could not be determined. Third, there are no preclinical studies of animals undergoing warming and the SKNA response of normal subjects undergoing TTM is unknown. Hence, not having a reference group might add uncertainty to interpreting the data presented. Lastly, this is a small pilot study where analysis of SKNA data was performed retrospectively. To practically demonstrate that SKNA can be used as a predictor of neurological recovery, prospective data analysis that is simultaneous with the recording using enhanced equipment and applied to a larger patient cohort is needed in future studies.
Conclusion
In patients undergoing TTM who did not achieve neurological recovery, low SKNA activity was detected. Furthermore, SKNA lacked a significant positive association with heart rate throughout warming. These preliminary findings provide insight into how altered sympathetic tone in patients undergoing TTM post-cardiac arrest may have the potential to be used as a complementary biomarker to assist in predicting neurologic outcome.
Supplementary Material
Acknowledgments
We thank Dr. Amit Dey, MBBS for assistance with editing the manuscript.
Sources of Funding
This study was supported in part by NIH Grants R42DA043391, U18TR002208-01, R01HL139829, OT2OD028190-01, OT2OD028183-01, a Medtronic-Zipes Endowment, the Charles Fisch Cardiovascular Research Award endowed by Dr Suzanne B. Knoebel of the Krannert Institute of Cardiology, and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative.
Footnotes
Disclosures – None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation January 27 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 2.Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. The New England journal of medicine February 21 2002;346:549–556. [DOI] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. The New England journal of medicine February 21 2002;346:557–563. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. The New England journal of medicine December 5 2013;369:2197–2206. [DOI] [PubMed] [Google Scholar]
- 5.Callaway CW, Soar J, Aibiki M, et al. Part 4: Advanced Life Support: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation October 20 2015;132:S84–145. [DOI] [PubMed] [Google Scholar]
- 6.Arrich J, Holzer M, Havel C, Mullner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. The Cochrane database of systematic reviews February 15 2016;2:CD004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greer DM, Rosenthal ES, Wu O. Neuroprognostication of hypoxic-ischaemic coma in the therapeutic hypothermia era. Nature reviews Neurology April 2014;10:190–203. [DOI] [PubMed] [Google Scholar]
- 8.Yacono CS, Eider S. Understanding therapeutic hypothermia. JAAPA : official journal of the American Academy of Physician Assistants February 2017;30:29–34. [DOI] [PubMed] [Google Scholar]
- 9.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Critical care medicine March 2009;37:1101–1120. [DOI] [PubMed] [Google Scholar]
- 10.Schenone AL, Cohen A, Patarroyo G, Harper L, Wang X, Shishehbor MH, Menon V, Duggal A. Therapeutic hypothermia after cardiac arrest: A systematic review/meta-analysis exploring the impact of expanded criteria and targeted temperature. Resuscitation November 2016;108:102–110. [DOI] [PubMed] [Google Scholar]
- 11.Doytchinova A, Patel J, Zhou S, Chen LS, Lin H, Shen C, Everett THt, Lin SF, Chen PS. Subcutaneous nerve activity and spontaneous ventricular arrhythmias in ambulatory dogs. Heart rhythm March 2015;12:612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doytchinova A, Hassel JL, Yuan Y, et al. Simultaneous noninvasive recording of skin sympathetic nerve activity and electrocardiogram. Heart rhythm January 2017;14:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabir RA, Doytchinova A, Liu X, Adams D, Straka S, Chen LS, Shen C, Lin SF, Everett THt, Chen PS. Crescendo Skin Sympathetic Nerve Activity and Ventricular Arrhythmia. J Am Coll Cardiol December 26 2017;70:3201–3202. [DOI] [PubMed] [Google Scholar]
- 14.Uradu A, Wan J, Doytchinova A, Wright KC, Lin AYT, Chen LS, Shen C, Lin SF, Everett THt, Chen PS. Skin sympathetic nerve activity precedes the onset and termination of paroxysmal atrial tachycardia and fibrillation. Heart rhythm July 2017;14:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusayama T, Wan J, Doytchinova A, Wong J, Kabir RA, Mitscher G, Straka S, Shen C, Everett THt, Chen PS. Skin sympathetic nerve activity and the temporal clustering of cardiac arrhythmias. JCI Insight February 21 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet March 1 1975;1:480–484. [DOI] [PubMed] [Google Scholar]
- 17.Greaney JL, Kenney WL, Alexander LM. Sympathetic regulation during thermal stress in human aging and disease. Auton Neurosci 2016;196:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietrichs ES, Tveita T, Smith G. Hypothermia and cardiac electrophysiology: a systematic review of clinical and experimental data. Cardiovasc Res March 1 2019;115:501–509. [DOI] [PubMed] [Google Scholar]
- 19.Khan JN, Prasad N, Glancy JM. QTc prolongation during therapeutic hypothermia: are we giving it the attention it deserves? Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology February 2010;12:266–270. [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Xu DZ, Wu AZ, et al. Concomitant SK current activation and sodium current inhibition cause J wave syndrome. JCI Insight November 15 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bro-Jeppesen J, Kjaergaard J, Stammet P, et al. Predictive value of interleukin-6 in post-cardiac arrest patients treated with targeted temperature management at 33 degrees C or 36 degrees C. Resuscitation January 2016;98:1–8. [DOI] [PubMed] [Google Scholar]
- 22.Miyatake H, Fujino K, Tanaka S, Tsujita Y, Horie M, Eguchi Y. Association between lymphocyte count and neurological outcomes in post-cardiac arrest patients treated with mild therapeutic hypothermia. Acute Med Surg January 2019;6:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Na MK, Kim W, Lim TH, Jang B, Cho Y, Choi KS, Shin HG, Ahn C, Lee J, Kim JG. Gray matter to white matter ratio for predicting neurological outcomes in patients treated with target temperature management after cardiac arrest: A systematic review and meta-analysis. Resuscitation November 2018;132:21–28. [DOI] [PubMed] [Google Scholar]
- 24.Park JH, Wee JH, Choi SP, Oh JH, Cheol S. Assessment of serum biomarkers and coagulation/fibrinolysis markers for prediction of neurological outcomes of out of cardiac arrest patients treated with therapeutic hypothermia. Clin Exp Emerg Med March 2019;6:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riker RR, Sawyer ME, Fischman VG, May T, Lord C, Eldridge A, Seder DB. Neurological Pupil Index and Pupillary Light Reflex by Pupillometry Predict Outcome Early After Cardiac Arrest. Neurocrit Care May 8 2019. [DOI] [PubMed] [Google Scholar]
- 26.Sekar K, Schiff ND, Labar D, Forgacs PB. Spectral Content of Electroencephalographic Burst-Suppression Patterns May Reflect Neuronal Recovery in Comatose Post-Cardiac Arrest Patients. J Clin Neurophysiol March 2019;36:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eertmans W, Tran TMP, Genbrugge C, Peene L, Mesotten D, Dens J, Jans F, De Deyne C. A prediction model for good neurological outcome in successfully resuscitated out-of-hospital cardiac arrest patients. Scand J Trauma Resusc Emerg Med November 9 2018;26:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.