Abstract
Purpose:
Anaplastic lymphoma kinase (ALK) aberrations are a promising target for patients with neuroblastoma. We assessed the activity of first generation ALK inhibitor crizotinib in patients with no known curative treatments and whose tumors harbored an activating ALK alteration.
Procedures:
Twenty patients with relapsed/refractory ALK-positive neuroblastoma received crizotinib at the recommended phase 2 dose of 280 mg/m2/dose. A Simon two-stage design was used to evaluate the anti-tumor activity of crizotinib monotherapy. Response evaluation occurred after cycles 1, 3, 5, 7, and then every 3 cycles. Correlation of ALK status and response was a secondary aim of the study.
Results:
The objective response rate for patients with neuroblastoma was 15% (95% CI: 3.3%,34.3%): two with partial responses and 1 with a complete response. All three patients had a somatic ALK Arg1275Gln mutation, the most common ALK hotspot mutation observed in neuroblastoma and the only mutation predicted to be sensitive to ALK inhibition with crizotinib. Two patients had prolonged stable disease (10 and 13 cycles, respectively); both harbored an ALK Arg1275Gln mutation. Three patients with ALK Phe1174Leu mutations progressed during cycle 1 of therapy, and one patient with an ALK Phe1174Val received 3 cycles before disease progression. The two patients with ALK amplification had no response. The most common adverse event was a decrease in neutrophil count.
Conclusions:
Despite limited activity seen in this trial, we conclude that this is more likely due to an inability to reach the higher concentrations of crizotinib needed to overcome the competing ATP affinity.
Keywords: crizotinib, neuroblastoma, ALK, pediatric oncology, Phase 2
INTRODUCTION
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase (RTK) that is known to be oncogenically activated by point mutations within the tyrosine kinase domain, copy number amplification, or chromosomal translocations(1–4). ALK mutations were first identified in both the germline of familial neuroblastoma patients(4) and somatically in the sporadic form of the disease(1, 2, 4, 5). In addition, recurrent neuroblastoma demonstrates an increased frequency of ALK mutations(3, 6, 7). These mutations, as well as ALK amplifications(8), are a promising therapeutic target.
The discovery of ALK rearrangements in 3–5% of patients with non-small cell lung cancer (NSCLC) (9) drove early-phase clinical studies of crizotinib, a first-in-class dual ALK/Met/ROS1 small molecular tyrosine kinase inhibitor(10, 11). The dramatic response rates in NSCLC validated ALK as a therapeutic target and led to the expedited FDA approval of crizotinib in August 2011 for use in patients with ALK-rearranged lung cancer(12, 13).
A pediatric phase 1/2 trial of crizotinib (ADVL0912, NCT00939770) established the recommended phase 2 dose (RP2D) as 280 mg/m2/dose twice daily(14). Pediatric patients with ALK fusions, most notably those with anaplastic large cell non-Hodgkin lymphoma (ALCL) and inflammatory myofibroblastic tumors (IMT), showed robust and durable complete anti-tumor responses in the majority of cases with proven ALK rearrangements(15). These data supported the portfolio of data required to obtain FDA breakthrough designation in May of 2018 for crizotinib in the context of relapsed ALCL(14, 15). This report describes the outcomes in children with relapsed or refractory ALK-positive (activating mutation or amplification) neuroblastoma treated with crizotinib at the RP2D.
MATERIALS AND METHODS
Patients
Patients >12 months and ≤ 21 years old who had relapsed or refractory ALK-positive neuroblastoma were treated at the recommended phase 2 dose (RP2D) of 280 mg/m2/dose twice daily in cycles of 28 days duration. ALK status was reported by each enrolling institution using a Clinical Laboratory Improvement Amendments (CLIA) certified FISH or DNA sequencing assay. Standard Children’s Oncology Group (COG) organ function requirements and performance scores were utilized (14). Patients with central nervous system metastases were excluded. Patients were recruited from member sites in the COG Phase 1 consortium, now known as the Pediatric Early Phase-Clinical Trial Network (PEP-CTN), and could have either measurable or evaluable disease. Patients were removed from protocol therapy if they had progressive disease, drug toxicity despite protocol specified dose modification, parental or provider preference, or development of a secondary neoplasm. This trial received the approval of the institutional review boards at participating sites. Consent was obtained from patients or legal guardians, and assent was obtained when appropriate.
This study was conducted in compliance with the Declaration of Helsinki, the International Conference on Harmonization, Guideline for Good Clinical Practice, and applicable national and local regulatory requirements. Institutional Review Boards at participating institutions approved the study. Informed consent was obtained from patients, ages 18 years or older, or from parents/legal guardians of children aged less than 18 years, with child assent when appropriate, according to institutional policies.
Drug Supply
Drug was supplied as capsules and a dosing nomogram was use to minimize interpatient dosing variability. An oral liquid formulation (25 mg/mL) was subsequently developed and administered at the same dose as the capsule formulation based on bioequivalence studies in adults(17).
Study Design
A Simon two-stage design was used to evaluate the activity of crizotinib monotherapy in patients with relapsed neuroblastoma harboring an ALK alteration. Patients with ALK aberrant neuroblastoma who were enrolled in the phase 2 component of the trial, as well as 6 patients from the phase 1 component who were treated at the RP2D (and also with ALK aberrations) were included in the analysis. Ten patients were included the first stage, including six enrolled in the phase 1 component (three that were previously reported in the Phase 1 trial, and three enrolled on the expansion cohort of the phase 1 trial, cohort A2, and not previously reported). The borrowing of these patients with known ALK positive mutation status and treated at the RP2D was part of the study design, a strategy warranted when studying a rare subset of patients. The pre-defined criteria of at least 1 response amongst the Stage 1 patients was met. The trial proceeded to stage 2 during which an additional 10 patients were entered. This 10+10 Simon two-stage design provides 88% power to reject the one-sided null hypothesis that the proportion of patients with a response is <0.05 in favor of the alternative hypothesis that the proportion is at least 0.25 with a type 1 error rate of 0.07. Correlation of the impact of ALK mutation or amplification status and response to crizotinib in patients with relapsed neuroblastoma known to harbor an ALK alteration was a secondary aim of the study.
Safety Evaluation
The Common Terminology Criteria for Adverse Events version 4.0 was used to grade adverse events (AE). All patients who received at least one dose of crizotinib were evaluated. The relative frequency of each AE considered possibly, probably or likely related to crizotinib was estimated as the proportion of all toxicity-evaluable cycles in which such toxicity was observed.
Response Assessment
The Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 was used to evaluate patients with measurable disease. Patients with evaluable but not measurable MIBG positive lesions and disease present in bone marrow only were evaluated based on the International Neuroblastoma Response Criteria(16). Tumor disease evaluation occurred after cycles 1, 3, 5, 7, and then every 3 cycles. Objective responses had to be sustained for a minimum of two consecutive imaging evaluations at least 4-weeks apart. Imaging studies for patients with a reported objective response or for those with prolonged stable disease (at least six cycles) underwent central radiographic review.
Pharmacokinetic studies
Plasma samples for pharmacokinetics were obtained at steady state and analyzed as previously described(17).
Statistical Analyses
Participant demographics and clinical characteristics were summarized by medians with minimum and maximum values or frequencies with percent. Best objective responses were summarized by frequencies overall and stratified by ALK status. The percent of responders (CR or PR) with 95% confidence interval was estimated using methods described by Koyama and Chen(18).
RESULTS
Patients
Twenty patients with relapsed/refractory ALK-positive neuroblastoma, treated at the RP2D, were enrolled between March 2012 and July 2015; all were eligible and evaluable for response. Of the twenty patients, six patients were treated at the RP2D in phase 1 component of the study(14), and fourteen patients were enrolled in the phase 2 expansion cohort. Patient characteristics are summarized in Table 1. All ALK mutations were somatic; no patients with germline mutations were enrolled in this cohort.Two (R1275 and F1174) of the three (F1245 not observed in this cohort) neuroblastoma-specific ALK hot spot mutations were represented in this cohort, in addition to ALK amplification. The median number of cycles (28-days) received was 1.5 (range 1–16).
Table 1.
Characteristics of the Patients at Baseline
| NBL 280 mg/m2/dose | ||
|---|---|---|
| Characteristic | Value | (n = 20) |
| Age at enrollment (years) | Median (min,max) | 5 (2, 14) |
| Sex | ||
| Female | 7 (35%) | |
| Male | 13 (65%) | |
| Race | ||
| White | 18 (90%) | |
| Black | 1 (5%) | |
| Asian | 1 (5%) | |
| Unknown | 0 (0%) | |
| No. of prior therapies | ||
| 1–2 | 3 (15%) | |
| 3–4 | 5 (25%) | |
| 5–6 | 4 (20%) | |
| 7+ | 8 (40%) | |
| No. of patients with at least one prior therapy | ||
| Chemotherapy | 19 (95%) | |
| Radiotherapy | 16 (80%) | |
| Transplant | 11 (55%) | |
| Surgery | 15 (75%) | |
| Immunotherapy | 5 (25%) | |
| Prior Therapy NOS | 9 (45%) | |
| Disease status | ||
| Evaluable | 7 (35%) | |
| Measurable | 13 (65%) | |
| Cohort | ||
| Phase 1 | 6 (30%) | |
| Phase 2 | 14 (70%) | |
| ALK Aberration | ||
| Amplification | 2 (10%) | |
| Arg1275Gln | 12 (60%) | |
| Arg1275Gln; Phe1174Leu |
1 (5%) | |
| Phe1174Leu | 3 (15%) | |
| Phe1174Val | 1 (5%) | |
| Tyr1278Ser | 1 (5%) |
Toxicity
The most common grade 3 or 4 drug-related adverse event were hematologic and predominantly manifested as a decrease in neutrophil count, with one dose limiting toxicity amongst all twenty patients (Supplementary Table 1). All other ≥ grade 3 toxicity experienced occurred in 1 patient each.
Response
The objective response rate for patients with ALK-positive neuroblastoma enrolled at the single agent RP2D of crizotinib was 15% (95% CI: 3.3%,34.3%; Table 2). Three patients, all with RECIST measurable disease and an ALK Arg1275Gln mutation, experienced an objective response, two with partial responses and one with a complete response (Figure 1). Figure 2A shows the CT and 123I-MIBG SPECT/CT images at time of enrollment, partial response at cycle 5 and complete response at cycle 13; Figure 2B shows MRI images at baseline, end of cycle 5 and end of cycle 10, as well as whole body MIBG planar images at baseline and end of cycle 10 with near complete resolution of skeletal disease. Two additional patients with an ALK Arg1275Gln mutation, one with RECIST measurable and one with MIBG evaluable disease, had prolonged stable disease (13 and 10 cycles, respectively). The remaining seven patients with an ALK Arg1275Gln mutation received crizotinib for 1–5 cycles without evidence of response, and six of the patients came off protocol therapy for progression of disease (PD). One patient with a composite ALK Arg1275Gln/Phe1174Leu mutation and evaluable disease received therapy for 3 cycles prior to PD. Three patients with ALK Phe1174Leu mutations progressed during cycle 1 of therapy, and one patient with an ALK Phe1174Val stayed on therapy for 3 cycles prior to PD. Two patients with ALK amplification received only 1 cycle of therapy prior to PD. One patient with an ALK Tyr1278Ser mutation, a rare but activating mutation (19), received 3 cycles of therapy prior to PD. No patients with an ALK F1245 mutation, the third most common neuroblastoma-specific ALK hot-spot site, were enrolled on this study.
Table 2.
Overall Best Response.
| Best Overall Response | Alk Status | Total | |||||
|---|---|---|---|---|---|---|---|
| Amplification | Arg1275Gln | Arg1275Gln; Phe1174Leu |
Phe1174Leu | Phe1174Val | Tyr1278Ser | ||
| Complete Response | 1 | 1 | |||||
| Partial Response | 2 | 2 | |||||
| Stable Disease | 2 | 2 | |||||
| Progressive Disease | 2 | 6 | 1 | 3 | 1 | 1 | 14 |
| ★Invevaluable for OBR | 1 | 1 | |||||
| Total | 2 | 12 | 1 | 3 | 1 | 1 | 20 |
This patient was removed from protocol therapy after one cycle of treatment due to toxicity.
Figure 1. Response characteristics in patients with ALK-mutant or amplified neuroblastoma treated with crizotinib at the recommended phase 2 dose.
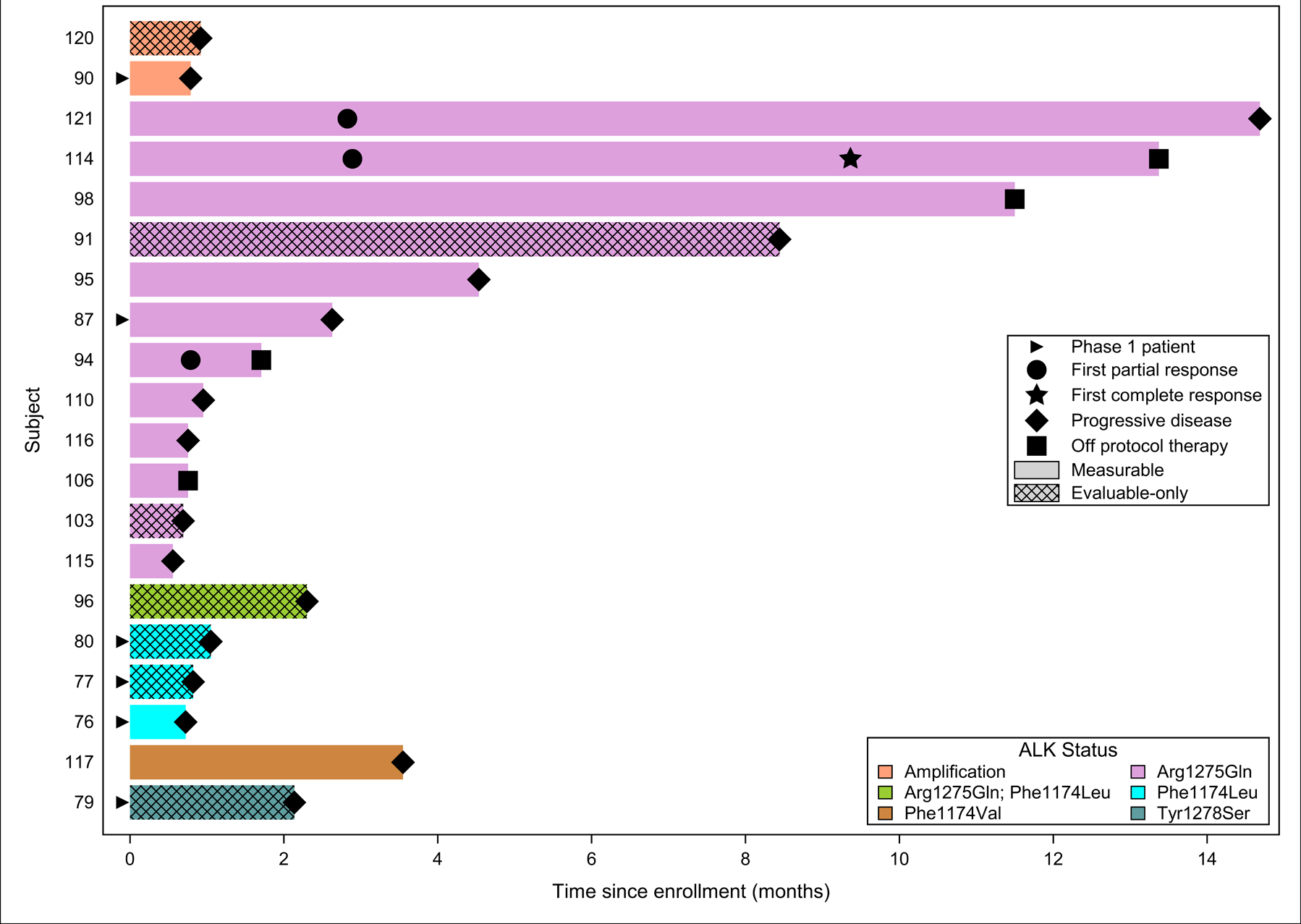
This panel shows response onset duration along with exact ALK mutation for patients with neuroblastoma treated with crizotinib. The length of the bar shows the time until the patient had a PR, CR or came off protocol therapy due to disease progression. Therapy duration did not exceed 15 months. PR, partial response; CR, complete response.
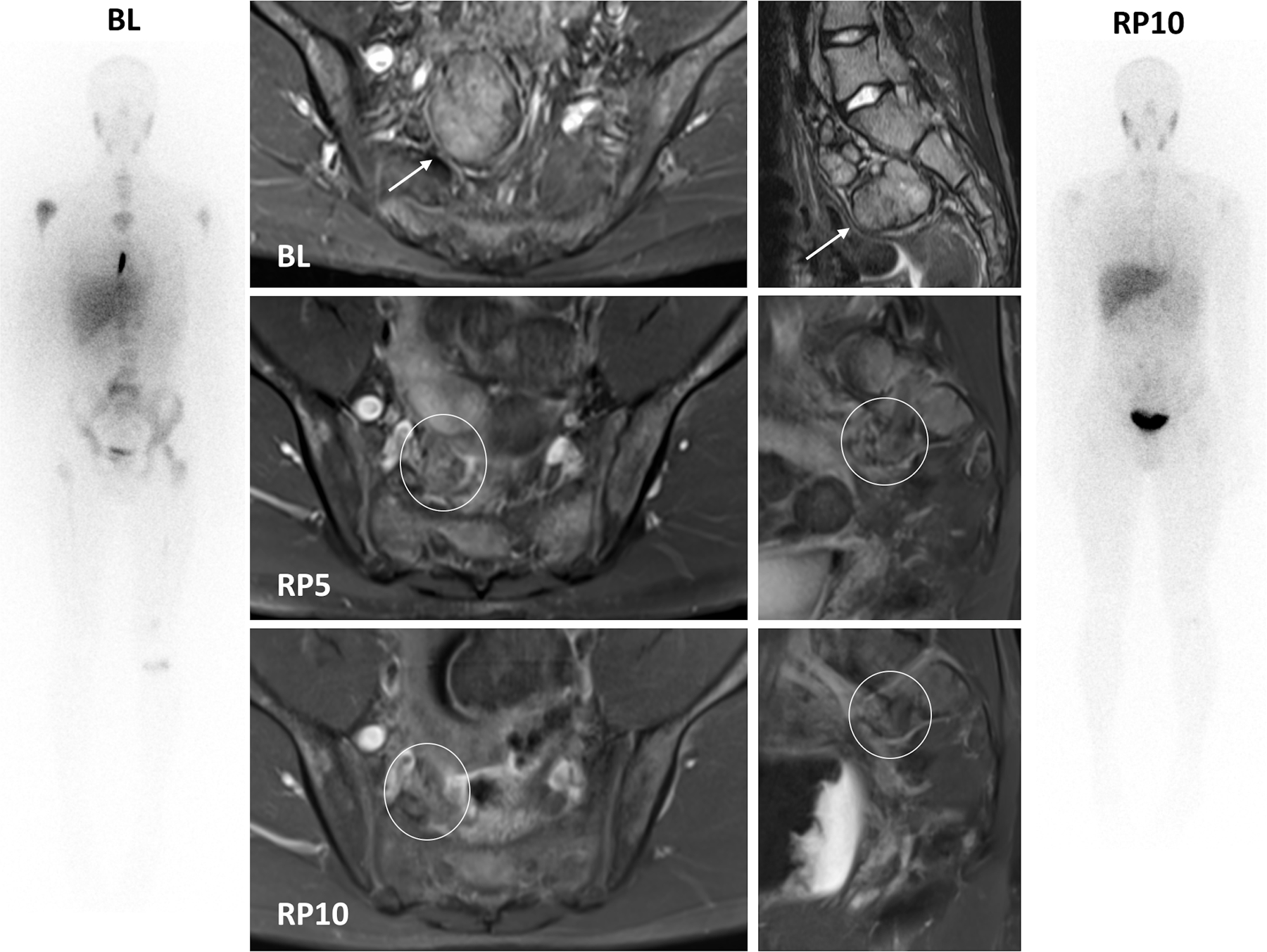
Figure 2. Representative responses to Crizotinib monotherapy in patients with activating Arg1275Gln ALK mutation.
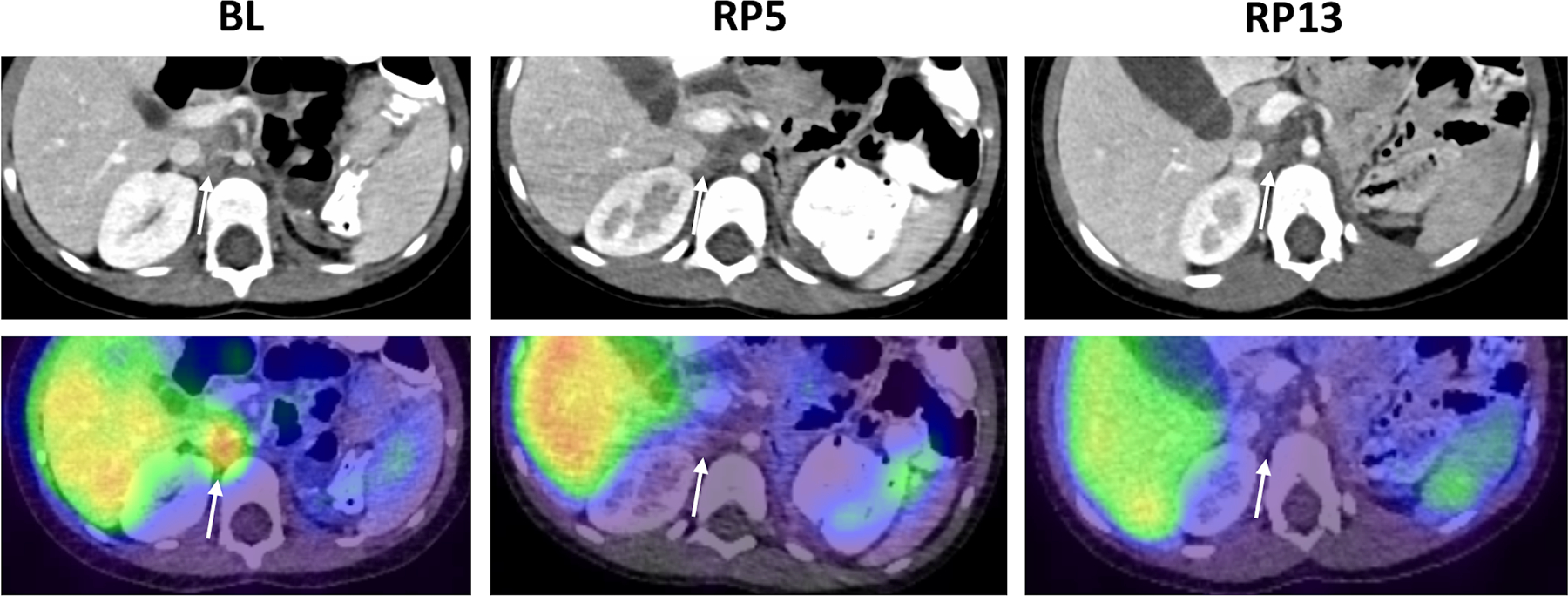
A. Patient 114. Diagnostic CT (upper row) and 123I-MIBG SPECT/CT images (lower row) show response of an enlarged right aortocaval lymph node (arrows) to Crizotinib therapy. Images obtained at baseline and after 5 and 13 cycles of therapy, respectively, show a partial response at RP5, and a complete response at RP13, with no residual lymphadenopathy or MIBG uptake at RP13. A similar pattern of response was also shown for a separate site of retroperitoneal disease (not shown).
B. Patient 121. Whole body MIBG planar images (outer panels), axial (inner left panels) and sagittal (inner right panels) fat-suppressed T2 weighted MRI images show partial response of measurable disease by MRI and MIBG-avid evaluable disease to Crizotinib therapy. The pre-sacral mass present at baseline (BL, arrow) has decreased significantly in size, with only a small focus of measurable disease (circle) remaining by RP10. Multiple sites of MIBG avid disease, including the pre-sacral soft tissue mass, left parietal skull, entire vertebral column, proximal humeri, bony pelvis, proximal femurs, distal left femur and proximal left tibia, have either resolved (left parietal skull) or are significantly decreased in signal intensity, with only faint uptake remaining at sites of baseline MIBG avid disease.
Pharmacokinetics
PK analysis was available for 13/20 patients, with a median age of 6 years (range 2 −14 years). The median steady state concentration of crizotinib was 665 ng/mL (range 298 – 1040 ng/mL). Of the three patients with a response (1CR, 2PR), one patient with a PR had steady state concentration data available (923 ng/mL). These values are similar to those previously reported from the phase 1 component of the clinical trial(17).
Discussion
Survival rates for the childhood cancer neuroblastoma have improved incrementally from 25% to 50% over three decades with dramatic escalation in therapy intensity. We and others discovered in 2008 that activating mutations in the tyrosine kinase domain of ALK are the cause of hereditary neuroblastoma, and that these mutations can also be somatically acquired(1, 2, 4, 5). This work drove the early-phase clinical trial of crizotinib in patients with relapsed/refractory pediatric tumors, with emphasis on enrollment of ALK-driven cancers. ADVL0912 (NCT00939770) opened to accrual within 18 months of the initial discovery of recurrent ALK mutations in a neuroblastoma, thus rapidly translating these seminal preclinical molecular findings into the clinic. Notably, this is the first clinical demonstration that ALK is a tractable molecular target in neuroblastoma, as well as demonstration that the preclinical cellular, biochemical and in vivo data accurately predicted the differential sensitivity of the ALK R1275 versus F1174 mutations, albeit the small number of patients treated.
Early preclinical studies showed that crizotinib had activity against neuroblastoma cell lines and xenografts harboring activating ALK mutations, with mutations at two of the three hotspots demonstrating intrinsic resistance to ALK kinase inhibition with crizotinib (19, 20). The relative resistance in F1174- and F1245-ALK neuroblastoma mutations result from an increase in ATP-binding affinity(20) and less binding of crizotinib to the target. Preclinical experiments demonstrate that neuroblastomas harboring an F1174-ALK mutation may benefit from ATP-competitive ALK inhibitors such as crizotinib if an increase in dosage to overcome the relative affinity for ATP compared with R1275-mutated ALK was possible (19, 20). In Phase 1, we maximized the dose of crizotinib in pediatric patients in order to achieve higher plasma concentrations to overcome the increased ATP-binding affinity of de novo resistant ALK mutations. We ultimately defined a crizotinib recommended phase 2 dose in children that is nearly 200% of the adult maximum tolerated dose(14).
In the phase 1 portion, we previously reported five patients with neuroblastoma and known ALK mutations treated at dose levels other than the RP2D (100 – 365 mg/m2/dose twice daily), and six patients treated at the RP2D who are included in the Phase 2 trial, three previously reported without response and three not previously reported upon. Of these patients, one had a complete response and had a germline Arg1275Gln mutation (treated above the RP2D at 365 mg/m2/dose); two had prolonged stable disease at dose levels below the RP2D, one of whom had a germline Arg1275Gln mutation and resolution of MIBG-avid disease(14). The other three borrowed patients treated in the Phase 1 trial at the RP2D have not been previously reported; they were enrolled on the A2 expanded cohort for patients with confirmed ALK alterations that could enroll at any time. In the phase 2 portion of the trial, where crizotinib was administered orally twice daily as monotherapy at the RP2D of 280 mg/m2/dose twice-daily, three out of twelve patients with an activating Arg1275Gln ALK mutation had objective responses (1 CR, 2 PR), two had prolonged stable disease, and six of the remaining seven patients had early disease progression (Figure 1; Table 2). No responses were seen in patients harboring ALK F1174 mutations or ALK amplifications.
At the 280 mg/m2 dose level in the phase 1 portion of ADVL0912 (NCT00939770), the steady state average plasma concentration (Cave) was 580 ± 173 ng/mL or 1290± 380nM (mean ±SD) (17). The protein binding of crizotinib is 90%. Therefore, the Cave steady state free drug concentration is estimated to be 129 nM which is equivalent to the IC50 for F1174L ALK mutation (130±10 nM) and only marginally higher than the IC50 for R1275Q ALK mutation (85 ± 8 nM). This suggests that 50% inhibition of ALK mutations may be insufficient to produce a therapeutic effect.(19,20) In the phase 2 component of the study, the mean steady state Cave was variable, ranging from 298 ng/mL (660 nM) to 1040 ng/mL (2310 nM); one patient with a PR had a steady state concentration of 923 ng/mL (2050 nM). PK data was not available for the other 2 patients who had a response. Thus, given the protein binding of crizotinib (90%), it appears likely that the free drug concentrations of crizotinib achieved in plasma even at the 280 mg/m2 dose level, may be insufficient to inhibit ALK kinase activity. Early on in the era of precision medicine, blockade of BRAF-mutant melanoma, using tyrosine kinase inhibitors demonstrated that complete pathway inhibition is necessary for objective anti-tumor activity(21). Our preclinical data suggest the same is true for ALK-aberrant neuroblastomas and that the reduced sensitivity to crizotinib is caused by a heightened ATP-binding affinity in tumors with an F1174 of F1245 mutation. In the context of neuroblastoma-specific ALK mutations, crizotinib is likely just good enough to have some effect for only the R1275 mutations because ATP binding is weaker in this context(20), and without complete abrogation of ALK signaling, the remaining kinase activity is likely sufficient to maintain tumor growth, even in the setting of an R1275 mutation.
In summary, crizotinib is active against a subset of Arg1275Gln ALK mutated neuroblastomas, with possible prolonged utility in the setting of a germline mutation, a context where ALK is likely the initiating truncal mutation in the disease; however, crizotinib has no objective single agent activity in neuroblastomas harboring other hotspot ALK mutations or amplification. High-risk neuroblastomas are characterized by extensive intra-tumoral and stroma-derived heterogeneity and harbor both pre-existing and acquired subclonal populations, as well as spatial intra-tumoral heterogeneity and temporal clonal evolution, that confer therapy resistance, rendering early phase studies challenging, even in the context of exploiting an oncogenic driver. We have shown that crizotinib in combination with chemotherapy can overcome the intrinsic resistance of ALK hotspot mutations by a synergistic interaction through the intact p53 pathway(22). These data provided the preclinical rationale for the ongoing COG phase 3 trial ANBL1531 (NCT03126916).
As the field has moved forward, lorlatinib has been identified as a highly specific and potent third generation ALK inhibitor that overcomes de novo resistance and exerts unprecedented activity in neuroblastoma patient derived xenografts harboring all hotspot mutations(23). Lorlatinib is now completing Phase 1 (NCT03107988) evaluation in children and adults with relapsed/refractory ALK-driven neuroblastoma, and has confirmed anti-neuroblastoma activity with minimal observed toxicity(24). Lorlatinib will be studied in the upfront setting for patients with ALK aberrant neuroblastoma, replacing crizotinib in the current Phase 3 trial (NCT03126916) for patients with high-risk neuroblastoma. Thus, ALK inhibition is an important strategy for improving the outcomes of children with neuroblastoma.
As we learn more about how cancers evolve under the selective pressure of anti-tumor therapy, we are recognizing that this does not occur through a linear stepwise selection of genetic changes. Genomic alterations occurring during neuroblastoma progression and the resulting genomic heterogeneity and mutational diversity between intra-patient tissues remain poorly understood, but it is likely that ALK driver mutations can occur as a branched or truncal mutation, as seen by the increased frequency of ALK mutations at relapse (3, 6, 7), whereas MYCN amplification is always a truncal event and completely shared between lesions. The overall effect of these factors on the efficacy of treatment is daunting; however, multi-regional sequencing, single-cell sequencing, and serial profiling of plasma circulating tumor DNA are all emerging technologies that will allow us to more precisely dissect the subclonal complexity and spatio-temporal genomic heterogeneity in order to develop more effective personalized therapies.
Supplementary Material
Statement of Translational Relevance.
Gain-of-function mutations in the ALK oncogene occur in 15% or more of newly diagnosed patients with high-risk neuroblastoma. This discovery positioned ALK as the first tractable molecular target for patients with this disease. However, crizotinib showed limited anti-tumor activity in this phase 2 trial for patients with relapsed ALK+ neuroblastoma. The preclinical mechanism underlying this observation revealed that two of the three hot spot mutations in ALK confer intrinsic resistance to crizotinib due to preferential affinity for ATP binding that could potentially be overcome by higher drug exposures. The observed responses occurred in patients with the most common both germline and somatic hot spot mutation at residue R1275. Despite limited activity and lack of objective responses in patients harboring de novo resistant ALK mutations, we conclude that, while this was possibly a limitation of the number of patients enrolled, this is more likely due to an inability to reach the higher concentrations of crizotinib needed to overcome the competing ATP affinity. Emerging data with the third generation ALK inhibitor lorlatinib shows promise for patients with ALK-driven neuroblastoma.
Acknowledgments
Research was supported by the NCI of the NIH under award number UM1 CA097452 and UM1 CA228823, Cookies for Kids’ Cancer Foundation, the Children’s Oncology Group Foundation and Pfizer, Inc.
Footnotes
Conflicts of Interest:
Yael P Mossé, MD- the senior author on this manuscript, is an active consultant for Pfizer.
Keith Wilner, PhD is an employee of Pfizer and stockholder at the company.
REFERENCES
- 1.Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature 2008;455(7215):971–4. [DOI] [PubMed] [Google Scholar]
- 2.George RE, Sanda T, Hanna M, Frohling S, Luther W, 2nd, Zhang J, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 2008;455(7215):975–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eleveld TF, Oldridge DA, Bernard V, Koster J, Daage LC, Diskin SJ, et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet 2015;47(8):864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 2008;455(7215):930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janoueix-Lerosey I, Lequin D, Brugieres L, Ribeiro A, de Pontual L, Combaret V, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 2008;455(7215):967–70. [DOI] [PubMed] [Google Scholar]
- 6.Padovan-Merhar OM, Raman P, Ostrovnaya I, Kalletla K, Rubnitz KR, Sanford EM, et al. Enrichment of Targetable Mutations in the Relapsed Neuroblastoma Genome. PLoS Genet 2016;12(12):e1006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schleiermacher G, Javanmardi N, Bernard V, Leroy Q, Cappo J, Rio Frio T, et al. Emergence of New ALK Mutations at Relapse of Neuroblastoma. J Clin Oncol 2014;32(25):2727–34. [DOI] [PubMed] [Google Scholar]
- 8.George RE, Attiyeh EF, Li S, Moreau LA, Neuberg D, Li C, et al. Genome-wide analysis of neuroblastomas using high-density single nucleotide polymorphism arrays. PLoS ONE 2007;2(2):e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448(7153):561–6. [DOI] [PubMed] [Google Scholar]
- 10.Crystal AS, Shaw AT. New targets in advanced NSCLC: EML4-ALK. Clin Adv Hematol Oncol 2011;9(3):207–14. [PubMed] [Google Scholar]
- 11.Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. The lancet oncology 2011;12(11):1004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw AT, Solomon B, Kenudson MM. Crizotinib and testing for ALK. J Natl Compr Canc Netw 2011;9(12):1335–41. [DOI] [PubMed] [Google Scholar]
- 13.Chabner BA. Early accelerated approval for highly targeted cancer drugs. N Engl J Med 2011;364(12):1087–9. [DOI] [PubMed] [Google Scholar]
- 14.Mosse YP, Lim MS, Voss SD, Wilner K, Ruffner K, Laliberte J, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol 2013;14(6):472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosse YP, Voss SD, Lim MS, Rolland D, Minard CG, Fox E, et al. Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children’s Oncology Group Study. J Clin Oncol 2017;35(28):3215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JR, Bagatell R, Cohn SL, Pearson AD, Villablanca JG, Berthold F, et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol 2017;35(22):2580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balis FM, Thompson PA, Mosse YP, Blaney SM, Minard CG, Weigel BJ, et al. First-dose and steady-state pharmacokinetics of orally administered crizotinib in children with solid tumors: a report on ADVL0912 from the Children’s Oncology Group Phase 1/Pilot Consortium. Cancer Chemother Pharmacol 2017;79(1):181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Stat Med 2008;27(16):3145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bresler SC, Weiser DA, Huwe PJ, Park JH, Krytska K, Ryles H, et al. ALK Mutations Confer Differential Oncogenic Activation and Sensitivity to ALK Inhibition Therapy in Neuroblastoma. Cancer Cell 2014;26(5):682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bresler SC, Wood AC, Haglund EA, Courtright J, Belcastro LT, Plegaria JS, et al. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci Transl Med 2011;3(108):108ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 2010;467(7315):596–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krytska K, Ryles HT, Sano R, Raman P, Infarinato NR, Hansel TD, et al. Crizotinib Synergizes with Chemotherapy in Preclinical Models of Neuroblastoma. Clin Cancer Res 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Infarinato NR, Park JH, Krytska K, Ryles HT, Sano R, Szigety KM, et al. The ALK/ROS1 Inhibitor PF-06463922 Overcomes Primary Resistance to Crizotinib in ALK-Driven Neuroblastoma. Cancer Discov 2016;6(1):96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldsmith KC, Kayser K, Groshen SG, Chioda M, Thurm HC, Chen J, et al. , editors. Phase I trial of lorlatinib in patients with ALK-driven refractory or relapsed neuroblastoma: A New Approaches to Neuroblastoma Consortium study. American Society of Clinical Oncology; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


